Abstract
Thermophysical properties of pulp and rind of papaya (Carica papaya L., cv. Maradol) were measured at 20, 30, 40, 50, and 60°C and modeled as a function of temperature. Thermal conductivity, specific heat capacity, and apparent density were measured using the line heat-source probe, differential scanning calorimetry, and the liquid displacement method. For pulp, the values of thermal conductivity, specific heat and apparent density ranged from 0.668 to 0.755 W/m K, from 3019 to 3732 J/kg K, and from 991 to 1036 kg/m3, respectively. The corresponding values for rind of papaya ranged from 0.651 to 0.714 W/m K from 2756 to 3282 J/kg K and from 1019 to 1043 kg/m3. Although thermal conductivity and apparent density of pulp and rind of papaya were significantly (p < 0.05) dependent on temperature, specific heat capacity and thermal diffusivity of pulp and rind of papaya were not affected by temperature in the measurement range.
Keywords:
INTRODUCTION
Knowledge of the thermophysical properties of foodstuffs has fundamental relevance for storage and refrigeration equipment design as well as for the estimation of processing time for freezing, heating, blanching, and drying. In addition, it is required for calculations of quality preservation in hot water immersion treatments and for the control of decay. Thermal conductivity, specific heat capacity, and density allow the optimization and simulation of food processing operations involving heat and mass transfer.Citation[1]
The thermal properties of foodstuff can be determined by direct measurement or can be estimated using predictive equations, based on the chemical composition of the foodstuff. One of the most commonly used procedures to measure thermal conductivity in many food applications is the line heat-source method due to its experimental short time, simplicity, low cost, adequacy for small sample sizes and suitability for the low values of food thermal conductivity.Citation[2] Analytical techniques such as the finite element method can account for non-uniform thermal properties, which change with time, temperature, and location as a food product is heated or cooled.Citation[3] This greatly increases the demand for more accurate thermal properties data and more sophistication in the sense that now it is necessary to know how thermal properties change during a process.Citation[3]
Among the existing methodologies to determine specific heat capacity for foods, differential scanning calorimetry is usually applied to evaluate the effect of temperature since it allows the handling of a wide range of temperatures in a single determination. Apparent density is based on the apparent volume of the material, which includes the pore space within each unit of the material and can be measured by either air or liquid displacement methods.Citation[4] This method can be done either by immersing the sample in water and then weighting the water displaced, or by precisely measuring the volume displaced by the sample.Citation[1]
Thermophysical properties of biological materials have been reported in the literature. Thermal properties depend strongly on temperature and composition of the product. Thoméo et al.Citation[5] found that the effective thermal conductivity of beans increased linearly with increasing moisture content, using a steady-state concentric cylinder equipment. FasinaCitation[6] measured the thermophysical properties of sweet potato at freezing and refrigeration temperatures and reported that after freezing, the specific heats of restructured and non-restructured sweet potato puree were 3.70 and 3.40 kJ/kg K, respectively. Gabas et al.Citation[7] studied the changes of thermal conductivity, thermal diffusivity, and specific heat of plums during drying, and reported that these properties increased with the moisture content from 14.2 to 80.4% (w.b.). Telis et al.Citation[8] found that thermal conductivity of mango and papaya pulps above the freezing point (−1.69 and −1.24°C, respectively) was almost independent of temperature, and that in the frozen state, it increased with decreasing temperature. Tavman et al.Citation[9] reported specific heat capacity of different meat products at temperatures ranging from −60°C to +40°C and found that specific heat capacity of all samples increased with increasing temperature. Farinu et al.Citation[10] measured thermal properties of sweet potato as a function of temperature and moisture content, and reported thermal conductivity and specific heat values of 0.49 W/m K and 3600 J/kg K, respectively.
The proximate composition of papaya cv. Maradol is 88.5% water; 0.90% protein; 0.04% fat; 0.73% ash; 0.95% crude fiber; and 8.88% carbohydrates.Citation[11] However, little information is available on experimentally measured thermophysical properties of papaya fruit, especially for its rind. The objective of this work was to measure the thermophysical properties of pulp and rind of papaya (Carica papaya L., cv. Maradol) at the following selected temperatures: 20, 30, 40, 50, and 60°C. Knowledge of these properties can then enable heat transfer to be modeled in hot water immersion treatments for control of decay.
MATERIALS AND METHODS
Fresh ripe papayas (Carica papaya L. cv. Maradol) were manually harvested at a mature-green stage. The fruits were immediately brought to the laboratory, selected for uniformity of size, color and absence of defects. Fruits presented the following physicochemical characteristics: pH = 5.97, 9.5° Brix; and color parameters L* = 43.3, a* = − 11.1, b* = 27.3.
