Abstract
In this study, acidic whey (AW), a natural tofu-coagulant in China, was investigated for the micro flora and chemical changes of AW during tofu whey natural fermentation. The gel properties of AW tofu coagulated with AW were also studied. Throughout the fermentation stages, the change of lactic acid bacteria (LAB) was found to be similar to that of total mesophilic aerobic bacteria (TMAB), implying that LAB is the most important role that works on AW fermentation. The numbers of enterobacteriaceae, yeast and mould (fungi) were too low to detect. Bacterial spores (spores) and acetibacteria was found to increase slowly with fermentation time. The pH value, protein, carbohydrate, organic acid changes during the production of acidic whey were studied. It was found that the pH value of acidic whey had a significant effect on the gel properties of AW tofu. The microbiological findings in this study have clearly demonstrated the presence of the high counts of LAB investigated and a large amount L-lactic acid produced by LAB must have act as the main tofu coagulant.
INTRODUCTION
Tofu (soybean curd), the most widely accepted soy food, is a versatile protein-rich food and has traditionally been used as a source of protein or meat substitute in Eastern diets.[Citation1 Citation2] For the basic tofu production, soymilk is coagulated with salts or acids (coagulants) either alone or in combination to produce a protein gel which traps water, soy lipids, and other constituents in the matrix.[Citation3] Coagulation of soymilk is the most important step in the tofu-making process. Calcium sulphate, calcium chloride, glucono-δ-lactone (GDL), magnesium sulphate and magnesium chloride are many of the different tofu coagulants used on an industrial scale for the preparation of tofu.[Citation4] The effects of types of coagulants on viscoelastic properties of tofu were investigated.[Citation5,Citation6] The effect of different coagulants on the function and properties of tofu was also investigated.[Citation4] Besides of some tofu coagulants generally used in tofu process, the potential of chitosan as a coagulant in commercial tofu preparation was investigated.[Citation7]
Acidic whey (AW) produced by tofu whey naturally fermented, has been used as a traditional tofu coagulant for 400 years in the history in China, especially in the rural area of China. For centuries, acidic whey (AW) tofu with its characteristic flavour and taste has been widely accepted and enjoyed by Chinese people as a staple food. The Chinese have a long history of using micro organisms to convert agricultural commodities into fermented foods.[Citation8] Acidic whey is usually made from tofu whey, a liquid by-product of the soybean product tofu, naturally fermented by its normal micro biota. However the production process of the AW is not completely understood. Traditionally, acidic whey was maintained by inoculating fresh tofu whey with a small volume of the already fermented acidic whey. The processing method for producing the acidic whey tofu (.) is similar with the tofu made using magnesium chloride as the coagulant. The gelling mechanism of acidic whey tofu similar with the soft-tofu made using glucono-δ-lactone (GDL) in Japan and acetic acid tofu made using acetic acid in Indonesia is mainly that the pH induced precipitation of soy protein.[Citation9] Acidic whey tofu is apparently considered to be more organoleptically superior to similar commercially products in China because of its special flavour, light sweet taste and good texture properties.[Citation10] It is also perceived to be “naturally made.”
In the manufacture of tofu, soymilk is coagulated by heating, in combination with salts, or acid, and enzymes.[Citation11] This generates a liquid by-product called “tofu whey (TW)”, which is usually used as a nutritious drink for animals such as cows and pigs.[Citation12] It contains nutrients such as soluble proteins, lipids, soluble sugar, vitamins and minerals. It also contains some functional factors such as soybean oligosaccharides, isoflavone and saponin.[Citation13] This suggests that tofu whey has so many nutrients that it can be used as a natural medium for the micro organism growth, especially lactic acid bacteria. Tofu whey was reported that it was used as a growth medium for the production of lactic starters and substituting for expensive basal medium for the production of L- Lactic acid by lactic acid bacteria.[Citation14,Citation15]
Tofu whey naturally fermented is a tofu coagulant which has been the subject of a limited number of studies. After fermentation, acidic whey contains large amounts of various micro organisms. Five strains of lactic acid bacteria were isolated from two sorts of acidic whey.[Citation10] A strain of acid-producing bacteria isolated from acidic whey was identified as Lactobacillus acidophilus.[Citation16] A strain of flavour-producing micro organism isolated from acidic whey was identified as yeast.[Citation17] These results suggest that it is possible that lactic acid bacteria is the dominating microbial community in an acidic whey and play a major fermentative role affecting the aroma and acidity of the product. There maybe exist a strong relation between the quality of the acidic whey tofu and the quality of the acidic whey used to make the AW tofu.
