Abstract
The coagulation of ewe's milk was studied by using plant source coagulants namely the artichoke, Cynara scolymus L. cv. Blanca, and latex from the fig tree (Ficus carica L.). A turbidimetric method was used to evaluate and compare the coagulation properties of the novel coagulants with chymosin treated samples. Syneresis capacity and sensory evaluation of resultant cheese samples were studied and it was found that both cynara and chymosin produced sigmoidal increase in turbidity to the milk with three distinct phases. The coagulation kinetics was affected substantially by both coagulants. Plant coagulant induced shorter gelation time compared to chymosin however required longer time for restructuration (end of coagulation). The coagulum obtained with the latex of Ficus carica had a higher yield, owing to its high water retention capacity. With the exception of color, the overall sensory attributes did not show significant differences among coagulants.
INTRODUCTION
The calf rennet is still the most commonly used enzyme in cheese making. However, due to an increase in world cheese production, coupled with a world shortage of calf rennet, proteases derived from other sources are currently being explored by many researchers for their potential use in cheese manufacture. Several plant based proteases have been found to coagulate milk.[Citation1] These are generally extracted from the leaves and stems of bedstraw, the flowers of artichoke and cardoon, the latex of fig and the leaves of papaya and zucchini.[Citation2] Some of these preparations are still used to produce gourmet food. Telem, a traditional Turkish product, is manufactured by using ficin as a coagulating enzyme.[Citation3,Citation4] Serra da Estrada and Serpa, two popular Portuguese cheeses, are made from ewe's raw milk using coagulants extracted from the stylet and stigma part of the flowers of cardoon, Cynara cardunculus L. and Cynara humilis L..
Milk coagulation, which is expressed by the formation of a gel, is the result of physicochemical modifications occurring on the casein micelles.[Citation5] Cheese making conditions such as milk composition, incubation temperature, calcium and phosphate addition, culture, enzyme type and concentration influence coagulation properties.[Citation6,Citation7,Citation8,Citation9]
Coagulation properties are of interest because they influence cheese yield and quality. Enzymatic milk coagulation consists in several phases. Decreasing enzyme activity decreases the rate of all phases of the coagulation process but does not affect their sequence.[Citation10] Thus, the type of coagulant is a critical parameter, which greatly influences the characteristics of the final product.
Plant coagulants have many features similar to chymosin; they are aspartic proteinases and they have similar specifications in the cleavage sites, as they hydrolyze the Phe105-Met106 bond of κ-casein.[Citation11] Several artisanal cheeses, manufactured in the Mediterranean countries from raw ewes' and goats' milk utilized plant extracts. These cheeses are appreciated for high quality, exotic flavors and texture.[Citation12] Recent studies of cardoon extracts revealed that good quality semi-hard cheeses can be made from ovine milk.[Citation13] However, plant originated coagulants are generally not very much suitable for cheese making due to the low yield, off-flavor (e.g. bitterness) and soft texture of the ripened cheeses.[Citation14] These are found to be excessively proteolytic relative to their milk-coagulation activity.[Citation15]
Figs and artichokes are two important crops produced in Tunisia. These are not utilized as coagulant extracts since the cheese making follows the conventional procedures. In addition, plant based coagulants have not yet characterized well for food additives. Only few studies have been carried out by Esteve et al.,[Citation11,Citation12,Citation16] Silva and Malcata,[Citation17] and Tavaria et al.[Citation18] on the characterization of the coagulation and proteolytic activity of extracts from cardoon flowers Cynara cardunculus L. and Cynara humilis L.. Limited research works have been conducted on cynarase extracts obtained from the flowers of artichokes, Cynara Scolymus L. cv. Blanca. In addition, cynarase activity is variable and depends on the cultivar, maturity stage of the plant, part of the flower used, drying time, etc.[Citation19] The objective of this work was, therefore, to study the coagulation of ewe's milk using coagulants from a range of sources namely latex from the fig tree and extracts of Cynara. The properties of coagulation using the novel coagulants were compared to those when chymosin was used to induce coagulation.
MATERIAL AND METHODS
Milk Samples
Ewes' milk was collected from the farm of Ecole Supérieure d'Agriculture de Mateur, Tunisia. Milk was produced by ewes (8 in total) of Sicilia Sarde race. In order to prevent microbial proliferation, milk samples were stabilized by the addition of 0.06 % (w/v) potassium dichromate, followed by storage at –20οC until use. Before experiment, milk samples were defatted at 2000 × g for 15 min. The operation was repeated thrice.