Thermal Conductivity
The line heat source method was used to measure papaya thermal conductivity, k. Temperature T(r, t) of the heat source can be calculated from the Fourier Field equation assuming a constant thermal diffusivity, α. The governing partial differential equation that describes the one-dimensional unsteady state conduction in cylindrical coordinates is given asCitation[12]:
A uniform temperature distribution is used as the initial condition:
The heat source provides a constant heat transfer, QL per unit of length:
Therefore, the first boundary condition is:
As the solid is considered infinite, the temperature gradient in the outer side of the solid is zero. Therefore the second boundary condition is:
The analytical solution is given as:
Over the linear part of the temperature versus natural log of time curve a regression analysis can be performed to obtain the most suitable value of the slope, m (K), which is used to calculate the conductivity.
The constant heat transfer per unit of length QL (W/m) can be calculated from the supplied current intensity I (A), resistance of the probe R (Ω), and length of the probe L (m):
Pieces of pulp and rind of papaya (about 2 cm in length) were skewered side by side on a thermal conductivity probe, taking care to eliminate spaces between the pieces (). Probe and samples were immersed in water having been coated with plastic films to avoid moisture transfer. Food samples and probe were initially equilibrated at 20, 30, 40, 50, and 60°C before a step input of thermal energy generation within the probe. Current intensity, resistance, and length of the probe used were 140 mA, 69.62 Ω, and 14.7 cm, respectively. Temperature of the heat source against time was recorded using a scanning thermometer (Digi-Sense Thermocouple Scanning Thermometer, EW-92000-00, Cole-Parmer, USA). Temperature was registered every 5 s for 2 min. The probe was calibrated with a 0.4% agar gel at 20°C. The calibration factor was obtained relating the published thermal conductivity of 0.4% agar gel and the experimental one. To validate the second boundary condition given by EquationEq. 5, a T type thermocouple was inserted in the outer side of the sample and temperature was recorded during the entire test. This temperature did not change throughout the 2 min of evaluation. The quoted accuracy of the meter was 3% over a range of measurement from 0.1 to 2 W/m K. Five replicates were taken at each working temperature.
Specific Heat Capacity
A Differential Scanning Calorimeter (TA Instruments Thermal Analysis and Rheology, DSC 2010, USA) was used to measure specific heat capacity, Cp . The enthalpy change, ΔH is given on the DSC curve of heat flow against temperature by the difference in heat flow between the baseline and test material curves. The amount of heat flow, dQ/dt absorbed to increase the temperature is proportional to the enthalpy change:
The calibration constant, E can be obtained using a calibrating reference at the temperature of interest. At a constant pressure, the amount of heat is:
Combining Equationequations 9 and Equation10, the specific heat capacity can be obtained:
For the latter measurements, sapphire was taken as the calibrating reference, the sample size was about 15 mg and the rate of temperature scanning was 20°C/min. Specific heat capacity was measured over a range from 20 to 60°C. Four replicates were done for each treatment.
Apparent Density
The apparent density, ρ was determined by the liquid displacement method; distilled water being the liquid medium. For each determination, mass of about 5 g was obtained using a compact balance (AND EW-3000 A, A&D Company Limited, Japan). For the determination of volume, several pieces in the form of strips of pulp and rind of papaya were coated with a thin plastic film and equilibrated at 20, 30, 40, 50, and 60°C. The coated samples were immersed in water and placed into a 50 mL graduated cylinder. The volume displaced by the product was measured by reading the meniscus and the density calculated as the ratio of mass to volume. Ten replicates were taken for each temperature.
Thermal Diffusivity
Thermal diffusivity, α was calculated from experimentally measured values of thermal conductivity, k specific heat capacity, C p and density, ρ:
Experimental Design
A completely random design was used and an analysis of variance was performed for every response variable using temperature as the factor. Levels for temperature were 20, 30, 40, 50, and 60°C. The least significant difference, LSD was used as the multiple range tests to obtain differences between means.
