Abstract
The antioxidant activity of mulberry (Morus Indica L.) leaves was evaluated in rice bran oil (RBO). The oil was subjected to accelerated oxidation at 100° C for 5 days and heat treatment at 180°C for 1 h. In order to examine its potential antioxidant activity, the oxidative stability of the oils was evaluated by employing peroxide value (PV), radical scavenging activity (RSA), thiobarbituric acid reactive substances assay (TBARS), and percent free fatty acids (FFA). Mulberry in two forms viz: the methanolic extract and powder were applied to RBO at 200 ppm and 0.05%, respectively, and results were compared with RBO treated with a synthetic antioxidant-BHT. The PV, RSA, TBARS, and FFA values indicated that both mulberry extract and powder were effective in inhibiting lipid oxidation when compared to synthetic antioxidant BHT. Thus, mulberry leaves may serve as a new source of natural antioxidant for use in food systems.
INTRODUCTION
Lipids are susceptible to oxidation on storage and frying processes. Characteristic changes associated with oxidative deterioration include development of unpleasant tastes and odors as well as changes in color, viscosity, specific gravity, and solubility. Oils that contain relatively high amounts of polyunsaturated fatty acids (PUFA) experience stability problems. During oxidation hydroperoxides are formed which again break down to form products like alcohols, aldehydes, ketones and hydrocarbons, which possesses offensive off flavors.[Citation1] Because of the high temperatures used, frying processes are usually accompanied by thermolysis, hydrolysis, oxidative degeneration and polymerization. There is also an increase in free fatty acids, cyclic fatty acids, foaming, color, viscosity, formation of polar materials and polymeric compounds.[Citation2]
In order to increase the shelf life of lipids, the commercial approach is the use of synthetic antioxidants, which quench free radicals, formed during oxidation and can prevent or delay oxidation thereby increasing its shelf life.[Citation3] The interest in the activities of natural antioxidants has grown in recent years due to the possible deleterious health effects from the ingestion of synthetic antioxidants.[Citation4] These considerations led to a screening of plants foods to meet current needs in a broad range of fats, oils and food products.[Citation5]
It is reported that green tea polyphenols in several edible oils and fried products have stronger antioxidative activity than BHA and tocopherols.[Citation6] Similarly it was found that the effect of ginger extract as an antioxidant in sunflower oil reflected a higher potency to suppress lipid oxidation.[Citation7] The hunt for alternatives to synthetic antioxidants resulted in hundreds of substances being evaluated over the years for their antioxidant capabilities. Of special interest are the uses of spices and herb extracts like rosemary, sage and green tea catechins extensively in lipid containing food systems like sausages, pie-crusts, snacks, dressings, pastries as well as food ingredients such as breads and flavors.[Citation3] Reports indicate that mulberry leaves contain proteins, carbohydrates, calcium, iron, ascorbic acid, β-carotene, vitamin B1, folic acid, and vitamin-D. Apart from their use as animal and insect feed, they have been shown to possess excellent antioxidative, diuretic, hypoglycemic and hypotensive activities.[Citation8] Many investigations have revealed the excellent antioxidative potential of mulberry leaves and they have been added in food systems to find its effect as an antioxidant.[Citation9,Citation8] Scientific evidences are available on the hypoglycemic and hypolipidemic effects of Morus indica in patients with type II diabetes[Citation10] as well as their hypoglycemic and antioxidant activity in streptozotocin-diabetic rats.[Citation11,Citation12] The antioxidant activity of various solvent extracts[Citation13] and free radical scavenging activity of flavonoids [Citation14] and other constituents of Morus alba leaves[Citation15,Citation16] have been reported.