Coagulants Preparation
Bovine rennet containing chymosin activity of 520 mg/l was obtained from Rhodia (Rhodia-Food Inc., Dangé-Saint-Romain, France) and was used at a dose of 0.1 ml/l of milk.[Citation20] Plant extracts containing the enzyme ficin and the coagulant of Cynara were obtained from fig latex and the stigma and flowers of artichoke, respectively. The coagulant of Cynara was extracted according to the method described by Esteve et al.[Citation12] Stigma and flowers of Cynara were mixed with distilled water (80 g/l), homogenized and then centrifuged at 3000 × g for 10 min. The supernatant was filtered through a Whatman filter paper #1. The crude extract was used for inoculation at a concentration of 50 ml/liter of milk. Crude fig latex was used without further treatment at a dose of 500 μl/liter of milk.
Coagulation Conditions
Ewes' skimmed milk was first heated to 40oC for 30 min. Chymosin/plant extracts were then added at the normal pH of the milk. Preliminary tests were carried out to optimize the amounts of Cynara extract and fig latex in order to achieve a gelation time (tg) of about 58 min which was the reference time for chymosin. These attempts failed to achieve desired results. The minimum concentration required to induce coagulation varied with the coagulant. A minimum of 500 μl/l of milk was required for fig latex and 50 ml/l of milk for Cynara extract. The minimum time required for coagulation also varied, the time was much shorter with fig latex (11 min) compared to 35 min when cynara extract was used.
Syneresis Capacity and Speed of Whey Expulsion
To study the syneresis capacity of cheese produced under the conditions mentioned above, coagulation of milk was stopped after 1.5 h, and the whey was separated from the curd by centrifugation at 4000 × g for 10 min without prior cutting. Syneresis was calculated as the ratio of the mass of whey obtained to the mass of initial milk.[Citation21] The speed of syneresis was also monitored. Curds obtained after one hour were cut into small cubes (1 cm3) and transferred into perforated cheese molds. The mass of the whey separated was measured at 15 min time interval for the first hour and every 30 min thereafter up to 4.5 h.[Citation22]
Turbiditimetric Milk Coagulation Assay and Conductivity Measurement
The turbidity and the conductivity of milk samples during coagulation were measured using a turbidimeter (Model Analite NEP 160, Mc Van Instruments PTY-LDT, Mulgrave, Australia) and a conductivity meter (Model 18.38 pH/EC meters, Eijkelkamp Agrisearch Equipment, Giesbeek, The Netherlands), respectively. Samples were initially monitored every 10 s for 30 min, since turbidity and conductivity change very rapidly at the beginning, then every 30 s until 1 h of coagulation and finally every one minute until 2 h. Turbidity and conductivity profiles were obtained by plotting these parameters as function of time. These profiles were used to define the different phases of milk coagulation by different coagulants.
Physicochemical Analysis
Titratable acidity, total solids and ash content were measured according to AFNOR methods.[Citation23] The pH of milk was measured using a pH meter (Model pH 315i/SET, WTW Inc., Weilheim, Germany). Different nitrogen fractions of milk and whey: total nitrogen (TN), non-protein nitrogen (NPN) and non-casein nitrogen (NCN) were extracted using the method of Rowland[Citation24] and determined by the Kjeldahl method[Citation23] after mineralization using a Büchi 435 digestion unit (Büchi Laboratory Equipment, Flawil, Switzerland). The concentrations of protein [PRO = (TN – NPN) × 6.38] and of casein [TCN = (TN – NCN) × 6.38] were determined.
Determination of Cheese Yield
Cheese yield (g/l) was expressed as the ratio of curd mass obtained after 4 h of drainage to the volume of milk used.
Sensory Analysis of the Curds
Sensory analysis was performed using 60 semi-trained panelists including laboratory staff and graduate students from Ecole Supérieure des Industries Alimentaires de Tunis. This test was carried out in a sensory analytical room. Panelists (23 males and 37 females, average age: 26 years) received sensory analysis training and they were informed about the sensory characteristics of cheese. Cheeses were assessed for color, odor, flavor, bitterness, after taste, presence of granules and overall appreciation. A hedonic scale from 1–10 was used by panelists to assess the attributes.
Statistical Analysis
Statistical analysis was performed using STATGRAPHICS PLUS 4.0.[Citation25] Analysis of variance was performed at the 5% confidence level. All tests were performed in triplicate.