RESULTS AND DISCUSSION
Thermal Conductivity
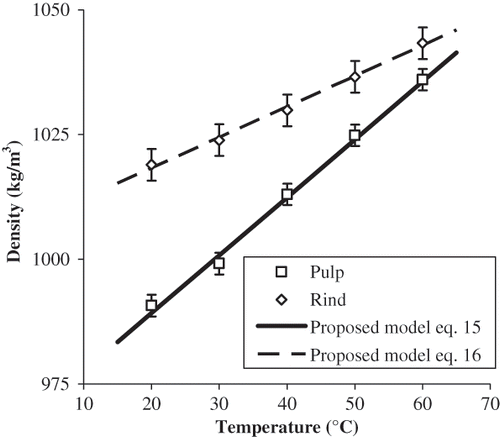
Temperature of the heat source increased as a function of Ln time almost linearly and the recorded data was fitted to a straight line (R 2 adj > 0.998). Thermal conductivity of pulp and rind of papaya as a function of temperature is shown in . Thermal conductivity values of pulp of papaya were 0.668, 0.691, 0.710, 0.726, and 0.755 W/m K at 20, 30, 40, 50, and 60°C, respectively. It can be observed that at the studied temperatures thermal conductivity of both materials increased almost linearly with temperature. Kurozawa et al.Citation[13] found that thermal conductivity of papaya ranged from 0.58 to 0.62 W/m K in the interval from 20 to 40°C. Gratzek and ToledoCitation[14] reported that thermal conductivity of potato and carrot increased significantly with temperature in the interval from 26 to 130°C.
Figure 2 Thermal conductivity of papaya cv. Maradol (LSD pulp = 0.028 W/m K; LSD rind = 0.027 W/m K, α = 0.05).
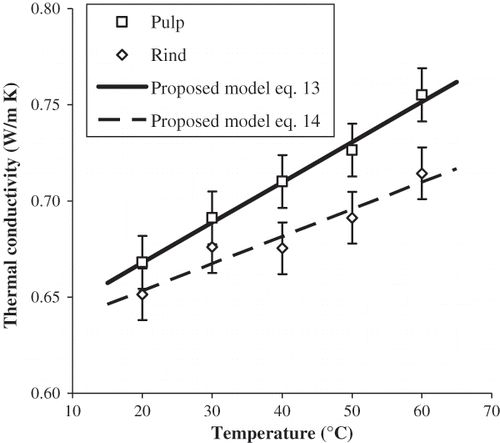
Thermal conductivity of rind of papaya was 0.651, 0.676, 0.675, 0.691, and 0.714 W/m K at 20, 30, 40, 50, and 60°C, respectively. No reports were found on thermal conductivity of rind of papaya. Thermal conductivity of pulp was higher than that of rind in the range from 40 to 60°C and the difference was higher as temperature increased. The average value of thermal conductivity for pulp of papaya (0.710 W/m K) was significantly higher (p < 0.05) than that for rind (0.682 W/m K) with a LSD = 0.012 W/m K. This could be due to the fact that moisture content of pulp (89%) is higher than that of rind (82%), and this is the compositional parameter contributed more to the value of thermal conductivity.
The magnitudes of the thermal conductivity were estimated using the equation proposed by Sweat.Citation[15] Experimental and estimated values of thermal conductivity of pulp and rind of papaya are shown in . These estimations were obtained with the corresponding moisture content of both materials, which were determined experimentally. It can be observed that the differences between experimental and estimated values were lower for pulp than for rind. It could be due to the fact that the equation was developed for pulp of fruits. Linear equations are proposed to predict thermal conductivity of pulp and rind of papaya cv. Maradol in the range from 20 to 60°C:
Table 1 Experimental and estimated thermal conductivity and specific heat capacity of papaya cv. Maradol
Specific Heat Capacity
Specific heat capacity of pulp and rind of papaya as a function of temperature is shown in . Specific heat capacity of both materials was not significantly different (α = 0.05) in the range from 20 to 60°C. Ali et al.Citation[16] have shown that specific heat capacity of green pepper, white radish, ginger and cassava was not affected by temperature from 20 to 80°C. Specific heat capacity values of pulp of papaya were 3019, 3051, 3298, 3493, and 3732 J/kg K at 20, 30, 40, 50, and 60°C, respectively. The corresponding value at 20°C reported by Hayes and YoungCitation[17] for papaya cv. Solo was 3433 J/kg K. Differences can be attributed to difference in cultivar and therefore in composition of the materials.
Figure 3 Specific heat capacity of papaya cv. Maradol (LSD pulp = 730 J/kg K; LSD rind = 411 J/kg K, α = 0.05).