Antioxidants affect the process of lipid oxidation at different stages due to differences in their mode of action. Because of the complexity of the oxidation process itself, the diversity of the substrates and the active species involved, the application of different test methods is necessary in the evaluation of antioxidants. Due to the high amount of unsaturated fatty acids rice bran oil is susceptible to oxidation[Citation17] and therefore needs to be stabilized. Since there are negligible literatures available on the use of mulberry as an antioxidant in edible oil, the present study was targeted to investigate the effect of addition of mulberry in different forms in inhibiting the oxidation of rice bran oil and the antioxidant potential of mulberry was compared with commercial antioxidants.
MATERIALS AND METHODS
Materials
Fresh Mulberry leaves (Morus Indica L.) were obtained from the Department of Studies in Sericulture, University of Mysore, India. Fresh, refined rice bran oil (RBO) that contained no added antioxidants (AOX) was obtained from a local oil company. Commercial brand of RBO (CRBO) was obtained from the local market. α, α - Bipiridyl was purchased from E Merck Ltd (Mumbai India ). ∞, ∞ - Diphenyl-β-picrylhydrazyl (DPPH) and α-tocopherol were purchased from Hi media Laboratories Pvt Ltd (Mumbai, India). Butylated hydroxytoluene (BHT) was purchased from Qualigens Fine Chemicals (Mumbai, India).
Preparation of Extract
Fresh mulberry leaves were cleaned and dried in an oven at 50°C (overnight), ground and passed through 60 mesh. A weighed portion (20 g) was taken for extraction in a conical flask. 100 mL methanol was added and kept in a mechanical shaker for 2 h. It was filtered and the residue was again extracted under the same condition and the filtrates were combined. The extract was concentrated using a rotary evaporator (Buchi laboritoriums-Technik, Flawil/Schweiz, Switzerland) by evaporating methanol at 60°C. The concentrated extract was stored at 4°C until use.
Oxidation Experiments
RBO added with mulberry extract (ME) and mulberry powder (MP) was compared with RBO added with synthetic antioxidant, commercial antioxidant and RBO without any added antioxidants (control). These oils were subjected to accelerated oxidative treatment at 100°C for 5 days. Samples (50 mL) were withdrawn each day for chemical analysis. In addition these oils were subjected to heat treatment at 180°C for 1 h. They were allowed to cool before analysis.
Experimental Variations
The following variations were used for the above treatments: (i) RBO without any added AOX, [RBO]; (ii) RBO + 200 ppm of synthetic AOX (BHT), [RBO + BHT]; (iii) RBO + 200 ppm of Mulberry Extract, [RBO + ME]; (iv) RBO + 0.05 % Mulberry powder (50 mg/100 ml), [RBO + MP]; and (v) Commercial RBO, [CRBO]. All samples were prepared and tested in duplicates.
Chemical Analysis
Antioxidant components in mulberry leaves and oxidation state in rice bran oil were determined. Ascorbic acid was determined according to reduction in the absorbance of 2, 6-dichlorophenol indophenol dye on reaction with ascorbic acid.[Citation18] α-Tocopherol was extracted by direct saponification of dried sample and estimated based on the formation of a red colored complex from the reaction of α,α - dipyridyl with ferrous ion due to reduction of ferric ion by Tocopherol.[Citation19] β-carotene was separated by liquid chromatography, followed by measuring the absorbance of the eluate at 450 nm against standard β-carotene.[Citation20] Reduced glutathione was determined based on the development of a yellow compound due to reaction of 5,5′-dithio (bis) nitro benzoic acid with compounds containing sulphydryl groups.[Citation21] Total phenols were extracted[Citation22] and analyzed by Folin-Ciocalteu micro method.[Citation23] In rice bran oil Vitamin E was estimated as mentioned earlier. The peroxide value (PV) and percent of free fatty acids (FFA) were determined in the oil variations.[Citation18]
Radical scavenging activity
The radical scavenging activity (RSA) was assessed using DPPH as a stable free radical.[Citation24] To 1 mL of the sample 3 mL of a 1 mM solution of DPPH in methanol was added, shaken well and kept in dark for 30 min. The optical density was measured at 517 nm. As blank methanol (4 mL) was taken. As control, 1 mL of CD (9 parts of cyclohexane + 1 part Diethyl ether) was added with 3 mL of DPPH reagent. The RSA was expressed as % inhibition. The RSA of the samples tested, expressed as percentage inhibition, was calculated according to the following formula:
where OD: optical density.