RESULTS AND DISCUSSION
Characterization of Ewe Milk
summarizes the mean values of the different physicochemical characteristics of ewes' milk. These values are similar to those reported earlier.[Citation2,Citation26,Citation27] The protein content (98.82% of the total nitrogen) was higher than the reported protein content for cows' (94.85%), goats' (92.5%) and camels' (96.45%) milks.[Citation28] Moreover, the caseins/nitrogen ratio was also relatively high (83.14%). The mean pH value (6.59) was similar to reported values.[Citation29,Citation30]
Table 1 Physicochemical characteristics of raw milk and expulsed whey
Physicochemical Characterization of Expelled Whey
illustrates the content of whey produced using different coagulants. Chymosin is included as the control for comparative purposes. The data in illustrates that when fig latex was used to induce coagulation a greater amount of curd was lost in the whey. In addition, the whey, following coagulation with fig latex, had the highest levels of total solids, total nitrogen, and casein. In contrast, the whey produced following coagulation with Cynara had low total solids content, but high levels of total nitrogen, protein, and casein. The variation in the whey content in response to the coagulant used confirm the observations of Low and co-workers[Citation31] who compared the coagulation of bovine milk by Cynara, ficin and chymosin. Those results revealed that ficin caused a complete degradation of casein, mainly the κ-casein fraction, during coagulation. Hydrolysis of the other casein fractions takes place at later stages.
Losses of fine curd particles in expelled whey significantly affect cheese yield. Since casein is a key factor in determining cheese yield, a comparison of the proportion of casein to total nitrogen in the whey may identify the role of casein in the yields obtained in this study. It appears from our results that using plant coagulants produced whey with the highest levels of caseins (63.49% for ficin, 66.57% for Cynara extract compared to 53.86% for chymosin). The losses in casein were thus 29.02, 26.73, and 19.39% for ficin, Cynara extract, and chymosin, respectively. These results indicate clearly that plant coagulants induced greater losses of fine particles in the whey and thus are less suited in this respect for cheese making compared to chymosin. shows that the level of non-protein nitrogen was low (between 1.70% and 3.15%) for all the coagulants used in this study. This could be attributed to the fact that these cheeses were not ripened. Nitrogen fractions undergo important changes during ripening with usually an increase in NPN in cheese.[Citation32,Citation33]
Sidrach et al.[Citation34] identified three proteases from artichoke flowers with coagulation activity; those were identified as cynarases A, B, and C. They were identified as aspartic proteases and had the same proteolytic activity as chymosin as they hydrolyze the 105–106 peptide bond of κ-casein. However, they revealed differences mainly in the hydrolysis of αs- and β-caseins. A similarity was established between the proteolytic activity of enzymes extracted from artichoke flowers and from Cynara cardunculus L.[Citation35] The latter is extensively used in cheese manufacture in Portugal.
It has been established that Cynara is more proteolytic than chymosin[Citation11,Citation36,Citation37,Citation38,Citation39] and that it hydrolyzes other peptide bonds in addition to the Phe105-Met106 bond of κ-casein. Macedo et al.[Citation40] reported that proteases of Cynara cardunculus revealed preference towards bonds between hydrophobic regions of αs-casein and β-casein. These bonds are less hydrolyzed by chymosin. In addition, it has been shown that these preferential hydrolysis sites are highly dependent on the milk origin.[Citation41]
Evolution of Turbidity of Milk Samples during Coagulation
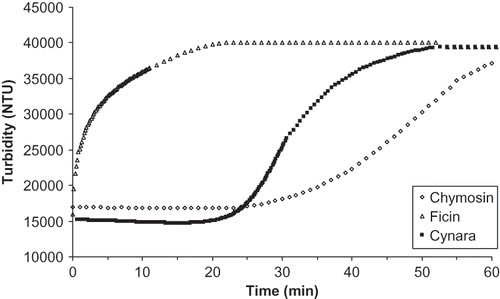
shows that turbidimetric signals obtained during coagulation of milk by Cynara extract and chymosin have a sigmoidal shape divided into three distinct phases. Scher and Hardy[Citation42] and Bornaz et al.[Citation20] obtained similar results and defined the 3 phases as (i) an initial phase or lag phase corresponding to the hydrolysis of κ-casein; (ii) a second phase characterized by an exponential increase in the signal, the result of the enlargement and aggregation of particles leading to coagulate; and (iii) a third phase where the signal reached a plateau indicating the final phase of gelation.
