Abstract
In this study effects of acid hydrolysis, autoclaving and storage (95°C for 2, 3, and 4 days) on resistant starch (RS) formation in corn starch were investigated and functional properties of RS preparations were determined. RVA peak and final viscosity values of the RS preparations decreased gradually for each storage period with increasing hydrolyzation level. RS contents increased to 13.6–16.7% as a result of storage. RS contents of the samples dried without storage were lower than those of the stored samples up to 3.5 h hydrolysis. Solubility and water binding values of RS preparations were higher than those of the native and hydrolysed samples. In contrast to RS preparations, the native and hydrolysed samples affected the emulsion properties of soy protein inversely.
INTRODUCTION
Resistant starch (RS) is the starch fraction that is not hydrolyzed in the small intestine but may be fermented in the colon. Due to its similar physiological properties, it is generally considered as a constituent of dietary fiber. RS contents in food range approximately between 0–4%. A higher amount of RS in the daily diet is recommended due to its preventative and therapeutical health effects.[Citation1] RS is a fermentation substrate for colonic bacteria and hence has potential for prebiotic applications. Fermentation of RS produces short-chain fatty acids such as acetate, propionate and butyrate. These fatty acids lower the overall pH of the colon, induce chemoprotective enzyme activity and hinder growth of harmful colonic bacteria and thus play a role in protecting against colorectal cancer. Other benefits of RS include lowering of plasma cholesterol and blood lipids as well as improving glucose tolerance.[Citation2,Citation3]
There are four types of resistant starches. RS1: physically inaccessible starch locked within cell walls, RS2: native granular starch, RS3: retrograded or crystalline starch and RS4: chemically modified starches.[Citation7–9] RS3 is the most common in the human diet because it is formed mainly as a result of food processing.[Citation8] Processing of raw materials in most cases destroys RS1 and RS2, but it can produce RS3. Most researches suggest that RS formed during processing is associated with amylose retrogradation.[Citation9,Citation10] The RS1 and RS2 are slowly but completely digested with appropriate pre-processing of foods, but RS3 totally resists digestion. The RS3 contents of foods are generally low; levels up to 3% have been reported in baked foods, pasta and processed cereals. RS3 content of starches can be increased by heating and cooling cycles.[Citation11] Flexible linear amylose molecules align themselves after gelatinization into tight linear configurations that form helices, making many of the α-1,4 glucosidic linkages inaccessible to amylase.[Citation7] Many factors may influence the crystallization process, and thus the formation of RS, such as amylose content and chain length, autoclaving temperature, storage time and temperature of the starch gels.[Citation12]
Food applications of resistant starch are of interest to product developers and nutritionists mainly due to fiber-fortification and the potential physiological benefits as well as unique technological properties. RS gives better cereal products, especially in terms of texture, mouthfeel, flavor, color and mineral bioavailability which are not attainable with traditional insoluble fibers.[Citation11,Citation13,Citation14] Besides their nutritional value starches have been used for their functional properties. There are some studies investigating the swelling, solubility and water binding capacity values[Citation15,Citation16,Citation17] and emulsion properties[Citation18,Citation19] of various starches. However, to the best of our knowledge, there are no studies investigating the effects of RS preparations on emulsion properties. The objective of this study was to investigate the effects of acid hydrolysis, heat treatment and subsequent storage on RS formation in corn starch. Functional properties of the RS preparations were also investigated.
MATERIALS AND METHODS
Materials
Normal corn starch with an amylose content of around 25% was obtained from Cargill Inc., Istanbul, Turkey.
Acid Modification
Corn starch (80 g) was suspended in 120 mL 1.64 M HCl and incubated at 40°C for various periods of time (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 h). After the incubation period, the pH of the suspension was adjusted to 6 with 10% NaOH. Then the samples were washed three times with distilled water and centrifuged (Heraus Labofuge, Germany) at 1000 rpm for 5 min.[Citation20] The washed samples were dried at 40°C and ground to pass through 212 μm sieve. Moisture contents of the samples were determined according to the standard AACC method.[Citation21]
Resistant Starch Formation
For resistant starch formation, a set of four samples for native and acid hydrolyzed starch samples were suspended in water (1:10) and gelatinized at 85°C for 15 min. Then they were autoclaved at 121°C for 30 min and three samples from each set were stored at 95°C for 2, 3, and 4 days. They were dried at 50°C, ground and sifted through 212 μm sieve. The fourth sample of each set was dried after autoclaving without storage and used as the control of the set. RS contents of the samples were determined by the enzymatic-gravimetric procedure.[Citation22]
Pasting Properties
Pasting properties of the samples were tested using a Rapid ViscoAnalyzer (RVA 4, Newport Scientific, Australia). A 4 g sample and 25 g distilled water were placed in an aluminum sample canister. The RVA pasting curve was obtained by using a 30 min test profile; initial equilibrium at 30°C for 10 min, heating to 95°C over 6 min, holding at 95°C for 5 min, cooling to 50°C over 5 min, and holding at 50°C for 4 min. The peak viscosity, breakdown viscosity and final viscosity values were evaluated with the data analysis software (Thermocline for Windows, Newport Scientific, Australia).
