Abstract
Investigations were carried out on the natural antioxidants in edible flours of small millets. Total carotenoids content varied from 78–366 μg/100 g in the millet varieties with an average of 199 + 77, 78 + 19, 173 + 25, and 366 + 104 μg/100 g in finger, little, foxtail, and proso millets respectively. Analysis of carotenoids by HPLC for the presence of β-carotene showed its absence in the millets. HPLC analysis of vitamin E indicated a higher proportion of γ−and α-tocopherols; however, it showed lower levels of tocotrienols in the millets. Total tocopherol content in finger and proso millet varieties were higher (3.6–4.0 mg/100 g) than in foxtail and little millet varieties (∼1.3 mg/100 g). Total antioxidant capacity in finger, little, foxtail and proso millets were 15.3 + 3.5, 4.7 + 1.8, 5.0 + 0.09, and 5.1 + 1.0 mM TE/g, respectively. From these studies it could be concluded that edible flours of small millets are good source of endogenous antioxidants.
INTRODUCTION
Small millets refer to a group of minor cereals among which a few are cultivated in India. The popular millet in India are finger millet (Eleusine coracana), foxtail millet (Setaria italica), kodo millet (Paspalum scrobiculatum), proso millet (Panicum miliaceum), little millet (Panicum sumatrens) and barnyard millet (Echinocloa frumenta). These are mainly grown in different regions of the country and their total production in world and India is about 26 and 8 million MT, respectively. They play an important role in food security and also in the economy of many of the less developed countries of the world. In India and Africa they are mainly used for food and allied purpose in the whole or decorticated flour form. Whereas in Japan and other developed countries, they are mainly used as bird feed.
Millets are rich source of nutrients and contain 60–70% dietary carbohydrates, 6–10% protein, 1.5–5% fat, 12–20% dietary fibre, and 2–4% minerals, and several other phytochemicals compared to rice or wheat[Citation1] and offer several health benefits to the consumers.[Citation2] However, the minor millets have remained staple food for the traditional consumers mainly because of lack of ready-to-use products similar to rice and wheat, and also due to the lack of awareness on their nutritional quality and health benefits. Hence, there is an immense potential for the development of a number of value added products with an edge on health benefits.
The foods we commonly consume contain phenolics, flavanoids, tocopherols and carotenoids, which serve as a good source of natural antioxidants,[Citation3,Citation4] and are reported to have health beneficial effects.[Citation5] Vitamin E functions as natural antioxidant[Citation6] to protect fat in membranes around cells such as nerves, heart, muscles and red blood cells from possible damage by oxygen and protect us from carcinogenesis, cardiovascular diseases and aging.[Citation7,Citation8] The naturally occurring tocopherols and tocotrienols constitute vitamin E group of compounds and report on these in millets are not available. Carotenoids are important in human nutrition and health. They are valuable as antioxidants,[Citation9] in the prevention of atherosclerosis,[Citation10] in the maintenance of immune function,[Citation11] in the health of eyes,[Citation12] and some are precursors of vitamin A. Presence of carotenoids was reported in grains such as maize, wheat and sorghum.[Citation13,Citation14] However reports on carotenoids in various minor millet varieties are scanty. Cereals and legumes contain a wide range of phenolics and act as good source of natural antioxidants.[Citation15] Presence of antioxidative phenolics have been reported in millets also.[Citation16,Citation17] Flavonoids like tannin and anthocyanins also have antioxidative potential.[Citation18] However, there are no reports on the nature of lipid soluble antioxidants of the minor millets, hence studies were undertaken to determine some of the antioxidants of the millets such as carotenoids, tocopherols and tocotrienols and also their total antioxidant potential.
