Abstract
The objective of this work was to develop a model meat emulsion in order to study the release of aroma-related compounds from the lipid and aqueous phases. Hexanal, octanal and nonanal were taken as indicators of lipid oxidation whereas 2-ethyl-3,5-dimethyl pyrazine and 2-methyl pyrazine were Maillard reaction indicators. Four systems were studied: I) a model meat emulsion; II) phosphate buffer; III) phosphate buffer + myofibrillar proteins; and IV) canola oil. Release of indicator compounds from each of the studied systems was analyzed in the headspace. It was concluded that pyrazines mainly contributed to aroma in lipid systems and in protein solutions but not in emulsions and non protein aqueous systems. Hexanal, octanal, and nonanal were minor aroma contributors in lipid media, whereas hexanal and nonanal were released in small amount from protein emulsions. Conversely, octanal can be considered of an important aroma contributor in emulsions; the three aldehydes showed a high release from aqueous systems, with and without protein.
INTRODUCTION
Meat aroma comprises a wide variety of chemical compounds. It is the result of a number of factors such as substrate composition, pH, water activity, processing and storage conditions, and gas atmosphere, among others. It has been reported as composed of approximately 700 chemical compounds, generated in the fat and lean meat.[Citation1] However, concentration and type of meat aroma compounds varies with species, breed, premortem handling, and processing, among other factors. Although type and concentration of volatiles present in meats result from diverse conditions and characteristics, aldehydes such as hexanal, octanal, and nonanal[Citation2,Citation3] and pyrazines[Citation4,Citation5] are indicators of meat aroma. Contribution of chemical compounds to meat aroma depends on their release from the food matrix which, in turn, depends on ion strength, temperature, presence and concentration of other compounds, and hydrophobicity. Proteins particularly affect aroma perception due to interactions with aroma-related compounds.[Citation6] Lipids greatly influence flavor through their effect on perception (mouth feel, taste and aroma), flavor generation and stability,[Citation7] whereas carbohydrates tend to increase retention in the matrix.[Citation8] Food structure is also associated to release of aroma compounds. In a two-phase system such as a protein emulsion, the concentration of these compounds in the lipid and aqueous phases, as well as the interface results in specific contributions due to diffusion to the gas phase.[Citation9]
The objective of this work was to develop a model meat emulsion in order to study the contribution of oil (disperse) and aqueous (continuous) phase to meat aroma by comparing the volatility of five compounds taken as indicators: three aldehydes (hexanal, octanal and nonanal) and two pyrazines (2-ethyl-3,5-dimetyl pyrazine and 2-methyl pyrazine).
MATERIALS AND METHODS
Raw Material
Pork was obtained from five animals 24 h after slaughtering in a certified local abattoir; no antemortem handling was recorded. Longissiumus dorsi muscles were excised and taken to our laboratory in a portable cooler at 6 to 8°C within 20 min. The meat was cut into 2 cm3 cubes, vacuum packed in 100 g portions using a Multivac D-8941 (Koch, Kansas City, OK) at −700 mBar vacuum, and stored at −20°C until used for experiments.
Meat pH was measured in 10 g meat homogenized with 90 mL distilled water for 1 min; the slurry was filtered and pH measured in the filtrate with a potentiometer (Beckman, Fullerton, CA).