Functional Properties
Solubility values of the samples were determined using a method based on Singh and Singh.[Citation23] A 0.5 g sample was added to 5 mL distilled water and vortexed for 15 sec every 5 min. After 40 min it was centrifuged (Heraus Labofuge, Germany) at 600 xg for 10 min. Supernatant was dried at 100°C and solubility was calculated as follows:
Precipitate was weighed and then dried at 100°C. Water binding was calculated as follows:
For the determination of fat binding capacity, 1 g sample was mixed with 10 mL corn oil in a centrifuge tube and vortexed for 1 min. After holding a period of 30 min, the tube was centrifuged (Heraus Labofuge, Germany) at 2100 xg for 10 min. Fat binding capacity was expressed as the amount (ml) of oil bound by 100 g sample.[Citation24]
Emulsion capacity and stability values were determined according to Ahmedna et al. [Citation25] and samples were prepared according to Abdul-Hamid and Luan.[Citation26] Five mL of 7% dispersion of the starch sample (prepared with 0.05% soy protein solution) was mixed with 5 mL of corn oil and homogenized at 23 500 rpm for 1 min (Art-Miccra D-8, Germany). Then it was centrifuged (Heraus Labofuge, Germany) at 2100 × g for 20 min. The ratio of the height of the emulsified phase to the height of total liquid was expressed as emulsion capacity (%). For the determination of emulsion stability, homogenized sample was incubated at 45°C for 30 min and then centrifuged (Heraus Labofuge, Germany) at 2100 xg for 20 min. The ratio of the height of the emulsified phase to the height of total liquid was expressed as emulsion stability (%).
Statistical Analysis
Data were analyzed for variance using the MSTAT-C statistical program. When significant differences were found, the LSD (Least Significant Difference) test was used to determine the differences among means.[Citation27]
RESULTS AND DISCUSSION
Pasting Properties
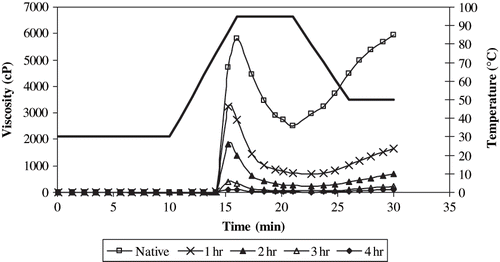
RVA pasting properties of native and acid modified starch samples are presented in and the pasting curves of selected acid modified starch samples (1, 2, 3, and 4 h hydrolysed) are shown in . All of the viscosity values of acid modified starch samples were found to be less than those of the native starch. As the level of acid modification increased, all of the RVA viscosity values of the hydrolysates significantly (p < 0.05) decreased. As indicated in the related literature, acid hydrolysis causes reduction in the molecular weight of starch.[Citation28] The decreases in the viscosity values observed in the present study might be due to the reduced molecular weight caused by acid hydrolysis.
Table 1 Pasting properties of native and acid modified corn starch samples
RVA pasting properties of RS preparations are presented in and the pasting curves of selected samples (native and 1, 2, and 4 h hydrolysed, stored for 0 and 3 days) are shown in . Peak and final viscosity values of the RS preparations decreased gradually for each storage period as the level of acid modification increased (). In general, RVA viscosity values of the samples dried without storage were significantly (p < 0.05) higher than those of the samples stored for 2, 3, and 4 days. The breakdown and final viscosity values of the 0, 2, 3, and 4 days stored samples produced from 4 h hydrolysed sample were not significantly different. On the other hand, there was generally no regular effect of storage period (2, 3, and 4 days) at 95°C on the peak and final viscosity values of RS preparations within each hydrolysis level. However, the difference in breakdown viscosity values of 2, 3, and 4 days stored samples were not significant (p < 0.05).