MATERIALS AND METHODS
Materials
Finger millet varieties (n = 14) were procured from Zonal Agricultural Research Station of the University of Agriculture Science, Bangalore, Karnataka State (India), whereas little millet, foxtail millet and proso millet were obtained from Tamilnadu Agriculture University, Coimbatore, Tamilnadu State (India). Except finger millet, the other millet were dehusked to remove inedible husk in centrifugal sheller (Kisan Krishi Yanthra Udyog, India) running at 2800–3200 rpm and aspirated to remove husk, followed by de-branning in a horizontal type friction polisher to 5–7% degree of milling, according to the procedure of Hadimani and Malleshi.[Citation1] The de-branned millets as well as cleaned finger millet were powdered in a Udy cyclone mill to 60 mesh (BSS) so as to obtain edible flours. Standard β-Carotene and tocopherols were purchased from ICN Biomedicals Inc. Aurora, Ohio and Sigma, USA and Tocovid capsule of Hovid Bhd, Malaysia used for tocotrienols. All other chemicals and solvents were of analytical grade procured locally and used without further purification.
Determination of Total Carotenoids
Each of the samples (5 g) was mixed with about 50 ml acetone and ground with pestle and mortar. The extract was filtered and the extraction repeated till colorless. The extracts were pooled and mixed with 50 ml petroleum ether and 400 ml distilled water in a separating funnel. The petroleum ether layer was separated and washed 2–3 times with water, dried with anhydrous sodium sulphate and made up to 100 ml with petroleum ether. The absorbance was measured at 452 nm and the total carotene content was calculated based on the molar extinction co-efficient of β-carotene.[Citation19] Linear response of carotenoid was in the range 0.5–10 ng.
Analysis of β-carotene
The solvent used for extraction of total carotenoid was evaporated using nitrogen at 40–50°C in a water bath and the residue was dissolved immediately in a known volume of methanol and stored at −20°C until analysis. The carotenoids were fractionated using HPLC system [Shimadzu HPLC with SCL-10A system controller, LC 10AT pump, C18 (250 × 4.6 mm, 5 μm) column and SPD, 10A UV- visible detector] and isocratic solvent system containing acetonitrile, chloroform, isopropanol and water (78: 16: 3.5: 2.5 v/v) set at a flow rate of 1 ml/min and the detector was set at 452 nm.[Citation20]
Extraction of Sample for the Total Antioxidant Activity and Vitamin E Analysis
The millet samples (0.1 g) were extracted with one ml of methanol for one hour with occasional mixing. The extract centrifuged at 3000 rpm and the supernatant was filtered, and then stored at –20°C until used for experiment.[Citation21]
Total Antioxidant Activity
The total antioxidant activities of the different varieties of small millets were quantified using the phosphomolybdenum reagent.[Citation22] An aliquot of sample (20 μL) in methanol was mixed with 1230 μl of the reagent in a microtube. The tubes were capped, shaken well and incubated at 90°C for 90 min in water bath and the absorbance was measured at 695 ηm against a reagent blank. Results were calculated and expressed as α-tocopherol equivalents (TE) per gram using the molar extinction coefficient of α-tocopherol. Linearity of reaction was found to be in the range 2 × 10−4 to 2 × 10−5 moles.
Vitamin E
Vitamin E (tocopherols and tocotrienols) content of millet varieties like finger millet (IND 8—brown colored variety), finger millet (IND 11—white colored variety), little millet (Paiyur 1), foxtail millet (TNAU 213), and proso millet (TNAU 143) were quantified by reverse phase HPLC (CBM-10A Shimadzu system with RF10AXL fluorescent detector, LC10AT pump). The Chromatograms were recorded and processed by LC-10A class software. The extracts were separated chromatographically on Shim-Pack Prep-ODS(H) column (250 × 4.6mm, 5μm) using a gradient solvent system consisting of acetonitrile, methanol, isopropanol and aqueous acetic acid [45:40:5:10] in pump A and acetonitrile, methanol, and isopropanol (25:70:5) in pump B.[Citation21] The Fluorescence detector was set at excitation and emission wavelengths of 298 and 328 nm, respectively. Standards of both tocopherol and tocotrienol exhibited a linear response in the range as follows; α 4–45 ng, γ 3−55 ng, δ 0.4–5ng.
Statistical Analysis
Values of mean + SD (standard deviation) of 3 independent determination were subjected to student t-test to study the level of significance at p < 0.05[23].