Myofibrillar Protein Extraction
Meat proteins were extracted according to the method reported by Ngapo et al.[Citation10] Homogenized samples in water and ice (1:1:1) were stirred for 10 min in an ice bath, the connective tissue was separated by filtration through a cheese cloth; 100 mL cold water (approximately 6°C) was mixed and stirring continued for 15 min. The homogenized mixture was centrifuged at 4,000 × g for 30 min, 4°C; the supernatant was discarded and the precipitate resuspended in 0.6 M NaCl, 50 mM phosphate buffer, pH 7. Protein concentration (9.112 mgprotein/mL) was analyzed by the method described by Peterson.[Citation11]
Protein Extract Characterization
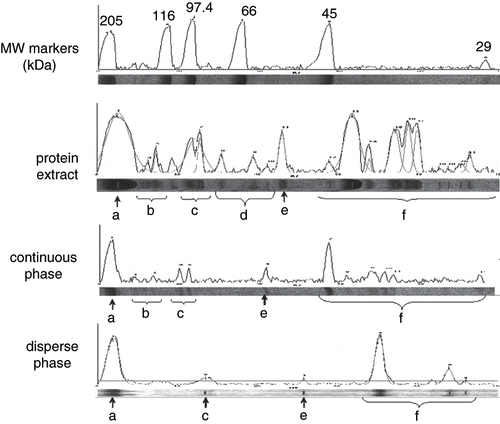
Molecular weight of proteins in the meat extracts was carried out by SDS-PAGE, according to the method described by García et al.,[Citation12] in 12% polyacrylamide separation gels and 4% stacking gels using a Mini-Protean II slab cell (Bio-Rad, Richmond, CA); 5 mg protein extract/mL and buffer solution (10% SDS+5% mercaptoethanol+0.05% bromophenol blue+10% glycerol+0.125% 0.5 M Tris-HCl, pH 6.8) (1:30) was heated at 97°C for 3 min. Fifteen microliters protein samples and molecular weight markers (205 KDa myosin, 116 kDa β-galactosidase; 97.4 kDa phosphorilase b; 66 kDa bovine serum albumin; 45 kDa egg albumin) (Sigma C3312) were applied to the gels. The analysis was carried out at 200 V constant voltage and 4 ± 1°C. The gels were stained with 0.1% Coomasie R-250+40% methanol+10% acetic acid for 30 min, destained in 40% methanol+10% acetic acid, and scanned in a Gel-Doc 2000 (Bio-Rad) fitted with a Quantity One™ software, version 4 (Bio-Rad).
Emulsion Formulation and Characterization
Emulsion formulation was attempted using excised pork fat, extracted with organic solvents and finally evaporating the solvent to obtain pure semisolid fat. However, due to the extremely low yield, it was decided to use canola oil as the lipid phase. Although canola oil is rich in oleic and linoleic acids (63 and 20%, respectively), whereas pork fat contain high amount of oleic and palmitic acids (47 and 25%, respectively), it was assumed that, being linoleic acid the most abundant in both lipids, oxidation reaction could proceed in a similar manner.
Preliminary tests on emulsion formulation were carried out using 5 to 25% canola oil and 15 to 25% protein extract in 0.1 M phosphate buffer, pH 7.5. Emulsions of 20 to 25% protein extract + 25% canola oil were stable after 24 h at room temperature. In subsequent experiments, emulsion formation was carried adding canola oil at 1 mL/min up to 25% final concentration to phosphate buffer with protein extract (20, 25, 30, and 35%) and dextrose, homogenizing in an ESGC homogenizer (Mettlen, Switzerland) at 13,000 rpm for 3 min at room temperature (approximately 18°C), the final protein concentration in the emulsion was analyzed by the method reported by Redinbaugh and Turley.[Citation13] Five percent dextrose was added to imitate a commercial meat emulsion formulation, as well as to promote Maillard reactions.
Emulsion capacity was analyzed according to the method reported by Swift et al.[Citation14] based on electric resistivity. An acrylic cylinder (32 mm diameter, 100 mm high), one end closed, was fitted with two copper wires passing through holes drilled 1 cm from the closed end and attached to a digital voltmeter (Tektronics CDM 250, Tektronics, Beaverton, OR). The protein extract was placed in the cylinder and canola oil added with continuous stirring (13,000 rpm). Emulsifying capacity was reported as milliliters of added oil per mg protein at the point were resistivity was interrupted, and related to g protein.
Particle size and size distribution were analyzed in a Malvern Mastersizer equipment, Series 2600 (Malvern Instruments, Malvern, England) applying a log normal model.[Citation15] Apparent viscosity (ηapp) was analyzed with a Brookfield LVDV-1 viscometer (Brookfield Engineering Laboratory, Middleboro, MA) at 0.6 rpm deformation rate, and calculated considering the torque in the linear region between 20 and 60%.[Citation16,Citation17]
Emulsion Disruption
The emulsion was disrupted with a Bransonic ultrasound bath (Danbury, CT) at 25 Hz for 2 h; phase separation was observed in a light microscope Olympus B201 (Melville, NY) at 200 × magnification, fitted to a 35 mm photographic camera (Olympus model SC35). The disrupted emulsion was centrifuged at 4000 × g for 30 min at 3°C to separate the continuous and disperse phases.[Citation16]
Density and Fraction Volume Analysis
Emulsion density before disruption, and density of the disperse and continuous phases after emulsion disruption were analyzed according to the method described by MacClemens[Citation15] and related to disperse phase fraction volume of the following equation:
where: Φdp is disperse phase fraction volume; ρe is emulsion density; ρ1 is continuous phase density; and ρ2 is disperse phase density, respectively.