Most traditional acidic whey is produced with different manufacturing technologies that are dependent on the geographical location. Thus there must be a significant variety exist. The quality of the acidic whey tofu varies depending on the predominant micro organisms in the acidic whey. It has to be noted that there is no standardized processing method for an acidic whey production to date. The overviews studies of acidic whey have been focused on the isolation and identification of the micro organisms in acidic whey samples investigated. There has been no study carried out on the microbiological aspects of acidic whey during natural fermentation and the effect of pH value and the organic acid changes of acidic whey fermented for different periods on the gelling properties of the acidic whey tofu. The aim of this study was to characterize these aspects to acidic whey and investigate the relationship between the pH value of the acidic whey used and the gelling properties of the resulted acidic whey tofu. This study could provide a better fundamental basis for a possible future standardized processing method of acidic whey and for developing starter cultures that can be used to produce safe products with reproducible quality and to capture the desirable attributes of the naturally fermented acidic whey in order to make high quality tofu products.
MATERIAL AND METHODS
Materials
Soybean (ZhongHuang 13) was purchased from the Institute of Crop Germplasm Resources, Chinese Academy of Agricultural Sciences and then stored at ambient temperature (25°C). Magnesium chloride and glucono-δ-lactone (GDL) were purchased from local food ingredient suppliers. Standards of the organic acids acetic, L-lactic, oxalic, citric, tartaric, formic and malic acids were obtained from Sigma Chemical Company (America), whereas ammonium dihydrogen phosphate (NH4H2PO4), phosphoric acids and high performance liquid chromatography (HPLC) grade acetonitrile were obtained from Beijing Chemical Reagents Company (Beijing, China).
Preparation of Soymilk
Soybeans (200 g) were washed, soaked overnight in water at room temperature, then drained, rinsed, and blended with 1600 mL of water (soybean to water ratio = 1:8 w/v) in a soymilk grinder (Model FSM-100, Shenyang Machinery No. 3 Factory, China) with automatic centrifugal 120 mesh filter to separate okara from raw soymilk. The resultant slurry was strained through four layers of gauze to obtain soymilk. 1500 mL soymilk was heated in ohmic-heating equipment.[Citation18] The soymilk was heated from 20°C to 100°C directly and kept for five minutes. The heating rate was set as 40°C/min.
Fermentation of Acidic Whey
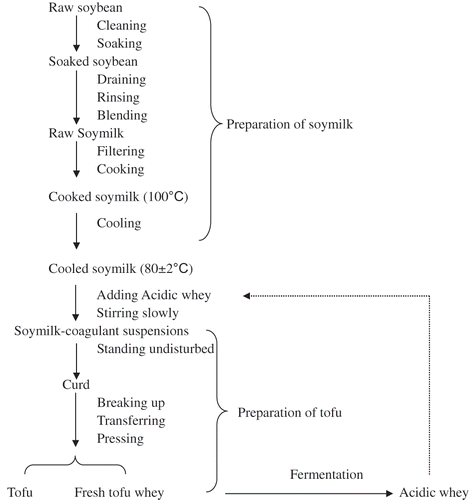
500 mL of fresh tofu whey was put into the steel pan cleaned with boiled water, cooled at ambient temperature. Then a small amount of already fermented acidic whey was added and stirred. In general, the already fermented acidic whey was stored at 4°C for one week if it was not used immediately. After mixing, the pan containing both fresh tofu whey and fermented acidic whey was immediately covered with lid, naturally fermented at ambient temperature for 72 h. Aliquots of 50 mL were withdrawn every 4 h of fermentation before 24 h fermentation and every 12 h of fermentation after 24 h fermentation for pH measurement, and subsequently stored at −20°C for organic acid, protein and carbohydrate analysis. Other aliquots of 50 mL were also withdrawn and put into a sterile bottle for microbiological analysis of acidic whey. The sample of 18 h fermentation was also withdrawn because the acidic whey of 18 h fermentation was generally used as coagulant in the manufacture.