It is evident from that significant differences in the enzymatic hydrolysis phase were observed among coagulant types. In fact, the time of this phase defined by Banon[Citation43] and Bornaz et al.[Citation20] as the time corresponding to the first 10 NTU change in turbidity (t10), was longer for milk clotted by chymosin (25 min) compared to the one clotted by Cynara extract (16.67 min), whereas it was not perceptible when fig latex was used (). This could be attributed to the high proteolytic activity of ficin contained in fig latex. Similar results were found by Fadyloglu[Citation3] who reported that ficin possesses a high proteolytic activity. These differences in the enzymatic phase announce disparity in the final characteristics of the gel. Indeed, Esteves et al.[Citation12] stated that the lag phase determines the amount of protein to be incorporated when the gel is initially formed and the size of the building blocks of the gel.
Table 2 Experimental values of characteristic points of the enzymatic coagulation of ewes' milk by different enzymes (expressed in min)
The duration of the chymosin hydrolysis time of the present work (25 min) is longer than that reported by Bornaz et al.[Citation28] for the same enzyme (4 min). This difference is probably due to the processing temperature (40 C) employed in this study compared to reported (35°C) by Bornaz et al.[Citation28] which is closer to the optimum temperature (32°C) for chymosin activity as prescribed by the supplier. Variability among three types of coagulants is also seen in the difference in ti seen in the curve as the time of the inflection point, characterizing the transition sol/gel of the milk.[Citation20] This time was 0.17 min, 35 min and 54 min for milks coagulated respectively by fig latex, chymosin, and Cynara extract.
The second characteristic of the aggregation phase is tg, which is characterized by a change in the slope or by the appearance of a second peak in the velocity curve.[Citation20] This time was rapidly achieved in milk coagulated by ficin extract. It was equal to 35 and 58 min for Cynara extract and chymosin respectively (). From , it appears that the duration of the aggregation phase (tg – t10) is very high with regard to milk coagulated by chymosin, whereas the solidification time (tg – ti) of the curd obtained by the action of Cynara extract is similar to that obtained by the action of chymosin. It was much higher when ficin latex was used.
Boudier and Luquet[Citation1] reported that the action of coagulants from animal and plant origins are very sensitive in terms of gelation time tg and (tg – ti). However, it appears that the solidification time of plant enzymes is slower and the time of transition sol/gel is shorter. The end coagulation time indicated by tc was achieved very rapidly for milk clotted by fig latex (17.67 min). This time was 4 times longer (> 62 min) for milk coagulated by chymosin. Esteves et al.[Citation11,Citation12] compared the rheological properties of milk gels made with the plant coagulants of Cynara cardunculus L. and Cynara humilis L. to those manufactured with chymosin. They found similar general patterns of gelation curves for the two plant extracts that they attributed to the similarity in the action of the enzymes in the extracts. However, according to these authors,[Citation11,Citation12] the gel obtained by the action of chymosin had slightly different rheological properties. In addition, chymosin gel was firmer than that obtained by the action of plant coagulants, probably owing to their extensive and non-specific proteolytic activity. Gel firmness is highly attributed to the composition of milk, the casein micellar structure and to the way caseins are hydrolyzed.[Citation27]
Follow-up of Milk Conductivity During Coagulation
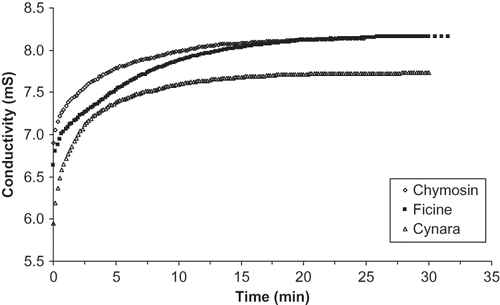
Evolution of the conductivity of milk coagulated by the three types of coagulants shows an exponential pattern (). The initial sharp increase in conductivity obtained after adding the coagulants to the milk is probably due to the liberation of ions from the caseins to the whey along with the increase of their degree of mobility following the hydrolysis.[Citation44] Thereafter, the migration of micellar ions decreased considerably and thus reached a plateau at the end of coagulation.
Final conductivities of curds obtained by the action of fig latex and chymosin were higher than those obtained from the curd made by Cynara extract. In fact, the ash content of the whey of Cynara extract (5.46 g/l) was lower than that of whey obtained by fig latex and chymosin (6.36 and 6.67 g/l, respectively). This variability could be attributed to differences in the proteolytic mechanism of the different enzymes.