Table 2 Pasting properties of resistant starch preparations
Figure 2 The pasting curves of resistant starch preparations obtained from native and acid hydrolysed starches (hydrolysis time: 1, 2, 4 hr). a: Unstored sample; and b: sample stored for 3 days.
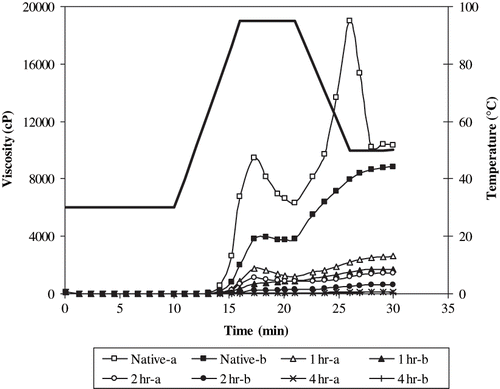
Since the starch is gelatinized prior to RS formation, it is expected to observe a viscosity value higher than “0” at the initial stage of RVA curve (before heating). This is referred to as cold peak viscosity in the related literature.[Citation29] However, in the present study, such cold peak viscosity values were not observed in the RVA curves () and the viscosity values increased as the temperature increased. These results are in agreement with those of Becker et al.[Citation30] The lack of cold peak viscosity might probably be due to loose rearrangement of starch chains with a few (small number of) H-bonds. This can be different from regular retrogradation of starch during RS formation. The loose rearrangement of starch chains might prevent water uptake at the initial stages of RVA test. Later on, viscosity increased as a result of water uptake by the system following H-bond disruption due to heating. Then a peak is formed. Although, the reason for the formation of this peak is different, its shape is similar to the one observed during starch gelatinization.
Resistant Starch Content
Resistant starch contents of the samples are presented in . While native and acid hydrolysed starch samples did not contain any RS (data not presented in ), the RS contents increased to 13.6–16.7% as a result of storage at 95°C for different periods after autoclaving. For each storage period (2, 3, and 4 days), the resistant starch content increased as the hydrolyzation degree increased up to 2.5 h hydrolyzation level. Above this level, RS contents decreased. On the other hand, as indicated by overlapping error bars, there was no significant difference between the RS contents of the samples hydrolysed at the same level but stored at different periods (2, 3, and 4 days) at 95°C after autoclaving.
Figure 3 Resistant starch contents of the acid hydrolysed samples autoclaved and stored at 95°C for different periods.
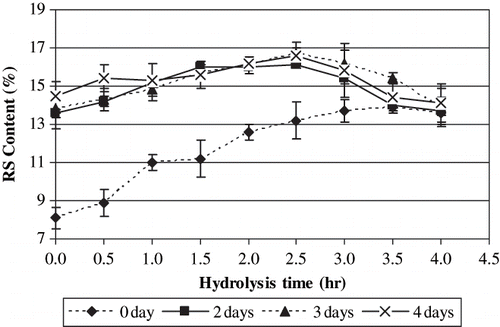
RS contents of the samples dried without storage increased from 8.1 to 13.9% as the hydrolyzation degree increased. RS contents of these samples were lower than those of the stored samples (2, 3, and 4 days) at all hydrolysis levels up to 3 h. However, RS contents of the samples dried without storage and those of the stored samples were comparable for 3.5 and 4.0 h hydrolysed samples. It has been also reported in earlier literature that acid-pretreated starch produced higher RS values than the native starch which might be attributed to increased rate of retrogradation in acid-hydrolysed starch.[Citation31] Partial acid hydrolysis produces short linear chains enhancing the mobility of the molecules. The shorter linear chains appear to participate in the formation of resistant portions through rearrangement and recrystallization of starch during autoclaving and cooling.[Citation32] In the present study, there are two major factors affecting RS formation: the molecular size (hydrolysis time) and storage time after autoclaving. Molecular size influences RS formation by affecting the mobility of starch chains. For the stored samples, because of the availability of adequate time for RS formation, the molecular size of the samples plays the major role in RS formation. Optimum RS content was achieved at 2.5 h hydrolysis time, and above this level, the RS contents decreased due to excessive hydrolyzation. In the samples prepared without storage, RS formation occurs only during drying. Although, at each hydrolysis time the stored and unstored samples have the same average molecular size, the time during drying of unstored samples is not enough for achieving (high) RS contents comparable to those of the stored ones. So, a steady increase in RS content was observed in the samples prepared without storage, as the hydrolysis time increased (upto 3.5 h hydrolysis). But the RS contents were still lower than those of the stored samples.