Preparation of Acidic Whey Tofu
The preparation of the acidic whey tofu is illustrated in . 700 mL hot soymilk (100°C) was put into a stainless steel container, and then cooled to 80 ± 2°C. The acidic whey (fermented) solution was slowly poured into the steel container, while stirring slowly and stop adding acidic whey until the soymilk-coagulant occurred. The volume of the acidic whey used is about 185 mL. The soymilk-coagulant suspensions were allowed to stand undisturbed for a period of 15 min to ensure that coagulation has occurred. The curds thus formed were broken and gently transferred to a perforated stainless steel container (11.1 × 7.1 × 5.5 cm depth) lined with a single layer of cheese-cloth. The tofu whey was drained off naturally for 10 min and the curd was pressed for 20 min using special bricks weighing 2.6 kg. After pressing, tofu and tofu whey were weighed separately. The tofu was transferred into a plastic bag and stored in a refrigerator for further analysis.
Microbiological Analysis of Acidic Whey
Total Mesophilic Aerobic bacteria (TMAB)
Sequential decimal dilutions of the acidic whey in sterile peptone water were plated in duplicates were spread on Plate Count Agar (PCA, Oxoid, England), the total mesophilic aerobic bacteria was enumerated after incubation at 30°C for 48 h. Results were expressed as log cfu/mL of acidic whey.
Lactic Acid Bacteria (LAB)
Lactic acid bacteria (LAB) were determined in pour-plates of de Man Rogosa Sharpe agar pH 5.5 (MRS, Merck, Germany) supplemented with 40 μg/mL cycloheximide (sigma).[Citation19] MRS plates were incubated under anaerobic conditions at 30°C for 48 h. Initial confirmation was based on positive Gram stain and negative catalase and oxidase tests. Carbohydrate metabolism of isolates of LAB was determined use API 50 CH strips and API 50 CHL medium (API system, Bio-Merieux, France) according to the manufacturer's instructions. Tentative identification based on phonotypical properties was done with APILABPLUS software (version 3.3.3, Bio-Merieux, France). Results were expressed as log cfu/mL of acidic whey.
Coli Forms
Coli forms enumeration was carried out in pour-plates of Violet Red Bile Agar (VRBA, Oxford, England) after incubation at 30°C for 24 h according to the method of Hatzikamari.[Citation20] Results were expressed as log cfu/mL of acidic whey.
Bacterial Spores (Spores)
AW Samples were pasteurized (80°C, 10 min) and spores were enumerated in pour-plates of nutrient Agar, after incubation at 37°C for 48 h. Results were expressed as log cfu/mL of acidic whey.
Yeasts and Moulds (Fungi)
Yeast and moulds were grown on Potato Dextrose agar (Oxford, CM139) acidified with 10 mL/L of 10% lactic acid (sigma) at 28°C for 3–5 days. Results were expressed as log cfu/mL of acidic whey.
Acetibacteria
The acetibacteria medium was prepared by mixing 10 g glucose, 10 g yeast extract, 15 g calcium carbonate, and 20 g agar, per litter. The pH value of solution was adjusted at 6.5 and the solution was sterilized at 121°C for 15 min. The acetibacteria plates were incubated at 30°C for 48 h. Results were expressed as log cfu/mL of acidic whey.
