Abstract
Total antioxidant activity (TAA) and cytotoxic effect of four solvent fractions of ethanol extract of Mentha spicata L. were estimated. Relative antioxidant activity (RAA) was also determined relative to quercetin and L-ascorbic acid. Polyphenolics (phenolics and flavonoids) and pigments (chlorophylls and carotenoids) were quantitated and expressed as mg/g of the ethanol fraction. The ABTS/HRP/H2O2 decoloration method was used to estimate the total antioxidant activity. Cytotoxic effect on human prostate cancer cell line (PC-3) was assessed by MTT assay. The TAA was highest for ethyl acetate fraction (83%) followed by aqueous (75%), chloroform (51%) and hexane (47%) fractions. RAAs of ethyl acetate and aqueous fractions were equal to quercetin and ascorbic acid but less in hexane and chloroform fractions. Phenolics and flavonoids were higher in ethyl acetate (54 and 22 mg/g), aqueous (32 and 24 mg/g) and chloroform (30 and 16 mg/g) fractions compared to the hexane (14 and 15 mg/g) fraction. Chlorophyll and carotenoids were higher in chloroform fraction (29 and 7 mg/g) than in ethyl acetate (13 and 5 mg/g), hexane (14 and 3 mg/g) and aqueous (5 and 0.9 mg/g) fractions. Cytotoxic effect against PC-3 cell line was found to be highest for the chloroform fraction and lower for the aqueous fraction. Polyphenolic content and TAAs of the ethanol fractions were positively correlated. Similarly pigment content and cytotoxic effect of PC-3 cells were positively correlated.
INTRODUCTION
Mentha spicata var. viridis L. (Lamiaceae), also known as spearmint, is an aromatic spice and is cultivated throughout the world. It is an herbaceous perennial with leafy stolons. In India, it is called as “Pudina” and mostly cultivated for culinary purposes.[Citation1] In Unani, leaves are used in the preparation of multicomponent herbal tea called “Zahraa'.[Citation2] In Danish folk medicine, it is known to enhance memory.[Citation3] This herb is considered to be a stimulant, carminative, antispasmodic, stomach ache and diuretic.[Citation4] Boiled leaf extract relieves hiccough, flatulence, giddiness and indigestion. It is used for gas pain, rheumatism, muscle pain and also as mouthwash.[Citation5] Its essential oil (spearmint oil) is used in food, cosmetic and pharmaceutical industries.[Citation6] The essential oil of M. viridis has been found to be toxic to certain insects.[Citation7] Mentha extracts are found to have antioxidant and antibacterial properties. The aqueous extract provides protection against radiation-induced chromosomal damage in bone marrow of mice and ethyl acetate fraction of methanol extract has been shown to have anti-histaminic.[Citation8,Citation9]
Antioxidant activity of M. spicata was previously estimated using ABTS with potassium persulphate decolorization assay.[Citation10] Presently antioxidant activity was estimated using ABTS/HRP/H2O2 method. This method differed from the earlier methods in the following aspects: ABTS .+ was generated enzymatically, high temperature was not required and the amount of radical production was controlled by H2O2. Antioxidant activity can be estimated over a wide range of pH values and is more accurate.[Citation11]
The objective of this report is to present the data on total antioxidant activity (TAA), relative antioxidant activity (RAA) of the hexane, chloroform, ethyl acetate and aqueous fractions of ethanol extract of dried leaf powder of M. spicata, their polyphenolic, pigments content and cytotoxic effect against PC-3 cell line.
MATERIALS AND METHODS
Mentha spicata L. was commercially purchased and identified (Herbarium voucher number-855, Centre for Advanced Studies in Botany, University of Madras). All solvents used in this study were of analytical grade (Sisco Research Laboratories Pvt. Ltd., India).