Syneresis Capacity and Draining Velocity
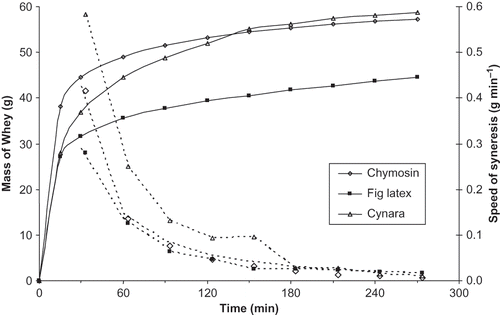
Coagula prepared by chymosin and fig latex had similar syneresis capacities that were lower than that of Cynara extract (). The mean syneresis capacity of Cynara coagulum was 89.8% with a variation coefficient of 0.40%. The variation curves of the mass of expelled whey with time revealed a high variability among the different coagulants (). The mass of expelled whey after 4.5 h was 44.45 g, 57.25 g, and 58.8 g for coagula obtained by fig latex, chymosin and Cynara extract respectively, indicating that the coagulum obtained by the action of fig latex had the highest water retention capacity. This variability is probably due to the differences in the physical properties of the gels.
Table 3 Cheese yield, total solids, and syneresis capacity
The velocity of syneresis decreased during the first hour and then it achieved a plateau regardless of the coagulant used (). Ability to syneresis is highly dependent on the rearrangement of the original micellar state after its destruction by proteolysis and on the establishment of new bonds after gelation.[Citation45] Moreover, the exudation of whey from the interior of the gel to the exterior is dependent on the permeability of the gel itself.[Citation46]
Cheese Yield
The use of fig latex as a coagulant gave the highest curd yield (509.5 g/l of milk) compared to that of Cynara extract and that of chymosin. Curd yields obtained from Cynaraextract and chymosin were almost alike (). However, Low et al.[Citation31] viewed this differently. They found that plant coagulants had an excessive proteolytic activity leading to losses of peptides in the whey thus lowering cheese yields. Even though, fig latex gave the highest cheese yield; it had the lowest syneresis capacity and a very low total solid in the coagulum (). In addition, it caused high losses in caseins and total nitrogen (). The high yield obtained could therefore be attributed to the high water retention by the coagulum. Curd obtained by the action of chymosin gave the lowest yield characterized by a relatively low content of total solids and a syneresis capacity similar to that of Cynara extract.
Sensory Evaluation
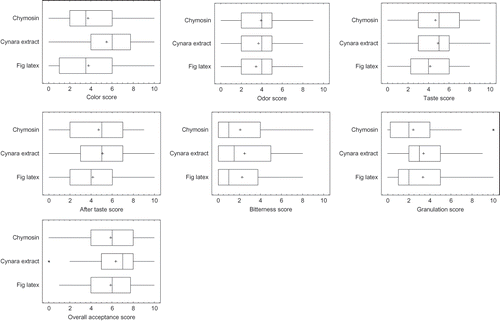
Sensory evaluation results of the three different types of cheese are presented in . The mean color score of fig latex curd was significantly (p > 0.05) higher than that of chymosin and Cynara extract curds. The color of the latter two curds was insignificant (p < 0.05). Color scores of fig latex curds showed less variation among panelists than the two other cheese types. No significant (p < 0.05) differences were observed among the three types of curds with regard to odor, taste, after taste, bitterness, texture and overall acceptance. clearly revealed that none had a perceptible bitterness as all of the curds scored below 3. This low bitterness could be explained by the fact that the curds did not undergo the ripening stage where more proteolysis takes place. In fact, Boudier and Luquet[Citation1] mentioned that perceptible changes in bitterness do not take place during the first cheese processing steps (coagulation, whey expulsion, salting …) but rather during ripening. However, curds obtained by the action of plant coagulants had slightly higher bitterness scores than those of chymosisn. Fadyloglu[Citation3] reported an excessive development of acidity and bitterness in cheese made with the action of plant coagulants than with the use of chymosin. This is due to the excessive proteolytic action of the former. Even though, no significant difference was observed among the three types of cheese with regard to overall acceptance. Cheese of Cynara extract had a higher score (6.35) than curds of chymosin and fig latex (5.87 for both).