Chemical Analysis of Acidic Whey
Organic Acids of Acidic Whey
5 mL aliquot of fermented tofu whey was centrifuged (14000 × g for 10 min) and then filtered using 0.22 μm pore size membrane into HPLC vial. Chromatographic analyses were conducted using a LC-10ATvp series high performance liquid chromatograph (Shimadzu, Japan) with the auto sampler (model SIL-10ADvp), the solvent delivery system (model LC-10ATvp), the variable wavelength ultraviolet visible (UV-VIS) detector (model SPD-10Avp) set at 214 nm, and thermostatically controlled column compartment set at 30°C. A DOCOSIL-B (4.6 × 150 mm) was used to separate organic acid. The separated conditions is using an isocratic flow rate of 0.4 mL min−1 of 1% acetonitrile-0.02 mol/L NH4H2PO4 (pH 2.7), which has been filtered through a 0.45 μm membrane and degassed using ultrasonic for 20 min. A 10 μL injection volume was used for both samples and standards with the retention time of L (+)-lactic acid and acetic acid at 6.5 and 7.4 min, respectively. A mixed standard stock solution of L (+)-lactic acid (1.0 g per 100 mL) and acetic acid (1.0 g per 100 mL) was prepared and diluted to 0.1, 0.075, 0.05, 0.035, 0.025, and 0.0125% of its concentration for multi-level calibration and organic acid levels were calculated back to milligram per 100 mL acidic whey. Three measurements were made at each sample and averaged.
pH of Acidic Whey
Changes in pH of acidic whey were recorded with a pH meter (F-23, Horiba, Japan). Three measurements were made at each sample and averaged.
Protein Content of Acidic Whey
The crude protein concentration of acidic whey was estimated according to the method of Lowry[Citation21] using bovine serum albumin as a standard. The absorbance employed at 660 nm. Three measurements were made at each sample and averaged.
Total Soluble Sugar Content of Acidic Whey
The total soluble sugar concentration of the acidic whey was determined according to the total soluble sugar assay of food as described by Nielsen.[Citation22] The mixture solution of 0.2 mL diluted samples with 0.8 mL distilled water and 5.0 mL grace alkone reagent was incubated at 100°C for 10 min. The reaction was terminated by cooling rapidly with cooling-water at room temperature for 10 min and the sugar concentration was determined from absorbance at 620 nm using glucose as a standard. Three measurements were made at each sample and averaged.
Reducing Sugar Content of Acidic Whey
The reducing sugar produced was determined by the method of Somogyi[Citation23] and Nelson[Citation24] using glucose as a standard. The absorbance was taken at 620 nm. Three measurements were made at each sample and averaged.
Chemical Analysis of Acidic Whey Tofu
pH of Acidic Whey Tofu
Five grams of tofu and 25 mL of deionised water were homogenized using a mortar and pestle, and then filtered through a single layer of cheesecloth to measure pH (F-23, Horiba, Japan).[Citation7] Three measurements were made at each sample and averaged.
Instrumental Texture Profile Analysis (TPA) of Tofu Samples
The texture analyses were conducted using rheometer (RT-2002-D.D, RHEO TECH Co., and Japan). Prior to testing, the tofu samples were equilibrated to room temperature for 30 min and were cut into 2.0 cm thick section, cored into a cylinder with a 2.5 cm diameter. The samples were compressed twice at a load speed of 60 mm/min to 70% of their original height using the 2.5 cm diameter cylindrical plate. From the resulting force/deformation curves, the textural parameters of hardness, cohesiveness, springiness, gumminess and chewiness were calculated.[Citation25] Four specimens each treatment was measured. Three measurements were made at different locations on each sample and averaged.