Protein Analysis in the Continuous and Disperse Phases
Protein molecular weight in the continuous and disperse phases were analyzed by SDS-PAGE as describe before, after emulsion disruption and phase separation.
Volatile analysis
From preliminary emulsion tests it was concluded that the most stable formulation was: continuous phase: 35% myofibrillar protein extract, 0.1 M phosphate buffer and 0.01% sucrose, pH 7.5, Γ = 0.6; disperse phase: canola oil. Three aldehydes (hexanal, octanal and nonanal, 98% purity, Sigma) and two pyrazines (2-ethyl-3,5-dimethyl pyrazine and 2-methyl pyrazine, 96% purity, Sigma) were used as aroma-release indicator compounds. They were added at 0, 2.0, 3.25, 5.0, 5.25, and 7.5 ppm to the following systems: I) canola oil; II) phosphate buffer; III) phosphate buffer+35% protein extract; and IV) model meat emulsion. Each one of the four systems were placed in 10 mL vials and left standing for 1.5 h at room temperature (around 20°C) to release added volatiles. The five indicator compounds were collected using solid phase microextraction (SPME) technique by static headspace analysis.[Citation18] They were adsorbed in PDMS/DVB fibers (Supleco, Bellefonte, PA) exposed 30 min in the headspace at 20°C. Volatile desorption was carried out placing the fiber in a GC-FID injector for 5 min at 200°C. Gas chromatography (Shimadzu GC-21010 equipment, Kyoto, Japan), was carried out at 31.69 psi, 4.1 mL N2/min total flow as carrier gas; 40°C oven initial temperature, 4 min ramp, 200°C final temperature; the equipment was attached with a HP-Wax-59 m column, 0.2 mm ID. Indicator compound concentration in the headspace, released from each system was compared to pure compounds; volatile release was calculated by the following equation:[Citation19]
where: Krel is release index; Cv is concentration of pure compound added to the system; CL is concentration of released compound from the system, respectively.
Experimental Design and Data Analysis
Emulsion formulation
Two experimental designs were studied to obtain the most stable emulsion formulation: a) Fixed variables: pH 7.5, 25% canola oil, Γ = 0.1; source of variation: protein extract concentration (20, 25, 30, 35%; 1.822, 2.278, 2.733 and 3.189 mgprot/mL, respectively); b) Fixed variables: 25% canola oil, Γ = 0.1, 35% protein extract (3.189 mgprot/mL); source of variation: pH (4.5, 5.5, 6.5, 7.5). The response variables for both designs were emulsion capacity and particle size.
Volatile analysis
Samples were assigned to a completely randomized design; the sources of variation were: indicator compounds concentration (2.5, 3.25, 5.0, 6.25, and 7.5 ppm) and studied system (canola oil, phosphate buffer, phosphate buffer+protein extract, and model meat emulsion); the response variable was the release index. Data were subjected to analysis of variance by ANOVA procedure and Duncan Multiple Range tests using a SAS program.[Citation20] All analysis were carried out at α = 0.05, in triplicate.
RESULTS AND DISCUSSION
Characterization of the Emulsion System
Data analysis of the two preliminary emulsion systems (pH as fixed variable, four protein extract concentration levels; protein extract concentration as fixed variable, four pH levels) gave the results reported in . As expected, emulsifying capacity increased with protein concentration (from 8.89 to 11.74 mLoil/gprotein); significant difference (P < 0.001) was observed from 30 to 35% protein extract; it also increased with pH (6.32 to 8.581 mLoil/gprotein at pH 4.5 and 7.5, respectively). Although myofibrillar protein isoelectric point is around 5.5, where emulsifying capacity was expected to be at a minimum, pH 4.5 and 5.5 showed non significant difference (P > 0.01) with respect to emulsifying capacity at fixed protein concentration; conversely pH 6.5 and 7.5 significantly increase (P < 0.001) oil emulsification.