Functional Properties
Solubility, water binding capacity, emulsion capacity, and stability of the native and acid hydrolysed starch samples and RS preparations are shown in . While the solubility values of the native and acid hydrolysed samples were less than 1%, the solubility values of the RS preparations were higher and increased up to 5.2% with increasing hydrolysis time and storage at 95°C (). Water binding values of the native and acid hydrolysed samples were low and the highest water binding value was obtained for the 4.0 hr acid hydrolysed sample (120.3%). However, the water binding values of RS preparations were much higher (at least two-fold) than those of the acid hydrolysed samples. The increase in water binding value is mainly due to the gelatinization caused by heating and autoclaving. RS formation does not play a major role in this respect. It is thought that these products could be used in food formulations in which relatively higher water binding properties are desired as compared to native starch. As the hydrolysis time increased, water binding values of RS preparations generally decreased ().
Figure 4 Functional properties of the native and acid hydrolysed starch samples and resistant starch preparations produced by autoclaving and storage at 95°C for different periods. a) Solubility; b) water binding; c) emulsion capacity; and d) emulsion stability.
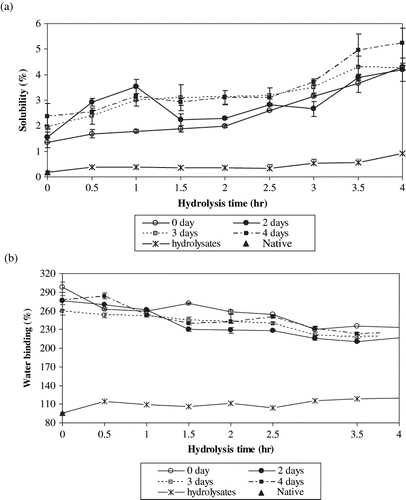
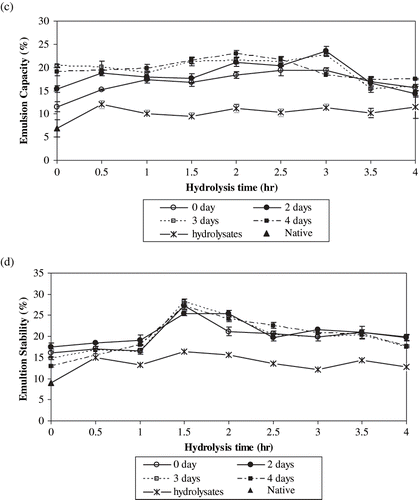
There are some studies investigating the solubility and water binding capacity values of different starches. Singh et al.[Citation17] found that pea starch had water binding capacity of 0.13–4.15 g/g and solubility of 12.5%. Aparicio-Saguilan et al.[Citation15] reported that solubility of native banana starch increased with increasing temperature. Autoclaved samples presented lower solubility than corresponding raw materials (native and lintnerized samples). Sandhu and Sing[Citation16] reported that solubility and water binding capacity values of starches from different corn lines were in the range of 15.3–22.4% and 77.6–88.5%, respectively. The water binding capacity results of the present study were comparable with those of Sandhu and Sing[Citation16] but the solubility values determined in the present study were lower. The difference might be due to the variations in method parameters (e.g., temperature).
Proteins are commonly used as emulsion forming and stabilizing agents. On the other hand, starch can not produce emulsion by itself, but might effect emulsion properties. Therefore, in the present study, effects of various starch preparations on the emulsifying properties of soy protein solutions were investigated. Emulsion capacity and emulsion stability values of soy protein solution (0.05%) was found to be 22% and 18%, respectively. Emulsion capacity and stability values of soy protein solution supplemented with the acid modified starch samples were lower than those of the soy protein solution on its own. The results indicated that the native and acid modified starch samples affected the emulsion properties of the soy protein inversely. On the other hand, RS preparations did not have a deteriorating effect on the emulsion capacity values of the soy protein solution. Emulsion capacity of soy protein solution supplemented with RS containing samples prepared from 3.5 and 4.0 hr hydrolysed samples (stored at 95°C for 2, 3, and 4 days) were lower than those of the other samples (). Emulsion stabilities of soy protein solutions supplemented with RS preparations were within the range of 13.0–28.2%. Most of them were higher than that of soy protein solution. An increase in emulsion stability value was observed for the starch samples with low hydrolyzation levels (up to 1.5 h) and then they gradually decreased (d). Although the effects of RS preparations on emulsion properties of soy protein were better than those of the hydrolysates, the RS preparations do not seem to have an improving effect on emulsion properties of soy protein. RS formation does not seem to affect fat binding properties of respective hydrolysates to a considerable extent. All of the RS preparations as well as the hydrolysates were found to have similar fat binding values of 85 ± 10mL/100g.