Colour of Acidic Whey Tofu
Colour values of tofu were measured with a CR-300 Minolta Chroma Meter (Minolta Camera Co. Ltd., Osaka, Japan). This instrument is a colour analyser with an 8‐mm diameter measuring area and glass light-projection tube for liquid. The L*, a*, b* colour scale was used to measure colour: L* (0, dark black) to L (100, white), +a* (red) to −a* (green), and +b* (yellow) to −b* (blue). The instrument was standardized using a standard white tile with L = 97.30, a* = 0.04 and b* = 1.65.[Citation26] Three measurements were made at different locations on each sample and averaged.
Sensory Evaluation
Recruitment, selection, and training of panelists were performed according to sensory evaluation procedure,[Citation27] ten panellists were screened from 15 potential panellists using basic taste identification test and trained with four commercial tofu products (tofu made using different coagulant) for 3 weeks to familiarize them with the product characteristics planned to be evaluated.The panelists evaluated each characteristic of the sample using a 5-point hedonic scale, where the sour taste was from one (1)(very weak) to five (5)(very strong); the flavour and overall acceptability were from one(1)(not good) to (5)(excellent). Before administration of the taste test, the tofu samples were coded with three randomly selected designs. At the tasting sessions which took place at room temperature (28°C) and under bright light. Each panellist evaluated all tofu samples on one occasion. To avoid prejudice during sensory evaluation, panellists were encouraged to take a few sips of cool water between tasting. To ensure accuracy in data collection panellists were seated in individual cubicles and no communication was permitted between the panellists during the tasting.
Statistical Analysis
For all tests except for sensory evaluation, the experiments were in triplicate and average values or mean ± SD are reported. Data were analysed using ANOVA with the Statistical Analysis System programmer (SPASS 12.0). The means comparisons were made by Tukey test in ANOVA. A significant level was defined as a probability of 0.05 or less.
RESULTS AND DISCUSSION
Microbial Changes
Soybeans are rich in proteins, lipids, carbohydrates, minerals and vitamins.[Citation28] Carbohydrates in soybeans are soluble. In the manufacture of tofu, soymilk is coagulated by heating, in combination with salts, acid or enzymes[Citation11] and then the soymilk-coagulant is pressed to produce a lot of tofu-whey, soluble carbohydrates, a small amount of protein and other nutritional factors could be lost in tofu whey, which is a good culture medium for various bacteria to grow.
presents the micro flora changes of acidic whey, where, as mentioned before, a small amount of already fermented acidic whey was added into fresh tofu whey, which are naturally fermented at room temperature (about 30 ± 2°C) for 72 h. The initial counts of TMAB, spores, acetibacteria and LAB were found to be around 104cfu/mL, but coli forms and fungi were not detectable in fresh tofu whey. Tofu whey's initial temperature around 65°C only some high temperature-tolerant bacteria could have survived. After the fresh tofu whey was cooled to room temperature, a small amount of already fermented acidic whey, which contained a large amount of LAB, was added to the pan with fresh tofu whey. The numbers of TMAB and LAB were measured and the results have shown that the growth rate of LAB was similar with that of TMAB; the viable counts of LAB were significantly higher than that of other bacteria during 24 h fermentation of tofu whey. LAB has shown faster growth in the tofu whey, attaining populations of about 107cfu/mL after 4 h. The strains attained maximum populations of about 109cfu/mL after 18–20 h fermentation similar to the TMAB. This implies that LAB was the dominating strains in the acidic whey. When the tofu whey was fermented for 24–72 h, there was a light reduction in the viable counts of LAB, but a slight increase in TMAB. This may be attributed to the reduction of some not acidic tolerance LAB and the increase of some spoilage organisms.
Figure 2 Changes of micro flora during tofu whey fermentation. TMAB: Total mesophilic aerobic bacteria; LAB: Lactic acid bacteria; spores: Bacterial spores; fungi: Yeasts and moulds. Arrows indicate that coli form and fungi counts fell below the detection limit. Values represent the means + standard deviation (SD) of n = 3 duplicate assays. Error bars were placed on only one curve to illustrate variation.