Correlation Between Cheese Parameters and Coagulant Types
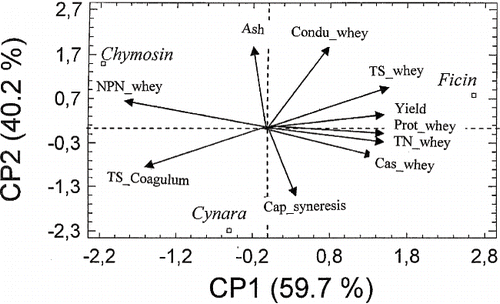
In order to determine the relationship between physicochemical parameters, PCA was applied. The obtained results were shown in . The space generated by CP1 and CP2 represented almost 100% of the information. CP1 positively correlated with the group containing total solids, total nitrogen, protein and casein-whey contents as well as cheese yield. However, it correlated negatively with total solids of the coagulum and the concentration of non-protein nitrogen of the whey. CP2 positively correlated with the ash content and the final whey conductivity. However, it correlated negatively with syneresis capacity and total solids of the coagulum. A high positive correlation was also observed between ash content and final conductivity of the whey. also shows that demineralization of the coagulum had a negative effect on syneresis capacity. Tarodo de la Fuente et al.[Citation46] reported that a highly mineralized coagulum is characterized by an easy circulation of the liquid phase from one pore to another. Moreover, a high positive relation between cheese yield and total solids of the whey is shown in the present study. However, the yield negatively correlated with total solids of the coagulum. This could be explained by the fact that curds with high yields have strong water retention capacities. In , further confirms that the curd obtained using Cynara extract is characterized by a high syneresis capacity, high total solids and high degree of mineralization of the coagulum, whereas the coagulum obtained by chymosin had high demineralization and high content of NPN in the whey. Finally, fig latex coagulum is characterized by high yield, high level of fine particles, and a low syneresis capacity.
CONCLUSION
The analysis of turbidimetric signals revealed differences in coagulation properties of skim ewes' milk as a function of the nature of the coagulants used. Gel obtained with Cynara extract had intermediary characteristics ranging from that of chymosin to that of fig latex. However, general coagulation with plant coagulants is characterized by shorter gelation time and longer restructuration one. Serum obtained with the use of plant extracts had higher casein content after 4.5 h of drainage indicating that plant coagulants are less suited for milk coagulation. Gel obtained by the action of fig latex had a high water retention capacity and thus a higher yield despite the great losses of curd fines in the whey. Sensory analysis showed that with the exception of color, overall sensory attributes did not show any significant differences among the three coagulants. In addition, the main component analysis showed that the curd obtained using Cynara extract was characterized by a high syneresis capacity, high total solids and a high degree of mineralization of the coagulum, whereas the coagulum obtained by chymosin had a high demineralization and a high content of NPN in the whey. Finally, fig latex coagulum was characterized by high yield, a high level of fine particles, and a low syneresis capacity.
REFERENCES
- Boudier , J.F. and Luquet , F.M. 1974 . “ Série Synthèses Bibliographiques N°3 ” . In Présure et succédanés de présure , 84 Paris , , France : Lavoisier .
- Ramet , J.P. 1985 . “ Serial FAO Animal Production and Health Paper N° 48 ” . In La fromagerie et les variétés de fromages du bassin Méditerranéen , 191 Rome , , Italy : FAO .
- Fadyloglu , S. 2001 . Immobilization and characterization of ficin . Nahrung Food , 45 ( 2 ) : 143 – 146 .
- Öner , M.D. and Akar , B. 1993 . Separation of the proteolytic enzymes from fig tree latex and its utilization in Gaziantep cheese production . Lebensmittel-Wissenschaft und Technologie , 26 ( 4 ) : 318 – 321 .
- Brulé , G. , Lenoir , J. and Remeuf , F. 2000 . “ The casein micelle and milk coagulation ” . In Cheesemaking , Edited by: Eck , A. and Gillis , J.C. 7 – 38 . Paris : Tec&Doc. Lavoisier .
- Okigbo , L.M. , Richardson , G.H. , Brown , R.J. and Ernstrom , C.A. 1985 . Variations in coagulation properties of milk from individual cows . Journal of Dairy Science , 68 ( 4 ) : 822 – 828 .
- Storry , J.E. and Ford , G.D. 1982 . Some factors affecting the post clotting development of coagulum strength in renneted milk . Journal of Dairy Research , 49 ( 3 ) : 469 – 477 .
- McMahon , D.J. , Brown , R.J. , Richardson , G.H. and Ernstrom , C.A. 1984 . Effects of calcium, phosphate, and bulk culture media on milk coagulation properties . Journal of Dairy Science , 67 ( 5 ) : 930 – 938 .
- Wium , H. , Pedersen , P.S. and Qvist , K.B. 2003 . Effect of coagulation conditions on the microstructure and the large deformation properties of fat-free Feta cheese made from ultrafiltered milk . Food Hydrocolloids , 17 ( 3 ) : 287 – 296 .
- McMahon , D.J. , Brown , R.J. and Ernstrom , C.A. 1984 . Enzymatic coagulation of milk casein micelles . Journal of Dairy Science , 67 ( 4 ) : 745 – 748 .