CONCLUSIONS
The results showed that the RS contents of acid-modified corn starch samples increased up to 16.7% after autoclaving and storage. RS preparations did not have a deteriorative effect on emulsion properties of soy proteins. Heating and autoclaving treatments caused an improving effect on the solubility and water binding properties. The RS preparations obtained in the present study seem to be suitable for the food products, which require relatively higher water binding properties. Further studies are needed to produce resistant starch preparations with improved functional properties.
ACKNOWLEDGMENTS
The authors wish to thank the Scientific and Technical Research Council of Turkey (Project No: TOGTAG-3027) for the financial support, Cargill Inc. for providing corn starch, and Novozymes for providing Termamyl.
Notes
27. Anonymous. User's Guide to MSTAT-C, a Software Program for the Design, Management and Analysis of Agronomic Research Experiments. Michigan State University, East Lansing, MI, 1988.
REFERENCES
- Lehmann , U. , Rössler , C. , Schmiedl , D. and Jacobasch , G. 2003 . Production and Physicochemical Characterization of Resistant Starch Type III Derived from Pea Starch . Nahrung/Food , 47 ( 1 ) : 60 – 63 .
- Niba , L.L. and Hoffman , J. 2003 . Resistant Starch and β-glucan Levels in Grain Sorghum (Sorghum bicolor M.) are Influenced by Soaking and Autoclaving . Food Chemistry , 81 : 113 – 118 .
- Voragen , A.G.J. 1998 . Technological Aspects of Functional Food-related Carbohydrates . Trends in Food Science & Technology , 9 : 328 – 335 .
- Eerlingen , R.C. , Crombez , M. and Delcour , J.A. 1993 . Enzyme-resistant Starch. I. Quantative and Qualitative Influence of Incubation Time and Temperature of Autoclaved Starch on Resistant Starch Formation . Cereal Chem. , 70 ( 3 ) : 339 – 344 .
- Guraya , H.S. , James , C. and Champagne , E.T. 2001 . Effect of Cooling and Freezing on the Digestibility of Debranched Rice Starch and Physical Properties of the Resulting Material . Starch/Stärke , 53 ( 2 ) : 64 – 74 .
- Shin , M. , Woo , K. and Seib , P.A. 2003 . Hot-water Solubilities and Water Sorptions of Resitant Starches at 25°C . Cereal Chem. , 80 ( 5 ) : 564 – 566 .
- Patil , S.K. 2004 . Resistant Starches as Low-carb Ingredients-current Applications and Issues . Cereal Foods World , 49 ( 5 ) : 292 – 294 .
- Kim , S-K. , Kwak , J-E. and Kim , W-K. 2003 . A Simple Method for Estimation of Enzyme- resistant Starch Content . Starch/Stärke. , 55 ( 8 ) : 366 – 368 .
- Berry , C.S. 1986 . Resistant Starch: Formation and Measurement of Starch that Survives Exhaustive Digestion with Amylolytic Enzymes during the Determination of Dietary Fibre . Journal of Cereal Science , 4 : 301 – 304 .
- Sievert , D. and Pomeranz , Y. 1990 . Enzyme Resistant Starch II. Differential Scanning Calorimetry Studies on Heat-treated Starches and Enzyme Resistant Starch Residues . Cereal Chem. , 67 ( 3 ) : 217 – 221 .
- Vasanthan , T. and Bhatty , R.S. 1998 . Enhancement of Resistant Starch (RS3) in Amylomaize, Barley, Field Pea and Lentil Starches . Starch/Stärke , 50 ( 7 ) : 286 – 291 .
- Eerlingen , R.C. , Jacobs , H. and Delcour , J.A. 1994 . Enzyme-resistant Starch. V. Effect of Retrogradation of Waxy Maize Starch on Enzyme Susceptibility . Cereal Chem. , 71 ( 4 ) : 351 – 355 .
- Yue , P. and Waring , S. 1998 . Resistant Starch in Food Applications . Cereal Foods World , 43 ( 9 ) : 691 – 695 .