Thiobarbituric acid reactive substances
TBARS were measured with small modifications.[Citation20] 10 g of the oil sample were added to 20 mL of aqueous thiobarbituric acid (0.67%) and 25 mL of benzene was shaken in a mechanical shaker for 2 h. The aqueous layer was withdrawn and placed in a boiling water bath for 30 min and after cooling the absorbance was read at 532 nm. The TBARS values were expressed as malonaldehyde (MDA equivalents μg/Kg oil). Inhibition of oxidation was calculated using the following equation:
where OD: optical density.
STATISTICAL ANALYSIS
The results are expressed as means ± SD with two replicate for each of the two duplicates. The results of the accelerated oxidative storage treatment were analyzed by Duncan's New Multiple Range test for Analysis of Variance.[Citation25] Results of heat treatment at 180°C were analyzed by the t-test for comparison of before and after treatment values.
RESULTS AND DISCUSSIONS
Antioxidative Components in Mulberry Leaf
The antioxidative components analyzed in dehydrated mulberry leaves (100 g) are presented in . It has 128 mg of ascorbic acid, 48 mg of α-tocopherol, 14.10 mg of β-carotene and 152 mmol of glutathione. The data indicates that mulberry can be considered as a natural source of antioxidant.
Table 1 Antioxidant components in dehydrated mulberry leaf
Chemical Properties of the Oil
The two oils, i.e., RBO and CRBO, used in the study were analyzed for peroxide value (PV), radical scavenging activity (RSA), free fatty acids (FFA), thiobarbituric acid reactive substances (TBARS), and vitamin E. The results are given in . It is evident from the values that both RBO and CRBO had good initial qualities, i.e., having PV of 7.3 and 5.0 meq O2/Kg oil and FFA of 0.06 and 0.05 % oleic acid, respectively. RBO had vitamin E content of 88 mg/100 g oil. These values are well within the desirable range (PV = 10 meq O2/Kg oil, FFA = 0.05% oleic acid) when compared to reported values. The RSA and TBARS values indicate the oxidative stability of oils. The RSA value was 68% for RBO and 70% for CRBO. The higher value of RSA in CRBO may be due to the addition of antioxidant. The TBARS value was lower for CRBO compared to RBO, which may be attributed to the addition of an antioxidant in the commercial brand.
Table 2 Analytical data of rice bran oils used in the study
Effect of Antioxidant Treatment on the Thermal Stability of Oils
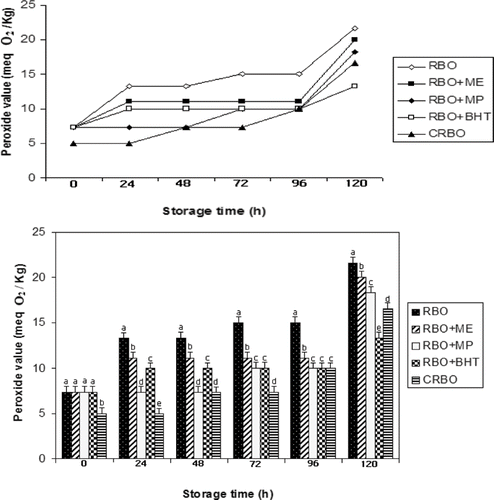
The oxidative stability of RBO during accelerated oxidative storage at 100°C for 5 days was investigated. Two-hundred mL of the oil variations were poured into 250 mL beakers and kept in an oven for accelerated oxidation at 100°C for 5 days. Development of both primary and secondary oxidation products in the oil devoid of any antioxidant was strongly enhanced (). However, addition of natural antioxidants prolonged the stability of the oil. The PV of all variations increased gradually during 5 days of storage. The induction time for the treated oils to reach a PV of 15 m eq O2/Kg oil was 72 h for the control and 120 h for the treated oils (RBO + ME, RBO + MP and CRBO). BHT treated oil had a significantly (p < 0.05) lower PV even after 120 h of oxidation (). At the end of storage, increases in PV were in the following order: RBO > RBO + ME > RBO + MP > CRBO > RBO + BHT. The oils that contained mulberry showed lesser PV when compared to the untreated RBO (control) thereby proving its efficacy in inhibiting lipid oxidation. Similar reports are available in the literature on addition of extracts viz, from red algae,[Citation26] ginger,[Citation27] and green tea polyphenols[Citation28] in edible oils.