- Esteves , C.L.C. , Lucey , J.A. , Wang , T. and Pires , E.M.V. 2003 . Effect of pH on the gelation properties of skim Milk gels made from plant coagulants and chymosin . Journal of Dairy Science , 86 ( 8 ) : 2558 – 2567 .
- Esteves , C.L.C. , Lucey , J A. and Pires , E. M. V. 2001 . Mathematical modelling of the formation of rennet-induced gels by plant coagulants and chymosin . Journal of Dairy Research , 68 ( 3 ) : 499 – 510 .
- Chen , S. , Agboola , S. and Zhao , J. 2003 . Use of Australian cardoon extract in the manufacture of ovine milk cheese—a comparison with commercial rennet preparations . International Journal of Food Science and Technology , 38 ( 7 ) : 799 – 807 .
- Fox , P.F. and McSweney , P.L.H. 1998 . Dairy Chemistry and Biochemistry , 496 New York : Kluwer Academic/Plenum Publishers .
- Walstra , P. , Geurts , T.J. , Noomen , A. , Jellama , A. and van Boekel , M.A.J.S. 1999 . Dairy Technology: Principles of Milk Properties and Processes , 752 New York : Marcel Dekker Ltd .
- Esteves , C.L.C. , Lucey , J A. and Pires , E.M.V. 2002 . Rheological properties of milk gels made using coagulants of plant origin and chymosin . International Dairy Journal , 12 ( 5 ) : 427 – 434 .
- Silva , S.V. and Malcata , F.X. 2005 . Studies pertaining to coagulant and proteolytic activities of plant proteases from Cynara cardunculus . Food Chemistry , 89 ( 1 ) : 19 – 26 .
- Tavaria , F. K. , Sousa , M. J. and Malcata , F.X. 2001 . Storage and lyophilization effects of extracts of Cynara cardunculus on the degradation of ovine and caprine caseins . Food Chemistry , 72 ( 1 ) : 79 – 88 .
- Verissimo , P.C. , Esteves , C.L.C. , Faro , C.J.F. and Pires , E.M.V. 1995 . The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities . Biotechnology Letters , 17 ( 6 ) : 621 – 626 .
- Bornaz , S. , Sammari , J. and Sahli , A. Turbidimetric Kinematics of milk during rennet coagulation and relation with composition . CD-ROM proceedings of ICEF9—Ninth International Congress on Engineering and Food. Modelling Tools for Design Understanding and Control . March 7–11 , Montpellier , France. pp. 162 – 167 .
- Remeuf , F. , Lenoir , J. and Duby , C. 1989 . Etude des relations entre les caractéristiques physicochimiques des laits de chèvre et leur aptitude à la coagulation par la présure . Lait , 69 ( 6 ) : 499 – 518 .
- Hocine , B. , Remeuf , F. , Schneid , N. and Lenoir , J. 2000 . Etude des caractères physico-chimiques et des aptitudes fromagères de poudres de lait . Industries Alimentaires et Agricoles , 117 ( 6 ) : 22 – 28 .
- AFNOR . 1993 . “ Milk and Milk Products ” . In Methods of Analysis Edited by: AFNOR . Paris , , France
- Rowland , S.J. 1938 . The determination o the nitrogen distribution of milk . Journal Dairy Research , 9 : 42 – 46 .
- 2001 . Statgraphics plus 4.0 , Herndon , VA : StatPoint, Inc .
- Assenat , L. 1985 . “ Composition et propriétés ” . In Lait et produit laitiers vache, brebis, chèvre; , Edited by: Luquet , F.M. 281 – 319 . Paris : Tec & Doc. Lavoisier .
- Pellegrini , O. , Remeuf , F. and Rivemale , M. 1994 . Evolution des caractéristiques physicochimiques et des paramètres de coagulation du lait de brebis collecté dans la région de Roquefort . Le Lait , 74 ( 6 ) : 425 – 442 .
- Bornaz , S. , Sahli , A. , Attalah , A. and Attia , H. Physicochemical characteristics and renneting properties of camels' milk: a comparison with goats’, ewes’ and cows’ milks . In CD-ROM Proceedings of IUFOST2006—XIIIth World Congress of Food Science and Technology, Interphase Food Technology - Food Chemistry . 17–21 , Nantes , France. September . pp. 1019 – 1020 .
- Maâmouri , O. , Rouissi , H. , Dridi , S. , Kammoun , M. , De Baerdemaeker , J. and Karoui , R. 2008 . Mid infrared attenuated total reflection spectroscopy as a rapid tool to assess the quality of Sicilo-Sarde ewe's milk during the lactation period after replacing soybean meal with scotch bean in the feed ration . Food Chemistry , 106 ( 1 ) : 361 – 368 .