Extraction and Fractionation
The extraction was carried out following the method described by Villasenor et al.[Citation12] Briefly, 150 g of leaves were shadow dried (90% moisture content), powdered and immersed in 500 ml of 95% ethanol with constant stirring (orbit shacker) for 24 h and filtered. The residue was further treated with ethanol twice. A final volume of 1.2 L filtrate was vacuum concentrated under reduced pressure at 40oC using rotary evaporator. Ethanol extract was partitioned using a mixture of hexane and water (6:1). The aqueous layer was further fractionated with chloroform (CHCl3) and ethyl acetate. The solvent fractions were collected and concentrated with vacuum rotary evaporator. The yields of these fractions were 3.0, 2.0 and 1.1 g respectively. The aqueous fraction was lyophilized (Flexi-Dry up at 50 MT and –85°C) and this fraction weighed 0.08 g.
Total Antioxidant Activity (TAA) and Relative Antioxidant Activity (RAA)
Total antioxidant activity was estimated by the method of Cano et al.[Citation13] with minor modification. The method is based on the capacity of biological compounds to scavenge the ABTS.+. The standardized was done in our laboratory at 25°C. The reaction mixture for hydrophilic compounds contained 2 mM ABTS, 150 μM H2O2 and 35 μM HRP in 50 mM phosphate buffered saline (PBS-pH 7.5) and for lipophilic compounds 1 mM ABTS, 120 μM H2O2 and 15 μM HRP in ethanol medium to a total volume of 1 ml. The reaction was monitored at 730 nm until stable absorbance was obtained. Known concentration of ethanol fractions (0–50 mg/ml) and pure compounds (0–20 μM/ml) were added to the reaction mixture. Decrease in absorbance, proportional to the ABTS .+ quenched, was noted after 5 min.[Citation11] The standard stock solutions of quercetin and L-ascorbic acid were prepared in ethanol and water respectively.[Citation14] Appropriate blanks were used and all the experiments were noted in five readings. The total antioxidant activity (TAA) and relative antioxidant activity (RAA) were calculated using the following equations[Citation15]:
Measurement of Total Flavonoids and Phenolics
Total flavonoids were estimated using the method of Lamasion and Carnat.[Citation16] One ml of 2% methanol aluminum chloride was mixed with 1 ml of ethanol fractions and immediately read at 430 nm. Total phenolics were estimated by Price and Butler[Citation17] method. Test tube containing 25 ml of deionised water and 250 μl of ethanol fraction were mixed with 3 ml of ferric chloride (0.1 M FeCl3 in 0.1 M of HCl). After 3 min, 3 ml of potassium ferricyanide (0.008 M K3 Fe (CN)6) was added and allowed to stand for 10–15 min at room temperature and the reaction mixture was measured at 720 nm. The values are expressed as mg/g of ethanol fraction using quercetin as standard.
Estimation of Total Chlorophyll and Carotenoids
Chlorophyll ‘a’, ‘b’ and carotenoids were measured at 646, 663, and 470 nm respectively by the method of Lichtenhaler and Wellburn.[Citation18] The pigments were expressed as mg/g of ethanol fraction. The total chlorophyll content was obtained by the addition of chlorophyll ‘a’ and ‘b’ values.
Cytotoxicity Assay
The in vitro cytotoxic effect on human prostate cancer cell line (PC-3) was assessed using 3-(4,5-dimethyl l-2-thiazol)-2,5-diphenyl-2H-tetrazolium bromide (MTT) dye. The assay is based on the reduction of the yellow dye to purple formazan crystals by the mitochondrial dehydrogenase enzyme.[Citation19] Cells (10000 cells/well) were pippetted into 96-well plates and allowed to adhere for 24 h. The ethanol fractions filtered through 0.2 μ Millipore filter, concentrated and resuspended in DMSO were added to a final concentration of 125, 250, and 500 mg/ml and incubated for 24 and 48 h at 37°C in humidified 5% CO2 atmosphere with appropriate control medium (RPMI-1640). After exposure, medium was replaced with 100 ml of fresh medium containing 100 mg of MTT (10 mg/10 ml). The cells were further incubated for 4 h. The formazan crystals were dissolved in 100 μl of 20% SDS (in 50% dimethyl formamide) and ODs of the solution was read at 620 nm using ELISA plate reader.