- Haralampu , S.G. 2000 . Resistant Starch—A Review of the Physical Properties and Biological Impact of RS3 . Carbohydrate Polymers , 41 : 285 – 292 .
- Aparicio-Saguilán , A. , Flores-Huicocheaa , E. , Tovarb , J. , García-Suáreza , F. , Gutiérrez-Meraza , F. and Bello-Péreza , L.A. 2005 . Resistant Starch-rich Powders Prepared by Autoclaving of Native and Lintnerized Banana Starch: Partial Characterization . Starch/Stärke , 57 : 405 – 412 .
- Sandhu , K.S. and Singh , N. 2005 . Relationships between Selected Properties of Starches from Different Corn Lines . International Journal of Food Properties , 8 : 481 – 491 .
- Singh , B. , Nagi , H.P.S. , Sekhon , K.S. and Singh , N. 2005 . Studies on the Functional Characteristics of Flour/starch from Wrinkled Peas (Pisum Sativum) . International Journal of Food Properties , 8 : 35 – 48 .
- Herceg , Z. , Režek , A. , Lelas , V. , Krešić , G. and Franetović , M. 2007 . Effect of Carbohydrates on the Emulsifying, Foaming and Freezing Properties of Whey Protein Suspensions . Journal of Food Engineering , 79 : 279 – 286 .
- Taherian , A.R. , Fustier , P. and Ramaswamy , H.S. 2006 . Effect of Added Oil and Modifed Starch on Rheological Properties, Droplet Size Distribution, Opacity and Stability of Beverage Cloud Emulsions . Journal of Food Engineering , 77 : 687 – 696 .
- Atichokudomchai , N. , Shobsngob , S. and Varavinit , S. 2000 . Morphological Properties of Acid-modified Tapioca Starch . Starch/Stärke , 52 : 283 – 289 .
- American Association of Cereal Chemists . 1990 . Approved Methods of AACC , St. Paul, MN : AACC .
- Association of Official Analytical Chemists . 1998 . Official Methods of Analysis , Arlingon, VA : AOAC .
- Singh , J. and Singh , N. 2003 . Studies on the Morphological and Rheological Properties of Granular Cold Water Soluble Corn and Potato Starches . Food Hydrocolloids , 17 : 63 – 72 .
- Lin , M.J.Y. , Humbert , E.S. and Sosulski , F.W. 1974 . Certain Functional Properties of Sunflower Meal Products . Journal of Food Science , 39 : 368 – 370 .
- Ahmedna , M. , Prinyawiwatkul , W. and Rao , R.M. 1999 . Solubilized Wheat Protein Isolate: Functional Properties and Potential Food Applications . J. Agric. Food Chem. , 47 ( 4 ) : 1340 – 1345 .
- Abdul-Hamid , A. and Luan , Y.S. 2000 . Functional Properties of Dietary Fibre Prepared from Defatted Rice Bran . Food Chemistry , 68 ( 1 ) : 15 – 19 .
- 27. Anonymous. User's Guide to MSTAT-C, a Software Program for the Design, Management and Analysis of Agronomic Research Experiments. Michigan State University, East Lansing, MI, 1988.
- Wang , L. and Wang , Y-J. 2001 . Structures and Physicochemical Properties of Acid-thinned Corn, Potato and Rice Starches . Starch/Stärke , 53 : 570 – 576 .
- Whalen , P.J. , Bason , M.L. , Booth , R.I. , Walker , C.E. and Williams , P.J. 1997 . Measurement of Extrusion Effects by Viscosity Profile Using the Rapid ViscoAnalyser . Cereal Foods World , 42 ( 6 ) : 469 – 477 .
- Becker , A. , Hill , S.E. and Mitchell , J.R. 2001 . Relevance of Amylose-lipid Complexes to the Behaviour of Thermally Processed Starches . Starch/Stärke , 53 : 121 – 130 .
- Wang , Y-J. , Truong , V-D. and Wang , L. 2003 . Structures and Rheological Properties of Corn Starch as Affected by Acid Hydrolysis . Carbohydrate Polymers , 52 : 327 – 333 .
- Shin , S. , Byun , J. , Park , K.H. and Moon , T.W. 2004 . Effect of Partial Acid Hydrolysis and Heat-moisture Treatment on Formation of Resistant Tuber Starch . Cereal Chem. , 81 ( 2 ) : 194 – 198 .