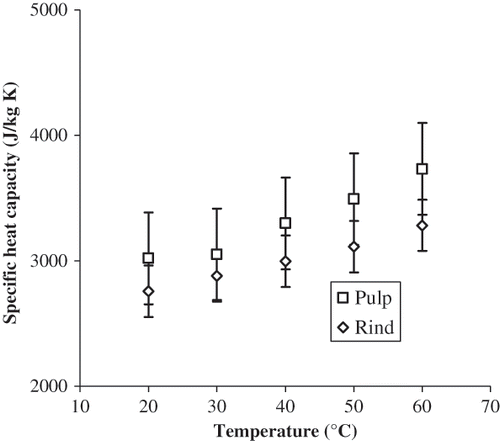
Specific heat capacity values of rind of papaya were 2756, 2879, 2996, 3112, and 3282 J/kg K at 20, 30, 40, 50, and 60°C, respectively. No experimental data concerning the dependence of the specific heat capacity of rind of papaya cv. Maradol on temperature was found from a literature search. The magnitudes of the specific heat capacity were estimated using the equation proposed by Alvarado.Citation[18] Experimental and estimated values of specific heat capacity of pulp and rind of papaya are shown in . Again, the differences between experimental and estimated values were lower for pulp than for rind of papaya.
Apparent Density
The apparent density of pulp and rind of papaya increased significantly (p < 0.05) with temperature in the range from 20 to 60°C (). For pulp, density values were 991, 999, 1013, 1025, and 1036 kg/m3 at 20, 30, 40, 50, and 60°C, respectively. There were no significant variations at consecutive temperatures for rind; however, apparent density from 40 to 60°C was significantly different (p < 0.05) to the corresponding values at 20°C. Apparent density values for rind of papaya were 1019, 1024, 1030, 1037, and 1043 kg/m3at 20, 30, 40, 50, and 60°C, respectively. Linear equations are proposed to predict apparent density of pulp and rind of papaya cv. Maradol in the range from 20 to 60°C:
Thermal Diffusivity
Thermal diffusivity exhibited a behavior similar to the specific heat capacity. In the range of temperatures of this study the variation of thermal diffusivity was not significant (α = 0.05) for both materials (). This behavior could be due to a self canceling effect between thermal conductivity and density, and to the great variability observed during the determination of the specific heat capacity, especially for pulp. This is in agreement with the results shown by Wang et al.Citation[19] which concluded that although large variations may exists in density, specific heat capacity and thermal conductivity of fruits, the effect on thermal diffusivity was minimum. In addition, Ali et al.Citation[16] found that from 5 to 20°C, the thermal diffusivity of cassava, ginger, green pepper, white radish, zucchini and eggplant increased steadily with increasing temperature, however beyond 20°C thermal conductivity appeared to remain constant.
Figure 5 Thermal Diffusivity of papaya cv. Maradol (LSD pulp = 3.2 × 10–8 m2/s; LSD rind = 4.8 × 10–8 m2/s, α = 0.05).
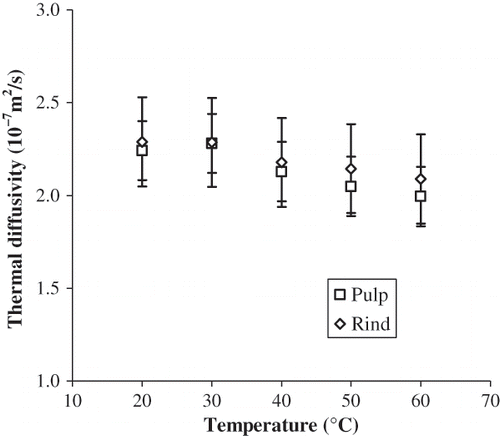
Thermal diffusivity from 20 to 60°C ranged from 1.99 × 10−7 m/s2 to 2.29 × 10−7 m/s2. Hayes and YoungCitation[17] reported the value of 1.52 × 10−7 m/s2 for thermal diffusivity of papaya cv. Solo. The variation could be attributed to the difference in cultivar and methodology used to determine thermal diffusivity. Rahman,Citation[20] Mohsenin,Citation[4] and Hayes and YoungCitation[17] reported values of thermal diffusivity in the range from 1.4 × 10−7 m/s2 to 1.7 × 10−7 m/s2 for apple, cherry, orange, papaya, pear and potato. The higher values obtained in this work could be ascribed to the higher thermal conductivity and lower specific heat capacity found in this study.
CONCLUSIONS
Thermophysical properties of papaya cv. Maradol were evaluated at 20, 30, 40, 50, and 60°C. Thermal conductivity and apparent density of pulp and rind of papaya increased (p < 0.05) with increasing temperature in the range from 20 to 60°C. However, specific heat capacity and thermal diffusivity of both materials were not affected by temperature in the studied temperature range. Differences between experimental and estimated values for thermal conductivity and specific heat capacity using the equations proposed by Sweat and Alvarado were 12 and 14% for pulp, and 15 and 18% for rind of papaya, respectively. Predictive models were developed for estimating thermal conductivity and apparent density of papaya cv. Maradol in the range from 20 to 60°C, with R 2 ≥ 0.927.