RESULTS
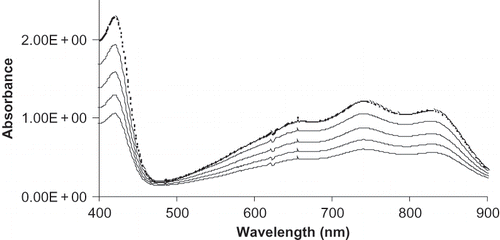
The absorbance spectrum of the ABTS radical in ethanol and phosphate buffered saline medium is shown in . The spectrum of the ethanol medium was taken at 30 second time intervals and that of the PBS medium at two min (dotted line). Absorption maxima were observed at 420, 740 and 827 nm in both media.
Figure 1 Absorbance spectrum of ABTS.+ generated in ethanol and sodium phosphate buffer (dotted line) medium.
The TAA increased with the increasing concentrations of the standard antioxidants (quercetin and ascorbic acid). Quercetin was found to possess the highest TAA (82%) at 5 μM and that of ASC (75%) at 20 μM (). Hexane, chloroform, ethyl acetate and aqueous fractions possessed the TAA of 47, 51, 83, and 75% at their maximum concentration: 50, 50, 15, and 30 μg/ml, respectively (). The RAAs of ethyl acetate fraction was more than one and that of aqueous fraction was almost equal to one ().
Figure 2 TAA of standards in decreased absorbance at 730 nm with increased concentration in both ethanol and phosphate buffered saline medium.
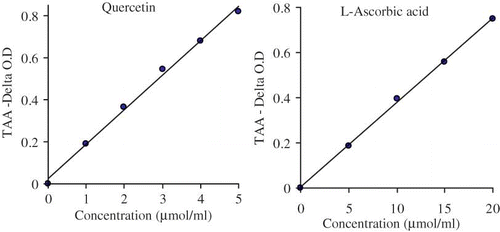
Table 1 Total antioxidant activity and relative antioxidant activity of ethanol fractions of Mentha spicata at different concentrations
Expressed as standard antioxidant equivalents (mM/g of ethanol fractions: quercetin for lipophilic compounds), the antioxidant activity (AA) of ethyl acetate fraction was higher (328 mM/g) compared to the hexane (57.60 mM/g) and chloroform (61.60 mM/g) fractions (). This can be accounted to the presence of greater amounts of polyphenolics in the ethyl acetate fraction compared to the hexane and chloroform fractions. The pigments (chlorophyll and carotenoids) were less in hexane and ethyl acetate fractions compared to the chloroform fraction (). The ascorbic acid equivalent for the aqueous fraction was 663.33 mM/g. Thus, it appears that, AA of ethyl acetate fraction was higher than that of the aqueous fraction. Highest phenolic content was observed in ethyl acetate fraction (54.00 ± 4.74 mg/g) followed by aqueous (32.00 ± 3.16 mg/g), chloroform (30.00 ± 2.28 mg/g) and hexane (14.00 ± 1.58 mg/g) fractions, respectively. On the other hand, flavonoids of aqueous fraction (24.20 ± 4.60 mg/g) were the highest compared to the ethyl acetate (22.00 ± 5.46 mg/g), chloroform (16.20 ± 3.38 mg/g) and hexane (15.00 ± 1.58 mg/g) fractions. In contrast, the pigment of aqueous fraction was the lowest compared to the other three fractions. The chloroform fraction possessed the highest amount of total chlorophyll (29.07 ± 0.76 mg/g) and carotenoids (7.17 ± 1.23 mg/g) compared to the hexane (14.40 ± 1.95, 3.07 ± 0.51 mg/g) and ethyl acetate (13.20 ± 1.40, 5.07 ± 1.41 mg/g) fractions. Overall, it appears that the TAA of ethyl acetate and aqueous fractions was mostly due to polyphenolics than due to the pigment compounds.