Table 1 Emulsion mean particle diameter (D3,2), apparent viscosity (ηapp), emulsifying capacity (EC) and fraction volume of the disperse phase (φdp), varying pH and protein extract concentration
The emulsification process denatured proteins in a certain degree, increasing the emulsifying capacity. As reported by Fischer and Widder,[Citation6] partial denaturation in an aqueous medium changes the protein tridimensional configuration, exposing reactive sites, either hydrophilic or dipole-dipole interaction promoters, and increasing its ability to form interfaces. Partial protein degradation, observed by MW peaks lower than 66 kDa () account for an increase in emulsifying capacity. Homogenization also had a profound effect on protein configuration, promoting partial degradation, but also contributing to the emulsifying capacity ().
Figure 1 SDS-PAGE densitograms of proteins in the extract, the continuous and the disperse phases. a) myosin (<200 kDa); b) 120 to 100 kDa; c) 100 to 80 kDa; (d) intermediate or regulatory proteins; e) 60 to 50 kDa; and f) degradation products (<45 kDa).
The model emulsion showed a monodisperse particle size distribution that significantly decrease (P < 0.001) with increasing pH and protein extract concentration (). Having similar particle distribution and being in the same medium ionic strength, differences in emulsion viscosity were due to particle shape and charge.[Citation9] Due myofibrillar proteins high molecular weight, they behave as particles in suspension,[Citation21] therefore the system stability depends on colloidal factors such as ionic strength and particle shape, surface charge and distribution.[Citation22] Viscosity of 35% protein extract system considerably increased with increasing pH, although values were consistently lower than those when protein concentration varied (fixed pH 7.5). A non significant decrease (P > 0.01) in ηapp (9.31 to 6.21 Pa s) was observed at pH 5.5 and 4.5, respectively, meaning that no net surface charge was generated. Conversely, pH increase to 6.5 resulted in net reduction of positive charges and reduced average particle size to D3,2 = 6.34 mm, while ηapp increased to 11.25 Pa s. This fact was more noticeable when pH was increased from 6.5 to 7.5; at this point ηapp significantly increased (P < 0.001) to 21.2 Pa s, and D3,2 significant decreased (P < 0.001) to 3.43 μm.
Effect of Myofibrillar Proteins
Increasing protein concentration from 20 to 35% significantly reduced (P < 0.001) average particle size and ηapp (P < 0.001) due to partial protein degradation, as observed in SDS-PAGE of the protein extract (). Emulsifying capacity was significantly different (P < 0.001) only at 35% protein extract concentration (3.189 mgprotein/mL); more protein was available to emulsify fixed oil amounts. Fraction volume of the disperse phase significantly increased (P < 0.001) with