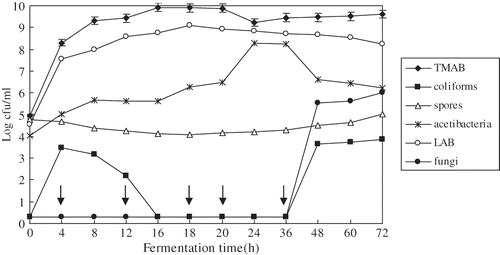
The number of bacterial spores was found to slightly decrease during 20 h fermentation of tofu whey, because spores do not germinate in low pH. But later the number of spores was found to increase slightly during 24–72 h fermentation of tofu whey. This result may be attributed to the increase of acidic whey pH, because the proteinase of some spores can hydrolyze the protein to produce alkaline amino acid.[Citation29] The number of acetibacteria gradually increased during 20 h fermentation, attaining 108cfu/mL after 24–36 h, later rapidly decreased.
Tofu whey was naturally fermented at room temperature, so it was easily contaminated by coli forms. And number of coli forms rapidly increased to 103cfu/mL after 4 h fermentation, but later decreased to being not detectable after 16–36 h. This indicates that the dominating strains of the tofu whey inhibited the growth of coli forms at low pH. Whereas the counts of coli forms rapidly increased 103cfu/mL after 48–72 h because of the increase in pH value of the tofu whey. The number of yeasts and moulds were not detectable during 0–36 h fermentation, but later changed in a similar way with coli forms. This implies that tofu-whey was spoilage as pH increases.
pH of Tofu-Whey
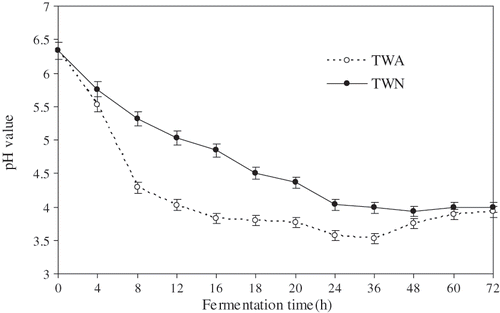
In , the pH changes are shown during the 72 h fermentation of tofu whey. In traditional process of the acidic whey, a small amount of already fermented acidic whey was always added to the fresh tofu whey before natural fermentation. The reason of this processing method could be accounted for by the explanation of monitoring pH value of tofu whey added and not added already fermented acidic whey during fermentation. Initial pH value of fresh tofu whey was 6.3, pH changes of tofu whey added already fermented acidic whey (TWA) rapidly decreased than that of tofu whey not added already fermented acidic whey (TWN) during 16–18 h fermentation. The pH of TWA attained 3.8 as expected, but only pH 4.5 of TWN. This implies that already fermented acidic whey contains a large of LAB, which can be used as starter cultures rapidly propagating in sufficient culture medium, fresh tofu-whey. The pH changes of TWN decreased slightly during 24–72 h fermentation and then remained in the range of 3.9–4.0. Whereas pH changes of TWA decreased slowly to around 3.5 during 20–36 h, and then increased slowly to 3.9 during 48–72 h fermentation. It is presumably due to the number of acetibacteria increased after 24 h () producing acetic acid thus lower pH. The number of fungi increased because of assimilating organic acid and the number of spoilage bacterium increased because of proteolysis leading to high pH. Therefore traditionally, the acidic whey after 16–18 h fermentation, which would contain a small amount of spoilage bacterium, has been used as tofu coagulant.
Carbohydrate and Protein Changes
The main soluble carbohydrates in the tofu whey are respectively glucose, fructose, sucrose, raffinose, and stachyose. The last two sugars are the α-galactosides of sucrose, comprising three and four sugar moieties, which are non-digestible due to the absence of a-galactosidase in the small intestinal mucosa.[Citation30] These α-galactoscyl oligosaccharides could be hydrolyzed by the strains producing a-galactosidase, which hydrolyses α-galactoside bond to produce α-galactose and sucrose. So reducing sugar in this study mainly refers to glucose, fructose and α-galactose.