Figure 1 Changes in peroxide values (meq O2/Kg) during accelerated oxidative storage (100°C, 5 days). Values bearing different subscripts a, b, c, d in each oil variation differ significantly (P < 0.05).
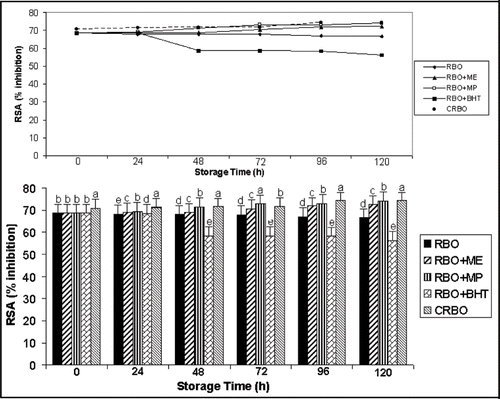
In this study, the radical scavenging model was based on DPPH radical. The synthetic nitrogen centered DPPH is often used as ‘indicator compounds’ in testing hydrogen-donation capacity and thus antioxidant capacity.[Citation29] Scavenging of different types of reactive oxygen and nitrogen species, mostly free radicals is thought to be one of the main mechanisms of the antioxidant action inhibited by phenolics phytochemicals.[Citation29] The antioxidant activity of mulberry (extract & powder) in the oil was assessed by its DPPH radical scavenging ability (RSA). The changes in RSA are reported in . The activity in untreated RBO (control) and RBO treated with BHT decreased during the accelerated storage period (5 days). This indicates that BHT is not stable at higher temperature and therefore is not suitable for use as an antioxidant in edible oils. It is interesting to note that the oil treated with mulberry RBO + MP, RBO + ME and CRBO showed increasing DPPH radical scavenging activity thereby proving the antioxidative effect of mulberry.
Figure 2 Changes in radical scavenging activity (% inhibition) during accelerated oxidative storage (100°C) Values bearing different subscripts a, b, c, d (in each column) oil variation differ significantly (P < 0.05).
The TBARS values (% inhibition) of treated oil variations before and after accelerated oxidative storage are presented in . The % inhibition against the formation of TBARS was less in CRBO (43.8%) when compared to control. Among the mulberry treated oils, the oil treated with mulberry extract showed a slightly higher % inhibition (24.6%) when compared to mulberry powder (22.5%) relative to control. The detection of malonaldehyde, a secondary oxidation product, has been widely used as a measure of the oxidation of polyunsaturated fatty acids in foods and animal tissues. The TBARS determinations confirmed that BHT was the weakest antioxidant as it showed poor inhibition of oil oxidation compared to other antioxidants and the oxidation inhibition was in the order of CRBO> RBO + ME> RBO + MP> RBO + BHT.