increasing pH and protein extract concentration, although a more noticeable increase was observed with increasing protein concentration at fixed pH 7.5, due to more availability of emulsifier in the system. The effect of increasing fraction volume was also observed in increasing ηapp and decreasing average particle size (). Due that protein is adsorbed at the emulsion interface by electrostatic interactions between charged aminoacid residues and polar heads of the lipid volatile binding to protein increased with protein concentration at the interface.[Citation23] When protein covers most of the interface, volatile release decreases; however, at low protein concentrations no effect is observed due to poor coverage of proteins at the fat globule surface.[Citation22]
Myosin (>200 kDa) (a); small peaks of 120 to 100 kDa (b); 100 to 80 kDa (c); 80 to 65 kDa, corresponding to intermediate (around 100 to 75 kDa) or regulatory proteins (80 to 60 kDa) (d); 60 to 50 kDa (e); and <45 kDa, related to degradation products (f); were observed in the extract (). As expected, proteins were more abundant in the continuous (buffer) than in the disperse (oily) phase due to their high solubility in medium ionic strength solutions such as phosphate buffer. However, myosin was present in similar amounts in the continuous and disperse phases; this protein adsorbed to the oil/water interface, and partially solubilized in the aqueous phase. Myosin, together with actin and actomyosin has been reported as the main responsible of functional properties of muscle systems.[Citation21]
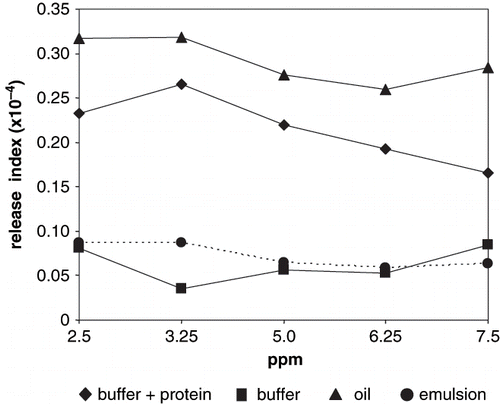
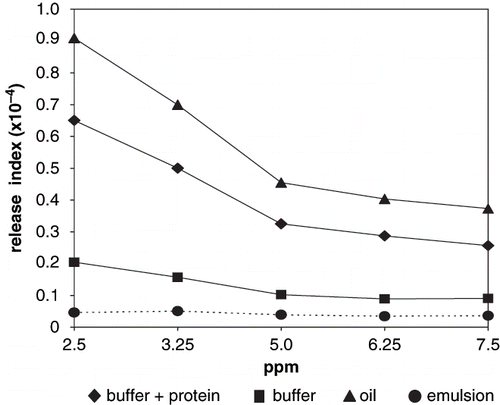
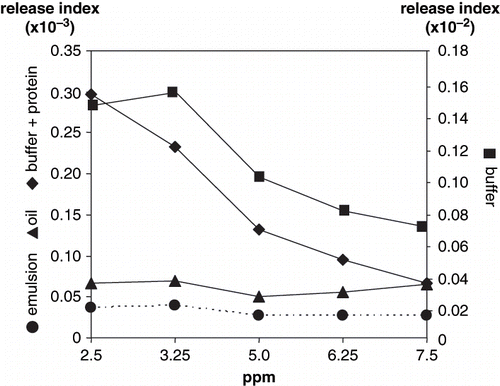
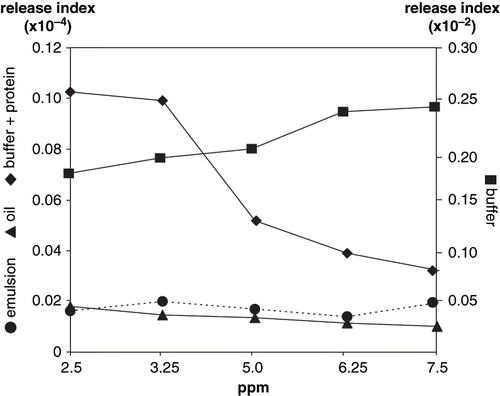
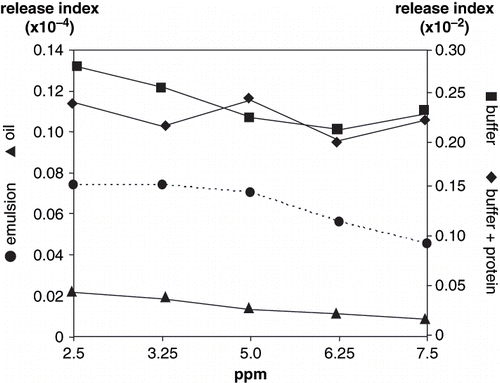
Smaller proteins of 100–80 kDa (c) and 80 to 65 kDa (d) were evident in disperse phase, probably bound to the interface. Myofibrillar proteins partitioned between the interface and the continuous phase; however, protein-volatile interaction varied depending on the solubility of aroma compounds in each phase. High protein concentration in the emulsion favors protein-protein concentration;[Citation23] protein unfolding at the interface allows interactions between non polar groups of the protein and lipids.[Citation24] On the other hand, lipid and aroma compound compete to protein binding at the interface.[Citation19] In our study, actin possibly unfold, liberating other associated proteins of the troponin-tropomyosin complex, partially accounting for the 45 kDa peak, and interacting with aroma compounds; binding reduces volatile liberation to the headspace, making them less available to nose and mouth receptors, as observed in volatile release index from the model emulsion for 2-methyl pyrazine (), 2-ethyl-3,5-dimethyl pyrazine (), hexanal () and nonanal (), but not for octanal ().