RESULTS AND DISCUSSION
Total Carotenoids
Wide variations with respect to total carotenoids contents among the different varieties of finger millet were observed (). The varieties MR 1, IND 5, IND 8, IND 11, and IND 9 contained higher (316–250 μg/100 g) levels; GPU 45, L 5, HR 911, GPU 28, GPU 26, and MR 6 contained medium level (212–134 μg/100 g) and PR 202, IND 15, and IND 7 contained lower levels of total carotenoids (112–78 μg/100 g). Even though, IND 11 was a white seeded variety, its carotenoid content was considerably high compared to a few colored varieties.
Table 1 Total carotenoid (μg/100g) in small millets
Similar to finger millet, the total carotenoid content of little millet also showed wide variations. It was high (104–87 μg/100 g) in CO 3, TNAU 101, and CO 2 varieties; medium (77–76 μg/100 g) in TNAU 81 and TNAU 99, and low (57–51 μg/100 g) in TNAU 91 and Paiyur 1 varieties. Likewise, the total carotenoid content in the foxtail millet varieties, TNAU 209, CO 7, CO 5, C0 6, and TNAU 213 ranged from 191 to 164 μg/100 g, but it was only 126 μg/100 g in TNAU 193 variety. Among the proso millet varieties, total carotenoid content was high (518–414 μg/100 g) in TNAU 149 and TNAU 143 varieties, medium (329–318 μg/100 g) in CO 14 and TNAU 137, and low in TNAU 151 (249 μg/100 g) varieties. These results indicate that the total carotenoids content is high in proso millet (366 μg/100 g), followed by finger millet (199 μg/100 g), foxtail millet [173 μg/100 g) and low in little millet (78 μg/100 g). These values are comparable with the carotenoids content of wheat 150–200 μg/100 g and sorghum 180–230 μg/100 g but significantly less than maize 1800–5500 μg/100 g.[Citation13]
Millets being the staple food of low income population their consumption will provide with good proportion of carotenoids. However, the β-carotene content in the millet varieties tested was not detected by HPLC analysis (), unlike in maize which is reported to contain about 15.7 ug/100 g.[Citation24] Even though, the presence of β-carotene could not be detected in small millets, contribution of other carotenoid types like xanthophylls (zeaxanthine, lutein, etc.) should not be overlooked. These are present in a wide variety of fruits and vegetables and also in corn.[Citation25] Lutein and zeaxanthin are reported to prevent eye diseases.[Citation26] Hence, detailed investigations on carotenoids of small millets will be very useful for their utilization in health foods.
Total Antioxidant Capacity
The total antioxidant capacity among the different varieties of finger millets was high (27–22 mM TE/g) in MR 6, MR 1, HR 911; medium (17–13 mM TE/g) in IND 15, IND 5, IND 7, GPU 28, L 5, IND 8, IND 9, and low (11–7 mM TE/g) in GPU 45, GPU 26, PR 202, IND 11 varieties (). The total antioxidant capacity was generally low in white variety (IND-11) and also in a few brown varieties. It shows that all colored varieties are not rich in antioxidants. Among the little millet varieties, total antioxidant capacity ranged from 6.3–3.5 mM TE/g, where as in foxtail millet and proso millet varieties, it ranged from 5.7–4.4 mM TE/g and 6.3–4.2 mM TE/g, respectively. On the other hand, among the different millets, the average values for the total antioxidant capacity was almost three-fold higher in finger millet flour (15.3 mM TE/g) as compared to the other millet flours. The lower levels of antioxidants in other millets could be due to the separation of husk and bran by milling.
Table 2 Total antioxidant capacity [mM TE (tocopherol equivalent)/g] in different small millets
Total antioxidant capacity is due to the presence of vitamin E, carotenoids, and polyphenols. Investigations in our lab also have shown that finger millets are rich in polyphenols[Citation27] and have free radical quenching ability.[Citation28] Phenolics like ferulic acid and coumaric acid in cereals are known to express high antioxidant activity. These phytochemicals contribute to an effective antioxidant potency. Antiradical properties of other cereals like sorghum,[Citation29] wheat,[Citation30] and rice[Citation31] have been reported. But these values are not comparable because different methods were followed to assay the antioxidant potency of different grains. Polished rice contains only 3mM TE/g, which is much less than in the fully polished small millets (unpublished data).