REFERENCES
- Rapusas , R.S. and Driscoll , R.H. 1995 . Thermophysical properties of fresh and dried white onions slices . Journal of Food Engineering , 24 ( 2 ) : 149 – 164 .
- Voudouris , N. and Hayakawa , K. 1994 . Simultaneous determination of thermal conductivity and diffusivity of foods using a point heat source probe: a theoretical analysis . Lebensmittel-Wissenschaft und-Technologie , 27 ( 6 ) : 522 – 532 .
- Sweat , V.E. 1994 . “ Thermal Properties of Foods ” . In Engineering Properties of Foods , 2nd Edited by: Rao , M.A. and Rizvi , S.S.H. 93 – 138 . Marcel Dekker , New York
- Mohsenin , N.N. 1980 . Thermal Properties of Foods and Agricultural Materials , New York : Gordon and Breach, Science Publishers, Inc .
- Thoméo , J.C. , Costa , M.V.A. and Lopes Filho , J.F. 2004 . Effective Thermal Conductivity of Beans via a Steady-State Method . International Journal of Food Properties , 7 ( 1 ) : 129 – 138 .
- Fasina , O.O. 2005 . Thermophysical Properties of Sweet Potato Puree at Freezing and Refrigeration Temperatures . International Journal of Food Properties , 8 ( 1 ) : 151 – 160 .
- Gabas , A.L. , Marra-Júnior , W.D. , Telis-Romero , J. and Telis , V.R.N. 2005 . Changes of Density, Thermal Conductivity, Thermal Diffusivity, and Specific Heat of Plums during Drying . International Journal of Food Properties , 8 ( 2 ) : 233 – 242 .
- Telis , V.R.N. , Telis-Romero , J. , Sobral , P.J.A. and Gabas , A.L. 2007 . Freezing Point and Thermal Conductivity of Tropical Fruit Pulps: Mango and Papaya . International Journal of Food Properties. , 10 ( 1 ) : 73 – 84 .
- Tavman , S. , Kumcuoglu , S. and Gaukel , V. 2007 . Apparent Specific Heat Capacity of Chilled and Frozen Meat Products . International Journal of Food Properties , 10 ( 1 ) : 103 – 112 .
- Farinu , A. and Baik , O. 2007 . Thermal Properties of Sweet Potato with its Moisture Content and Temperature . International Journal of Food Properties , 10 ( 4 ) : 703 – 719 .
- Pérez-León , O.L. 2003 . Calidad Nutrimental de Papaya “Maradol” tratada con 1-metilciclopropeno . MS. Thesis. Centro de Investigación en Alimentación y Desarrollo, A.C. Culiacán, Sinaloa, Mexico. ,
- Incropera , F.P. and DeWitt , D.P. 1990 . Introduction to Heat Transfer , New York : John Wiley & Sons .
- Kurozawa , L.E. , El-Aouar , A.A. , Simoes , M.R. , Azoubel , P.M. and Murr , F.E.X. 2005 . Determination of thermal conductivity and thermal diffusivity of papaya (Carica papaya L) as a function of temperature , 1 – 9 . Rio de Janeiro : ENPROMER-2nd Mercosur Congress on Chemical Engineering .
- Gratzek , J.P. and Toledo , R.T. 1993 . Solid food thermal conductivity determination at high temperatures . Journal of Food Science , 58 ( 4 ) : 908 – 913 .
- Sweat , V.E. 1974 . Experimental values of thermal conductivity of selected fruits and vegetables . Journal of Food Science , 39 ( 6 ) : 1080 – 1083 .
- Ali , S.D. , Ramaswamy , H.S. and Awuah , G.B. 2002 . Thermo-physical properties of selected vegetables as influenced by temperature and moisture content . Journal of Food Process Engineering , 25 ( 5 ) : 417 – 433 .
- Hayes , C.F. and Young , H. 1989 . Extension of model to predict survival from heat transfer of papaya infested with oriental fruit flies (Diptera: Tephritidae) . Journal of Economic Entomology , 82 ( 4 ) : 1157 – 1160 .
- Alvarado , J.D. 1991 . Specific heat of dehydrated pulps of fruits . Journal of Food Process Engineering , 14 ( 3 ) : 189 – 196 .
- Wang , S. , Tang , J. and Cavalieri , R.P. 2001 . Modeling fruit internal heating rates for hot air and hot water treatments . Postharvest Biology and Technology , 22 ( 3 ) : 257 – 270 .
- Rahman , S. 1995 . Food Properties Handbook , Boca Raton , FL : CRC Press .