Volatile Release
Differences were observed in pyrazines release indexes with respect to the studied systems, significantly higher (P < 0.001) in oil and buffer+protein. In these two systems, pyrazines release index decreased with volatile concentration, although a significant difference (P < 0.001) was observed for 2-ethyl-3,5-dimethyl pyrazine. High release from oil was due to no interaction with the non polar media. Non-protein buffer and emulsion systems showed significantly lower (P < 0.001) release indexes; non significant differences (P > 0.01) were observed for both pyrazine release indexes with respect to volatile concentration.
Protein solutions, as buffer+protein systems, are one-phase systems; protein solubility was promoted by ionic strength of the aqueous medium, therefore the tridimensional arrangement varied exposing hydrophilic groups, which were solvated by water molecules.[Citation2] In this situation, methyl and ethyl radicals cannot easily interact with proteins; therefore pyrazines were released to the headspace. Pyrazine volatility decrease in buffer+protein due to interactions with amino or hydroxyl groups;[Citation25] non significant differences (P > 0.01) were observed between 2-ethyl-3,5-dimethyl pyrazine and 2-methyl pyrazine release from emulsions. Conversely, release of 2-ethyl-3,5-dimethyl pyrazines was significantly higher (P < 0.001) than 2-methyl pyrazine in buffer+protein due to a stronger net hydrophobic interaction between the methyl radical and peptide chains.[Citation26] Hydrophobic interactions also accounted for low release from non protein buffer, although non significant differences (P > 0.01) were observed between the non protein buffer and the emulsion for both pyrazines.
Release of hexanal and nonanal from oil and emulsion systems showed non-significantly difference (P > 0.01) with respect to volatile concentration ( and ). Hexanal and nonanal indexes significantly decreased (P < 0.001) as volatile concentration increased in buffer+protein, and hexanal indexes from buffer. No significant differences (P > 0.01) were observed in nonanal release from non protein buffer with respect to volatile concentration. The lowest release indexes for hexanal and nonanal occurred in the emulsion and oil systems (P > 0.01). Hexanal release indexes, although following similar trends as nonanal in oil, buffer+protein and emulsion systems, were significantly higher (P < 0.001). In a two-phase system, such as a protein emulsion, hexanal and nonanal interact at the interface with unfolded proteins through mechanisms of hydrophobic nature, and cannot easily diffuse through the continuous phase.[Citation19] In addition, the shorter the aliphatic chain the higher the solubility as a result of its lower hydrophobicity.[Citation27] The decrease in hexanal release with increasing volatile concentration in buffer, with and without proteins, was due to its relative solubility in water;[Citation23] the concentration of solublilized hexanal increased with increased added volatile to the system. O'Neill and Kinsella[Citation28] reported a decrease in aldehydes volatility in the presence of proteins, this type of interaction mainly depends on carbonyl radicals reacting with amine or hydroxyl groups. Fischer and Widder[Citation6] also reported that esters show a slight but continuous volatility decrease with increasing protein content in protein solutions.
Octanal showed different release trends as compared to the other studied aldehydes. Conversely as pyrazines, hexanal and nonanal, octanal was not retained by emulsions; release indexes were significantly higher (P < 0.001) from emulsions as compared to oil (). Octanal release showed no significant differences (P > 0.01) with respect to volatile concentration in all the studied systems. However, in the same way as observed for hexanal and nonanal, the highest release indexes were observed in buffer with and without protein; release of all aldehydes from the oil was significantly lower (P < 0.001) than from buffer and buffer+protein.
Due to protein unfolding and degradation at the emulsion interface, reactive sites were exposed; therefore, chemical interaction seems the event most likely occurring for aldehydes retention in a protein emulsion. However, hydrophobicity is also a leading retention mechanism; Schirle-Keller et al.[Citation29] reported that small changes in lipid content had significant effects on vapor pressure of lipid-soluble compounds, whereas partial pressure of water-soluble compounds has little effect in the system. According to these authors, due to the non polar nature of the studied aldehydes, lipid presence in emulsion and pure oil systems considerable reduced aldehyde vapor pressure, therefore reducing the release indexes.