Table 2 Polyphenolics and pigments content of ethanol fractions of Mentha spicata
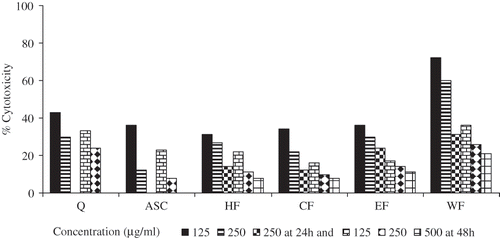
shows the percent cytotoxic effect of the ethanol fractions on PC-3 (Prostate cancer cells) cells at 125, 250, and 500 mg/ml concentration exposed to 24 and 48 h. The figure also shows cell growth inhibition of quercetin and ASC at 125 and 250 mg/ml concentrations. Irrespective of the duration of incubation and concentration, ASC was more effective than quercetin in inhibiting PC-3 cell growth. Increasing concentration and the incubation time enhanced the cytotoxic effect. Maximum cytotoxic effect (about 92% inhibition) was observed at 250 mg/ml concentrations, when incubated for 48 h. All the ethanol fractions had shown similar trends with respect to the concentration and duration of incubation. However, at 48 h incubation period the aqueous fraction showed comparable cytotoxic effect to that of quercetin. The cytotoxic effects of hexane, chloroform and ethyl acetate fractions were either comparable with the values of ASC or greater than the values observed for quercetin. Among the three, hexane and chloroform fractions had the highest cytotoxic effect.
DISCUSSION
The ABTS/HRP/H2O2 method is widely used for screening biological compounds, food products and extracts for both the lipophilic and hydrophilic antioxidant status.[Citation11] Of the two standards, quercetin had four fold higher TAA than that of ascorbic acid (TAA: 19%) at their corresponding concentration (5 μM/ml) (). This was attributed to the presence of hydroxyl groups with double bonds in the C ring at 2 and 3 positions and the 4-oxo groups in the polyphenolic structure. This structural advantage is responsible for enhanced antioxidant activity.[Citation20]
The TAA of solvent fraction was increased with the increasing concentration. Ethyl acetate fraction was having the highest TAA (83%) than the other solvent fractions at their maximum concentration and it was comparable with that of quercetin TAA (82%). Antioxidant activity of spearmint extract was reported to depend upon its concentration which was better than that of the coriander leaves and BHT.[Citation6] Quercetin equivalents antioxidant activity (AA) of ethyl acetate fraction was found to be the highest (328 mM/g) at the lowest concentration when compared to the aqueous fraction. The ethyl acetate fraction had TAA comparable to that of quercetin's TAA. This fraction had high content of polyphenolic compounds (phenolics and flavonoids) and this may be responsible for their higher AA. In an earlier study, Rice-Evans et al.[Citation21] reported that AA was due to the presence of polyphenolic compounds in the plant extract. Similar observation was also reported by Cakir et al.[Citation22] in the ethyl acetate fraction of Hypericum hyssopifolium (L.).
The lower AA of hexane (57.6 mM/g) and chloroform (61.6 mM/g) fractions can be attributed to the lower content of polyphenolics in these fractions (14.00 ± 1.58, 15.00 ± 1.58; 30.00 ± 2.28, 16.20 ± 3.83 mg/g) (). Chloroform fraction had 2‐fold higher chlorophyll and carotenoids content than the ethyl acetate and hexane fractions. In comparison with the aqueous fraction, the chloroform fraction had 5–7 fold higher amounts of pigments (). The lipophilic antioxidant activity is generally correlated to the pigments content and in particular, to the carotenoids. Moreover, this activity was not always directly proportional to the antioxidant activity.[Citation11] It means that polyphenolics have higher free radical scavenging ability than that of the pigments. This explains higher AA of the aqueous fraction even though it had the lowest amount of pigments. Polyphenolic content of the aqueous fraction was much higher (phenolic-32.00 ± 3.16; flavonoids-24.20 ± 4.60 mg/g) compared to hexane (phenolics-14.00 ± 1.58; flavonoids-15.00 ± 1.58 mg/g) and chloroform (phenolics-30.00 ± 2.28; flavonoids-16.20 ± 3.83 mg/g) fractions. Total phenolic content of the spearmint had been expressed as garlic acid equivalents and flavonoids were expressed as quercetin equivalent.