Table 3 Inhibitory action of mulberry extract and powder, and toward formation of TBARS in RBO on accelerated oxidative storage (100°C, 5 days)′
Changes in Oils During High Temperature Treatment
Five variations (oils treated with antioxidants and control) were subjected to heat treatment in an open pan at 180°C for 1 h. The results of chemical analysis, i.e., PV, FFA, and RSA, are as follows: The PV of untreated RBO after heat treatment was significantly higher (P < 0.05) than those of other variations (). Even though increases in PV were observed in oils treated with antioxidants it was lesser in mulberry treated variations (MP and ME) and the PV after heat treatment of RBO + ME was comparable (P < 0.05) to CRBO indicating the ability of mulberry extract to inhibit formation of peroxides.
Table 4 Changes in PV, FFA, and RSA of RBO variations after heat treatment (180oC, 1 h)
The RSA was found to decrease in all variations after heat treatment. The % reduction was the highest in RBO and the least in RBO + BHT (). The reductions in the activity of RBO treated with ME and MP were significantly (P < 0.05) lower compared to the untreated oil (control) again proving the efficacy of mulberry as an antioxidant in edible oils.
The results of FFA changes are given in . No differences were observed in the FFA values between samples after heat treatment. However, the FFA content in CRBO after heating was significantly (P < 0.05) lesser than the other variations. Perhaps, the commercial brand contained higher amounts of additives to impart stability to the oil
CONCLUSIONS
The mulberry leaves investigated contain components such as ∞-tocopherol, ascorbic acid, β-carotene, and glutathione with antioxidant abilities. The methods applied in this study considered the antioxidant properties of the mulberry extract and powder studied as determined by different testing methods. Mulberry showed a strong antioxidative activity in rice bran oil. Both temperature and source of antioxidant used, affected the rate of oxidative deterioration of rice bran oil. The order of antioxidant activity of the sources of antioxidants used in the study was mulberry extract> mulberry powder> BHT. Because mulberry had an antioxidant effect on rice bran oil, it can be considered as an excellent antioxidant for edible oil.
REFERENCES
- Shahidi , F.L. and Wanasundara , U.N. 1997 . “ Measurement of Lipid Oxidation and Evaluation of Antioxidant Activity. Natural Antioxidants ” . In The Chemistry, Ill Effects and Health Benefits , Edited by: Shahidi , F. 379 – 394 . Newfoundland , , Canada : St. John's .
- Abidi , S.L. and Rennick , K.A. 2003 . Determination of Non Volatile Components in Polar Fractions of Rice Bran Oils . Journal of American Oil Chemists Society , 80 : 1058 – 1062 .
- Giese , J. 1996 . Antioxidants: Tools for Preventing Lipid Oxidation . Food Technology , : 73 – 80 .
- Barbut , S. , Josephson , D.B. and Maurer , A.J. 1985 . Antioxidant properties of rosemary oleorisin in turkey sausage . Journal Food Science , 50 : 1356 – 1359 .
- Thompson , J.W. and Sherwin , E.R. 1997 . Investigation of Antioxidants for Polyunsaturated Edible Oils . Journal of American Oil Chemists .Society , 43 : 683 – 689 .
- Koketsu , M. and Satoh , Y. 1997 . Antioxidative Activity of Green Tea Polyphenols in Edible Oils . Journal of Food Lipids , 4 : 1 – 9 .
- Rehman , Z.U. , Salariya , A.M. and Habib , F. 2003 . Antioxidant activity of Ginger Extract in sunflower oil . Journal of Science Food and Agriculture , 83 : 624 – 629 .
- Sastri , B.N. 1962 . “ Raw Materials ” . In The Wealth of India (CSIR) Vol. 6 , 429 – 439 . New Delhi , , India
- Jung , M.Y. and Min , D.B. 1992 . Effects of Oxidised α-,γ - and δ-Tocopherols on the Oxidative Stability of Purified Soybean Oil . Food Chemistry , 45 : 183 – 187 .
- Andallu , B. , Suryakantham , V. , Srikanthi , B.L. and Reddy , G.K. 2001 . Effect of mulberry therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes . Clinica Chimica Acta , 314 : 47 – 53 .