CONCLUSIONS
Under the experimental conditions described in this paper, pyrazines were easily released from aqueous with proteins, and from oil; low pyrazine release was observed in emulsions and non protein aqueous systems, where aroma release decreased as pyrazines concentration increased. Pyrazines, therefore, mainly contributed to aroma in lipid systems and in protein solutions. They cannot be considered as aroma contributors in emulsions due to binding to unfolded protein at the interface, and aqueous systems without protein. Hexanal, octanal and nonanal were efficiently retained in the oil system; therefore, these volatiles are minor aroma contributors in lipid media. Hexanal and nonanal were also minor aroma components in meat emulsions. Conversely, octanal can be considered of an important aroma contributor in emulsions and in buffer with and without protein. In general, aldehydes showed high release indexes in aqueous systems, with or without added myofibrillar proteins. The effect of two phases (protein emulsion) or one phase (protein solution) was mainly due to hydrophobic interactions between proteins and ethyl/methyl pyrazines radicals. Release was significantly reduced for 2-methyl pyrazine, 2-ethyl-3,5-dimethyl pyrazine, hexanal, and nonanal in emulsions, due to interaction with unfolded proteins at the interface; this was not observed for octanal, were release indexes were significantly higher in emulsion as compared to the other volatiles.
ACKNOWLEDGMENTS
Author Herrera-Jiménez thanks the National Council of Science and Technology (CONACYT), Mexico for a graduate scholarship; and Universidad Autónoma Metropolitana for financial support during the doctoral thesis writing.
REFERENCES
- Shahidi , F. 1994 . “ Flavor of Meat and Meat Products-and Overview ” . In Flavor of Meat and Meat Products , Edited by: Shahidi , F. 1 – 3 . New York : Chapman and Hall .
- Kerscher , R. and Grosch , W. 1997 . Comparative Evaluation of Potent Odorants of Boiled Beef by Aroma Extract Dilution and Concentration Analysis . Z. Lebensmittel-Wissenschaft und Technologie Hunter. Forschung , 204 : 3 – 6 .
- Shahidi , F. and Pegg , R.B. 1993 . Hexanal as an Indicator of Meat Flavor Deterioration . Journal of Food Lipids , 1 : 177 – 186 .
- Mottram , D.S. 1998 . “ The Chemistry of Meat Flavour ” . In Flavour of Meat, Meat Products and Seafoods , Edited by: Shahidi , F. 5 – 26 . London, , England : Blackie Academic and Professional .
- Pérez-Chabela , M.L. , Escalona-Buendia , H. and Guerrero-Legarreta , I. 2003 . Physicochemical and Sensory Characteristics of Calcium Chloride-treated Horse Meat . International Journal of Food Properties , 6 ( 1 ) : 73 – 85 .
- Fischer , N. and Widder , S. 1997 . How Proteins Influence Food Flavor . Food Technology , 51 ( 1 ) : 68 – 70 .
- De Roos , K.B. 1997 . How Lipids Influence Food Flavor . Food Technology , 51 ( 1 ) : 60 – 62 .
- Goubert , I. , Le Querré , J.L. and Voilley , A. 1998 . Retention of Aroma Compounds by Carbohydrates: Influence of their Physicochemical Characteristics and on their Physical State . Journal of Agriculture and Food Chemistry , 46 : 1981 – 1990 .
- Miettinen , S.M. , Tuorila , H. , Piironen , V. , Vehkalahti , K. and Hyvönen , L. 2002 . Effect of Emulsion Characteristics on the Release of Aroma as Detected by Sensory Evaluation, Static Headspace Gas Chromatography and Electronic Nose . Journal of Agriculture and Food Chemistry , 50 : 4232 – 4239 .
- Ngapo , T. , Wilkinson , B. , Chong , R. and Haisman , D. 1992 . Gelation of Bovine Myofibrillar Protein Induced by 1,5-gluconolactone . Proceedings of the 38thInternational Congress of Meat Science and Technology . August 23–28 1992 , Clermont-Ferrand France.
- Peterson , G.L. 1983 . Determination of Total Protein . Methods in Enzymology , 91 : 95 – 119 .