[Citation23] In fact, flavonoids content of the aqueous fraction was the highest (24.20 ± 4.60 mg/g) compared to any other fraction. Therefore, the enhanced AA of the aqueous fraction can partly be attributed to the higher content of flavonoids. It will be relevant to identify the individual compounds of flavonoids and evaluate their AA. Polyphenolics are important components of the human diet and their intake is quite high (50 to 800 mg/day) compared to vitamin C (70 mg/day), vitamin E (7–10 mg/day) and carotenoids (β-carotenoid, 2–3 mg/day).[Citation24] The leafy part of the vegetable contains many active compounds that easily dissolve in water and hence can be used in reducing oxidative stress that mankind is often exposed to.[Citation25,Citation24]
The cytotoxic effect was higher in lipophilic fractions than the hydrophilic fraction. It indicates that the pigments are more effective on PC-3 cells than polyphenolics, although they have higher antioxidant activity. Similarly, vitamin C has higher cytotoxic effect compared to quercetin. Vitamin C regulates the cell proliferation via inhibition of the dioxygenase, deoxyhypusine hydroxylase and is considered to be a possible target for anti-cancer strategies.[Citation26] Hexane and chloroform fractions had greater cytotoxic effect than the other fractions. In an earlier study, methanol extract of Mentha has been found to be cytotoxic.[Citation27] Similar results have been reported by Ferraz et al.[Citation28] for both hexane and chloroform fractions of methanol extract of Hypericum species. These fractions have highest cytotoxic effect against HT-29 human colon carcinoma and H-460 non-small cell lung carcinoma cell lines. They isolated three benzopyrans in these fractions that were responsible for high cytotoxicity.
CONCLUSION
In this study, the antioxidant activity was entirely dependent on the amount of polyphenolics present and cytotoxic effect positively correlated with the amount of pigments present in various fractions. Therefore, it is essential to partially purify the solvent fractions and identify both the polar and non-polar compounds present in them.
ACKNOWLEDGMENT
This work was supported by a grant from University Grant commission, New Delhi, University of Madras (No. HS11).
REFERENCES
- Mhaskar , K.S. , Blatter , E. and Caius , J.E. 2000 . Indian Medicinal plant, their usage in ayurveda and unani medicines . Sri satyrur publications, Delhi , 8 : 2726 – 2727 .
- Carmona , M.D. , Llorach , R. , Obon , C. and Rivera , D. 2005 . “Zahraa”, a Unani multicomponent herbal tea widely consumed in Syria: Components of drug mixtures and alleged medicinal properties . J. Ethnopharmacol. , 102 : 334 – 350 .
- Adsersen , A. , Gauguin , B. , Gudiksen , L. and Jager , A.K. 2006 . Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity . J. Ethnopharmacol. , 104 : 418 – 422 .
- Saleem , M. , Alam , A. and Sultana , S. 2000 . Attenuation of benzoyl peroxide-mediated cutaneous oxidative stress and hyperproliferative response by the prophylactic treatment of mice with spearmint (Mentha spicata) . Food Chem. Toxicol. , 38 : 939 – 948 .
- Choudhury , R.P. , Kumar , A. and Garg , A.N. 2006 . Analysis of Indian mint (Mentha spicata) of essential, trace and toxic elements and its antioxidant behaviour . J. Pharmaceut. Biom. Analy. , 41 ( 3 ) : 825 – 832 .
- Kanatt , S.R. , Chander , R. and Sharma , A. 2007 . Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat . Food Chem. , 100 ( 2 ) : 451 – 458 .
- Papachristos , D.P. and Stamopoulos , D.C. 2002 . Repellent, toxic and reproduction inhibitory effects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) . J. St. Prod. Res. , 38 ( 2 ) : 117 – 128 .
- Samarth , R.M. and Kumar , Ashok . 2003 . Mentha piperita Linn., Leaf extract provides protection against radiation induced chromosomal damage in bone marrow of mice . Indian J. Exp. Biol. , 41 : 229 – 237 .
- Yamamura , S. , Ozawa , K. , Ohatani , K. , Kasai , R. and Kazuo . 1998 . Antihistaminic flavones and aliphatic glycosides from Mentha spicata . Phytochemistry. , 48 ( 1 ) : 131 – 136 .