Vitamin E Characterization
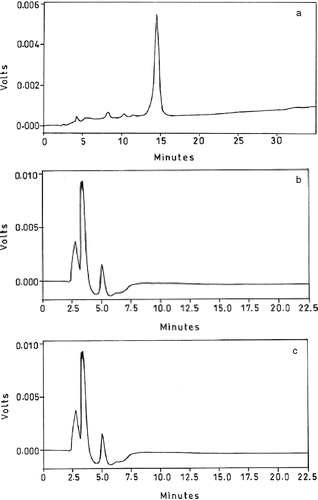
The characterization of vitamin E in small millets was carried out by reverse phase HPLC (). Vitamin E in the millets was found to be in the form of tocopherols as a major component and tocotrienols as a minor component. The content of total tocopherols () was highest in finger millet brown colored variety (4 mg/100 g) followed by finger millet white colored variety and proso millet (∼3.6mg/100 g). Content in little and fox tail millet were relatively low (∼1.3 mg/100 g).
Figure 2 HPLC chromatograms of (2a) standard; (2b) finger millet; (2c) proso millet; (2d) foxtail millet; and (2e) little millet, showing 1- δ, 2- γ, 3- α tocotrienols; 4-δ, 5− γ, 6− α tocopherols.
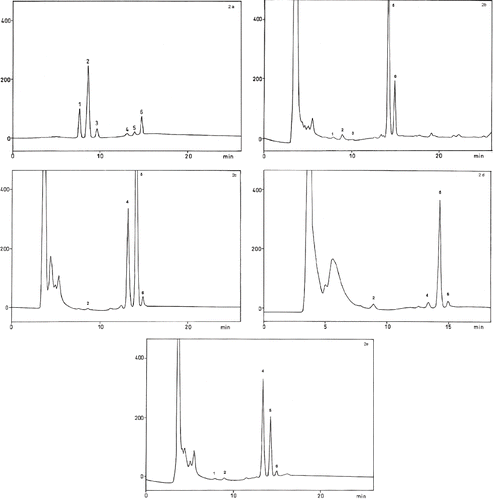
Table 3 Content of Tocopherols (mg/100 g) in small millets (Mean ± SD)
The prominent isomers of tocopherol in finger millet were γ and α, but δ and γ were prominent in little and proso millets. On the other hand, only γ isomer was the major tocopherol in foxtail millet. The content of δ was high in little and proso millets and γ was high in finger and proso millets. It was also observed that finger millet contained high proportion of α tocopherol also. Among the finger millet varieties, brown colored variety contained more δ and α tocopherols than the white colored variety, but γ tocopherol was same.
Among the various isomers of tocotrienols, δ, γ and α were observed in finger millet, δ and γ in little millet and γ only in foxtail and proso millets. Tocotrienols are not quantified since the contents are only in traces. Different isomers of vitamin E act as natural antioxidants and both tocopherols and tocotrienols do not have much difference in antioxidant potential against cholesterol oxidation.[Citation32]
Major vitamin E component in other cereals like wheat also is tocopherols, but rice contains tocotrienols in good quantity.[Citation33] Rice and wheat flours contain only 0.8 mg and 1.23 mg /100 g total Vitamin E, respectively, whereas the vitamin E content in millet flours is considerably higher than rice and wheat. Vitamin E has a number of health beneficial effects and the small millets could serve as a good source of these to the consumers.