- García , C.F.L. , Dimes , L.E. and Haard , N.F. 1993 . Substrate-gel Electrophoresis for Composition and Molecular Weight of Proteinases or Proteinaceus Proteinase Inhibitors . Analytical Biochemistry , 214 : 65 – 69 .
- Redinbaugh , M.G. and Turley , R.B. 1986 . Adaptation of the Bicinchoninic Acid Protein Assay for use with Microtiter Plates and Sucrose Gradient Fractions . Analytical Biochemistry , 153 : 267 – 271 .
- Swift , C.E. , Lockett , C. and Fryar , A.J. 1961 . Comminuted Meat Emulsions: The Capacity of Meats for Emulsifying Fats . Food Technology , 15 ( 11 ) : 468 – 471 .
- MacClemens , D.J. 1999 . Food Emulsions: Principles, Practice and Techniques , Boca Raton, Florida : CRC Press .
- Steffee , J.F. 1996 . Rheological Methods in Food Process Engineering , 428 East Lansing, MI : Freeman Press .
- Totosaus , A. , Gault , N. and Guerrero , I. 2000 . Dynamic Rheological Behavior of Meat Proteins During Acid-induced Gelation . International Journal of Food Properties , 3 ( 3 ) : 465 – 472 .
- Escalona , H. , Piggott , J.R. , Conner , J.M. and Paterson , A. 1999 . Headspace Concentrations of Alcohols and Aldehydes at Different Ethanol Concentrations . Italian Journal of Food Science , 11 : 241 – 248 .
- Seuvre , A.M. , Espinoza-Díaz , M.A. and Voilley , A. 2002 . Transfer Aroma Compounds Through the Lipidic-Aqueous Interface in a Complex System . Journal of Agriculture and Food Chemistry , 50 : 1106 – 1110 .
- Statistical Analysis System . 1999 . PC-SAS User's Guide , Cary, NC : SAS Institute, Inc .
- Li-Chan , E. , Nakai , S. and Wood , D.F. 1985 . Relationship Between Functional (Fat Binding, Emulsifying) and Physicochemical Properties of Muscle Proteins. Effects of Heating, Freezing, pH and Species . Journal of Food Science , 50 ( 4 ) : 1034 – 1040 .
- Dickinson , E. 2003 . Hydrocolloids at Interfaces and the Influence on the Properties of Dispersed Systems . Food Hydrocoloids , 17 : 25 – 39 .
- Gianelli , P. , Flores , M. and Toldrá , F. 2003 . Interactions of Soluble Peptides and Proteins from Skeletal Muscle on the Release of Volatile Compounds . Journal of Agriculture and Food Chemistry , 51 : 6828 – 6834 .
- Phillipe , E. , Seuvre , A.M. , Colas , B. , Langendorff , V. , Schippa , C. and Voilley , A. 2003 . Behavior of Flavour Compounds in Model Food Systems: A Thermodynamic Study . Journal of Agriculture and Food Chemistry , 51 : 1393 – 1398 .
- Signorini , M.L. , Ponce-Alquicira , E. and Guerrero-Legarreta , I. 2003 . Proteolytic and Lipolytic Changes in Beef Inoculated with Spoilage Microorganisms and Bioprotective Lactic Acid Bacteria . International Journal of Food Properties , 6 ( 1 ) : 147 – 163 .
- Boudaud , N. and Dumont , J.P. 1996 . “ Interactions Between Flavor Components and Beta-Lactoglobulin ” . In Flavor-Food Interactions , Edited by: McGorrin , R.J. and Leland , J.V. 90 – 97 . Washington : American Chemical Society .
- Langurieoux , S. and Crouzet , J. 1995 . “ Protein-Aroma interactions ” . In Food and Macromolecules , Edited by: Dickinson , E. and Lorient , D. 123 – 133 . Cambridge, , England : Royal Society of Chemistry .
- O'Neill , T.E. and Kinsella , J.E. 1987 . Binding of Alkaline Flavors to Lactoglobulin: Effects of Conformational and Chemical Modification . Journal of Agriculture and Food Chemistry , 35 : 906 – 909 .
- Schirle-Keller , J.P. , Reineccius , G.A. and Hatchwell , L.C. 1994 . Flavor Interactions with Fat Replacers: Effect of Oil Level . Journal of Food Science , 59 : 813 – 815 .