- Elortondo , F.J. , Albisu , M. and Barcian , Y. 1993 . Changes in the microflora of idiazabal cheese with the addition of commercial lactic starters . The Australian Journal of Dairy Technology , 48 ( 1 ) : 10 – 14 .
- Low , Y.H. , Agboola , S. , Zhao , J. and Lim , M.Y. 2006 . Clotting and proteolytic properties of plant coagulants in regular and ultrafiltered bovine skim milk . International Dairy Journal , 16 ( 4 ) : 335 – 343 .
- Guizani , N. , Kasapis , S. , Attabi , Z.H. and Ruzeiki , M.H. 2002 . Microbial, physicochemical, and biochemical changes during ripening of camembert cheese made from pasteurized milk . International Journal of Food properties , 5 ( 3 ) : 483 – 494 .
- Guizani , N. , Attabi , Z. , Kasapis , S. and Gaafar , O.M. 2006 . Ripening profile of semi-hard standard goat cheese made from pasteurized milk . International Journal of Food Properties , 9 ( 3 ) : 523 – 532 .
- Sidrach , L. , Garcia-Canovas , F. , Tudela , J. and Rodrguez-Lopez , J.N. 2005 . Purification of cynarases from artichoke (Cynara scolymus L.): enzymatic properties of cynarase A . Phytochemistry , 66 ( 1 ) : 41 – 49 .
- Silva , S.V. , Barros , R.M. and Malcata , F.X. 2002 . Hydrolysis of caseins by extracts of Cynara cardunculus precipitated by ammonium sulfate . Journal of Food Science , 67 ( 5 ) : 1746 – 1751 .
- Roseiro , L.B. , Gomez-Ruiz , J.A. , Garia-Risco , M. and Molina , E. 2003 . Vegetable coagulant (Cynara cardunculus) use evidenced by capillary electrophoresis permits PDO Serpa cheese authentication . Le Lait , 83 ( 4 ) : 343 – 350 .
- Sousa , M.J. and Malcata , F.X. 1997 . Comparison of plant and animal rennets in terms of microbiological, chemical and proteolysis characteristics of ovine cheese . Journal of Agricultural and Food Chemistry , 45 ( 1 ) : 74 – 81 .
- Silva , S.V. and Malcata , F.X. 1999 . On the activity and specificity of cardosin B, a plant proteinase, on ovine caseins . Food Chemistry , 67 ( 4 ) : 373 – 378 .
- Silva , S.V. and Malcata , F.X. 1998 . Proteolysis of ovine caseins by cardosin A, an aspartic acid proteinase from Cynara cardunculus L . Le Lait , 78 ( 5 ) : 513 – 519 .
- Queiroz-Macedo , I. , Faro , C.J. and Pires , E.M. 1996 . Caseinolytic specificity of cardosin, an aspartic protease from the cardoon Cynara cardunculus L.: action on bovine αs and β casein and comparison with chymosin . Journal of Agricultural and Food Chemistry , 44 ( 1 ) : 42 – 47 .
- Sousa , M.J. and Malcata , FX. 2002 . Advances in the role of a plant coagulant (Cynara cardunculus) in vitro and during ripening of cheeses from several milk species . Le Lait , 82 ( 2 ) : 151 – 170 .
- Scher , J. and Hardy , J. 1988 . Utilisation d'une méthode turbidimètrique pour étudier l'effet des différentes étapes de préparation des laits sur leur coagulabilité . Science des Aliments , 7 ( HS 8 ) : 159 – 165 .
- Banon , S. 1991 . Modification de la structure des micelles des caséines lors de l'acidification du lait par hydrolyse du Glucono-Delta-Lactone , 146 Nancy , , France : Thèse, Institut National Polytechnique de Lorraine .
- Attia , H. , Kheroutou , N. and Ayadi , J. 2000 . Acidification chimique directe du lait: corrélations entre la mobilité du matériel micellaire et les micro et macrostructures des laits acidifiés . Sciences des Aliments , 20 ( 3 ) : 289 – 307 .
- Ramet , J.P. and Scher , J. 1997 . “ Propriétés physiques du coagulum ” . In Le fromage , Edited by: Eck , A. and Gillis , J.C. 324 – 334 . Paris : Tec&Doc. Lavoisier .
- Tarodo de la fuente , B. , Lanlée , J. and Cuq , J.L. 1999 . Le Lait - coagulation et synérèse . Industries Alimentaires et Agricoles , 166 ( 6 ) : 19 – 26 .