- Arumugam , P. , Ramamurthy , P. , Santhiya , S.T. and Ramesh , A. 2006 . Antioxidant activity measured in different solvent fractions obtained from Mentha spicata Linn.: An analysis by ABTS.+ decolorization assay . Asia Pacific J. Clin. Nutr. , 15 ( 1 ) : 20 – 24 .
- Arnao , M.B. , Cano , A. , Alcoiea , J.E. and Acosta , M. 2001 . Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem . Anal. , 12 : 138 – 143 .
- Villasenor , I.M. , Echegoyen , D.E. and Angeladia , J.S. 2002 . A new antimutagen from Mentha cordifolia (Opiz) . Mutat. Res. , 515 : 141 – 146 .
- Cano , A. , Hernandez-Ruiz , J. , Garcia-Canovas , F. , Acosta , M. and Arnao , M.B. 1998 . An end-point method for estimation of the total antioxidant activity in plant material . Phytochem. Anal. , 9 : 196 – 202 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Rad. Biol. Med. , 26 : 1231 – 1237 .
- Arnao , M.B. , Cano , A. and Acosta , M. 1998 . Total antioxidant activity in plant material and its interest in food technology. Rec. Res. Develop . Agri. Food Chem. , 2 : 893 – 905 .
- Lamaison , J.N. and Carnat , A. 1990 . Teneur en principaux flavonoids des fleurs et des feuilles de Crataegus monogyna Jacq. et de Crataegus laevigata (Poiret) DC. (Rosaceae) . Pharmacol. Acta. Helv. , 63 : 315 – 320 .
- Price , M. and Butlar , L.G. 1977 . Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain . J. Agri. Food Chem. , 25 : 1268 – 1273 .
- Lichtenhaler , K. and Wellburn , A.R. 1983 . Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents . Biochem. Soc. Tran. , 11 : 591 – 592 .
- Mosmann , T.J. 1983 . Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays . J. Immolunol. Meth. , 65 : 55 – 63 .
- Miller , N.J. and Rice-Evans , C.A. 1996 . Spectrophotometric determination of antioxidant activity . Red. Rep. , 2 : 161 – 171 .
- Rice-Evans , C.A. and Miller , N.J. 1994 . Total antioxidant status in plasma and body fluids . Meth. Enzymol. , 234 : 279 – 293 .
- Cakir , A. , Mavi , A. , Yildirim , A. , Duru , M.E. , Harmandar , M. and Kazaz , C. 2003 . Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium (L.) by activity-guided fractionation . J. Ethnopharmacol. , 87 : 73 – 83 .
- Kumar , A. and Chattopadhyay , S. 2007 . DNA damage protecting activity and antioxidant potential of Pudina extract . Food Chem. , 100 ( 4 ) : 1377 – 1384 .
- Winston , J.C. 1999 . Health promoting properties of common herbs . Am. J. Clin. Nutr. , 70 : 491S – 499S .
- Yang , B. , Kotani , A. , Arai , K. and Kusu , F. 2001 . Estimation of the antioxidant activities of flavonoids from their oxidation potentials . Anal. Sci. , 17 : 599 – 604 .
- Caraglia , M. , Marra , M. , Giuberti , G. , Alessandro , D. , Budillon , A. , del Pretem , S. , Lentini , A. , Beninati , S. and Abbruzzese , A. 2001 . The role of Eukaryotic initiation factor 5A in the control of cell proliferation and apoptosis . Amino Acid. , 20 : 91 – 104 .
- Badisa , R.B. , Tzakou , O. , Couladis , M. and Pilarinou , E. 2003 . Cytotoxic activities of some Greek Labiatae herbs . Phytother. Res. , 17 : 472 – 476 .
- Ferraz , A. , Faria , D.H. , Benneti , M.N. , Brondani da Rocha , A. , Schwartsmann , G. and Henriques von Poser , G.L. 2005 . Screening for antiproliferative activity of six southern Brazilian species of Hypericum . Pytomedicine. , 12 : 112 – 115 .