CONCLUSION
Edible flours of small millet varieties contain 7–366 μg/100 g carotene with highest content in proso and lowest in little millet. Beta carotene was not detected in the millet flours. γ and α tocopherols were the major vitamin E components and tocotrienol was low in quantity. Total tocopherol content was in the range 1.3–4.0 mg/100g with higher content in finger and proso millet varieties and lower in foxtail and little millet varieties. Wide variations were observed in total antioxidant capacity in finger millet varieties, which ranged from 7–27 mM TE/g, and in other millet varieties it was only ∼5 mM TE/g. From these studies, it could be concluded that edible forms of small millet contain a number of phytochemicals like tocopherols, tocotrienols, and carotenoids which are antioxidants, and have fairly high total antioxidant capacity. Edible flours of small millets are also relatively superior to other cereals in the content of natural antioxidants.
ACKNOWLEDGMENTS
The authors thank Mr. S.N. Ram Rao, Scientist (rtd), for the expert advice during standardization for the carotenoid analysis and ICAR New Delhi, for financial support to under take this work under AICRP on Small Millets at CFTRI Mysore.
REFERENCES
- Hadimani , N.A. and Malleshi , N.G . 1993 . Studies on milling, physico- chemical properties, nutrient composition and dietary fibre content of millets . Journal of Food Science Technology , 30 : 17 – 20 .
- Jayaraj , A.P. , Tovey , F.I. and Clark , C.G. 1980 . Possible dietary protective factors in relation to the distribution of duodenal ulcer in India and Bangladesh . Journal of the British Society of Gastroenterology-GUT , 21 : 1068 – 1076 .
- Namiki , M. 1990 . Antioxidant/ antimutagens in food . Critical Reviews in Food Science and Nutrition , 29 : 273 – 300 .
- Gunger , N and Sengul , M. 2008 . Antioxidant activity, total phenolics content and selected physicochemical properties of white mulberry(Morus Alba L.) fruits . International Journal of Food Properties , 11 : 44 – 52 .
- Stanner , S.A. , Hughes , J. , Kelly , C.N. and Buttriss , J. 2004 . A review of epidemiological evidence for the antioxidant hypothesis . Public Health Nutrition , 7 : 407 – 422 .
- Ingold , K.U . , Burton , G.W. , Foster , D.O. , Hughes , L. , Lindsay , D.A. and Webba , A. 1987 . Biokinetics of and discrimination between dietary RRR- and SRR-α-Tocopherols in the male rat . Lipids , 22 : 163 – 172 .
- Theriault , A. , Chao , J. T. , Wang , Q. , Gapor , A. and Adeli , K. 1999 . Tocotrienol: - An overview of its therapeutic potential . Clinical Biochemistry , 32 : 309 – 391 .
- Traber , M.G. and Packer , L. 1995 . Vitamin E: beyond antioxidant function . American Journal of Clinical Nutrition , 62 : 1501S – 1509S .
- Palozza , P. and Krinsky , N.I. 1992 . Antioxidant effects of carotenoids in vivo and in vitro: an overview . Methods in Enzymology , 213 : 403 – 452 .
- Dwyer , J.H. , Navab , M. , Dwyer , K.M. , Hassan , K. , Shircore , A. , Hamalevy , H.G. , Wang , X. , Drake , T. , Merz , N.B. and Fogelman , A.M. 2001 . Oxygenated carotenoid lutein and progression of early atherosclerosis . The Los Angeles Atherosclerosis Study. Circulation , 103 : 2922 – 2927 .
- Hinds , T.S. , West , W.L. and Knight , E.M. 1997 . Carotenoids and retinoids - a review of research, clinical and public health applications . Journal of Clinical Pharmacology , 37 : 551 – 558 .
- Beatty , S. , Boulton , M. , Koh , H.H. and Murray , I.J. 1999 . Macular pigment and age related macular degradation . British Journal of Ophthalmology , 83 : 867 – 877 .
- Christopher , B.J. 1981 . “ Carotenoids as colorants and Vitamin A precursors ” . In In Carotenoids as food colors , 150 – 152 . New York : Academic Press INC .
- Julia , M.H. , Robin , D.G. and Daryl , J.M. 2004 . Application of reflectance colour measurement to the estimation of carotene and lutein content in wheat and triticale . Journal of Cereal Science , 40 : 151 – 159 .