- Chen , F. , Nakashima , N. , Kimura , I. , Kimura , M. , Asano , N. and Koya , S. 1995 . Potentiating effects on pilocarpine-induced saliva secretion by extracts and N-containing sugars derived from mulberry leaves in streptozotocin- diabetic mice . Biological and Pharmaceutical Bulletin , 18 : 1676 – 1680 .
- Andallu , B. and Varadacharyulu , N. 2003 . Ch. Antioxidant role of mulberry (Morus indica L. cv. Anantha) leaves in streptozotocin-diabetic rats . Clinica Chimica Acta , 338 : 3 – 10 .
- Yen , G. , Wu , S. and Duh , P. 1996 . Extraction and identification of antioxidant components from the leaves of mulberry (Morus alba L.) . Journal of Agricultural and Food Chemistry , 44 : 1687 – 1690 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoids contents in mulberry and their radical scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Kim , S.Y. , Gao , J.J. , Lee , W.C. , Ryu , K.S. , Lee , K. R. and Kim , Y.C. 1999 . Antioxidant flavonoids from the leaves of Morus alba . Archives of Pharmacological Research , 22 : 81 – 85 .
- Sharma , R. , Sharma , A. , Shono , T. , Takasugi , M. Shirata , A. 2001 . Mulberry moracins: Scavengers of UV stress- generated free radicals . Bioscience Biotechnology and Biochemistry , 65 : 1402 – 1405 .
- Mehta , B.V. August 2003 . “ Heart friendly cooking oil ” . In Saarc Oils and Fats Today August ,
- AOAC . 1970 . Official Methods of Analysis , 11th Vol. 129 , 769 Washington , D.C
- Freed , M. 1966 . Methods of Vitamins Assay Interactions , New York : John Wiley and Sons .
- Ranganna , S. 1999 . Handbook of analysis and quality control for fruits and vegetable products , 2nd , New Delhi, India : Tata McGraw-Hill Publishing Co Ltd .
- Beutler , E. and Kelly , B.M. 1963 . The effect of sodium nitrate on RBC Glutathione . Experienta , 19 : 96 – 97 .
- Vinson , J.A. , Hao , Y. , Su , X. and Zubic , I. 1998 . Phenol antioxidant quantity and quality in foods: vegetables . Journal of Agricultural and Food Chemistry , 46 : 3630 – 3634 .
- Slinkard , K. and Singleton , V.L. 1977 . Total phenol analysis: automation and comparison with manual methods . American Journal of Enology and Viticulture , 28 : 49 – 55 .
- Tsouklas , B. and Grosch , W. 1977 . Analysis of Fat Deterioration-Comparison of Some Photometric Tests . Journal of American Oil Chemists Society , 54 : 490 – 493 .
- Steele , R.G.D. and Torrie , J.H. 1960 . Principles and procedures of statistics. A Biometric approach , 2nd , New York : Mc Graw-Hill .
- Athukorala , Y. , Lee , K.W. , Shahidi , F. , Heu , M. , Kim , H. , Lee , J.S. and Jeon , Y.J. 2003 . Antioxidant efficacy of extracts of an edible red alga ( Grateloupia filicina) in linoleic acid and fish oil . Journal of Food Lipids , 10 : 313 – 327 .
- Rehman , Z. , Salaria , A.M. and Habib , F. 2003 . Antioxidant activity of ginger extract in sunflower oil . Journal of Science of Food and Agriculture , 83 : 624 – 629 .
- Koketsu , M. and Satoh , Y. I. 1997 . Antioxidative activity of green tea polyphenols in edible oils . Journal of Food Lipids , 4 : 1 – 9 .
- Dorman , H.J.D. , Peltokejo , A. , Hiltunen , R. and Tikkanen , M.J. 2003 . Characterization of the antioxidant properties of de-odorized aqueous extracts from selected Laminaceae herbs . Food Chemistry , 83 : 255 – 262 .