- Krings , V. , El-saharty , Y. , El-zeany , B.A. , Pabel , B. and Berger , R.G. 2000 . Antioxidant activity of extracts from roasted wheat germ . Food Chemistry , 71 : 91 – 95 .
- Mitsuru , W. 1999 . Antioxidative phenolic compounds from Japanese barnyard millet grains . Journal of Agricultural Food Chemistry , 47 : 4500 – 4505 .
- Chethan , S. and Malleshi , N.G. 2007 . Finger millet polyphenols: Characterization and their nutraceutical potential . American Journal of Food Technology , 2 : 582 – 592 .
- Guohua , C. , Eimin , S. and Ronald , L.P. 1997 . Antioxidant and pro-oxidant behaviour of flavanoids. Structure activity relationships . Free Radical Biology and Medicine , 22 : 749 – 760 .
- Ranganna , S. 1986 . “ Plant pigment analysis and quality control for fruit and vegetable products ” . In In Analysis of fruit and vegetable products , 2nd , 84 – 87 . New Delhi : Tata Mc graw Hill Publishing Company limited .
- Kaplan , L.A. , Miller , J.A. , Stein , E.A. and Stampfer , M.J. 1990 . Simultaneous HPLC analysis of retinol, tocopherols, lycopene and α-β-carotene in serum and plasma . Methods in Enzymology , 189 : 155 – 167 .
- Chen , M.H. and Bergman , C.J. 2005 . A rapid procedure for analyzing rice bran tocopherol, tocotrienols and gama oryzanol contents . Journal of Food Composition and Analysis , 18 : 312 – 331 .
- Pilar , P. , Manvel , P. and Mignel , A.I. 1999 . Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex . Analytical Biochemistry , 269 : 337 – 341 .
- Snedecor , G.W. and Cochran , W.G. Statistical Methods , 8th , Iowa : Iowa State Univ. Press .
- Scott , C.E. and Eldrige , A.L. 2005 . Comparison of carotenoid content in fresh, frozen and canned corn . Journal of Food Compositional Analysis , 18 : 551 – 559 .
- Mangels , A.R. , Holden , J.M. , Beecher , G.R. , Formam , M.R. and Lanza , E. 1993 . Carotenoid content of fruits and vegetables, an evaluation of analytical data . Journal of American Dietetic Association , 93 : 284 – 296 .
- Mares , J.A. , Millen , A.E. , Ficek , T.L. and Hankinson , S.E. 2002 . The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease: Overview . Journal of Nutrition , 132 : 518 – 524 .
- Chethan , S. and Malleshi , N.G. 2007 . Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability . Food Chemistry , 105 : 862 – 870 .
- Sripriya , G. , Chandrasekharan , K. , Murthy , V.S. and Chandra , T.S. 1996 . ESR Spectroscopic studies on free radical quenching action of finger millet . Food Chemistry , 57 : 537 – 540 .
- Vasudeva , G.K. , Chandrashekar , A. and Rajani , P.S. 2004 . Antiradical properties of sorghum flour extracts . Journal of Cereal Science , 40 : 283 – 288 .
- Ting , S. and Ho , C. 2005 . Antioxidant activities of buk wheat extracts . Food Chemistry , 90 : 743 – 749 .
- Iqbal , S. , Bhanger , M.I. and Farroq , A. 2005 . Antioxidant potential and components of some commercially available varieties of rice bran in Pakistan . Food Chemistry , 93 : 265 – 272 .
- Xu , Z. , Hua , N. and Godber , J.S. 2001 . Antioxidant activity of tocopherols, tocotrienols and γ-Oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2′-Azobis (2‐methlypropionamidine) Dihydrochloride . Journal of Agricultral Food Chemistry , 49 : 2077 – 2081 .
- Sakina , K. 2004 . Gopalakrishna, A.G. Fat soluble nutraceuticals and fatty acid composition of selected Indian rice varieties . Journal of American Oil Chemical Society , 81 : 939 – 943 .