The carbohydrates and crude protein changes are shown in for the 72 h fermentation of tofu whey. The initial total soluble sugar, reducing sugar and crude protein contents in tofu whey were about 5.57, 2.21, and 7.81 mg/mL respectively. Reducing sugar (RS) accumulated in tofu whey giving a slow rise during the first 16 h fermentation. This coincides with the decrease in total soluble sugar (TSS) content. TSS and RS levels in tofu whey decreased rapidly after 18 h, and then the rate of decrease reduced slowly till levels of 1.5 and 0.6 mg/mL, respectively; sugars in tofu whey were almost consumed by bacteria after 72 h fermentation.
Figure 4 Changes of carbohydrate and crude protein during tofu whey fermentation. Values represent the means + standard deviation (SD) of n = 3 duplicate assays. Error bars were placed on only one curve to illustrate variation.
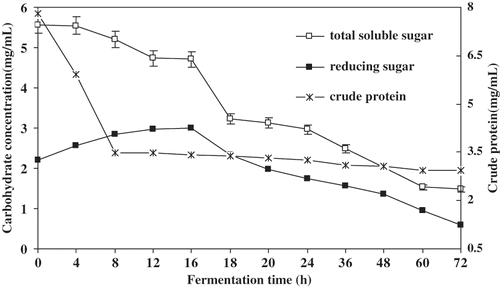
The reducing sugar in tofu whey mainly contains fructose (5.1%) and glucose (5.6%). The two sugars can be utilized directly by micro organism. As such, during the initial fermentation of tofu whey, glucose and fructose are preferentially metabolized by micro organism mainly referring to LAB and then sucrose utilization. RS concentrations were not subject to decrease before 16 h fermentation because only small amounts of glucose and fructose, which were present in tofu whey, were rapidly metabolized; whereas the large amount of glucose and fructose was produced during sucrose hydrolysis, and the growth rate of LAB was slow (). This suggests that the sucrose utilization was probably due to the induction of β-fructofuranosidase (invertase) of some strains in acidic whey.[Citation31]
The number of LAB attained the maximum (), which corresponded to the exponential phase of growth based on the O.D600 (optical density at 600 nm) of the culture (data not reported) after 18 h fermentation. Here the highest a-galactosidase activity of some of the LAB occurred. In our latest study (not publication), many lactic acid bacteria with highest a-galactosidase activity were isolated from the acidic whey after 18–48 h fermentation. Stachyose and raffinose in tofu whey were metabolized to produce a small amount of α-galactoses but a great amount of RS was found to be metabolized as well, the levels of RS and TSS, therefore, rapidly decreased after 18 h fermentation. The crude protein changes of tofu whey decreased rapidly before 8 h, and then decreased slowly to about 3.0 mg/mL. These results suggest that the large amount of micro flora proteinase was produced to hydrolyze protein in tofu whey to supply the rapid growth of micro flora before 8 h fermentation.
Organic Acid Changes
From the results of the organic acid analysis for acidic whey by HPLC, we have found that the acidic whey contain mainly the L (+)-lactic acid. Also, some other organic acids such as acetic acid and tartaric acid are in small amounts. The formic acid, malic acid, oxalic acid and citric acid are not detectable. It is desirable that fermented products contain only low quantities of acetic acid, because of its undesirable taste. This was why the contents of the L (+)-lactic acid and acetic acid changes in tofu whey during fermentation were investigated in this study. shows that the two organic acids contents varied during the 72 h fermentation of the tofu whey. These organic acids are intermediates and metabolites of a variety of biochemical processes that take place during the process, and are important secondary carbon sources for a number of microbial genera that proliferate during food fermentation.[Citation32]
Figure 5 Changes of L (+)-lactic acid and acetic acid during tofu whey fermentation. Values represent the means + standard deviation (SD) of n = 3 duplicate assays. Error bars were placed on only one curve to illustrate variation.
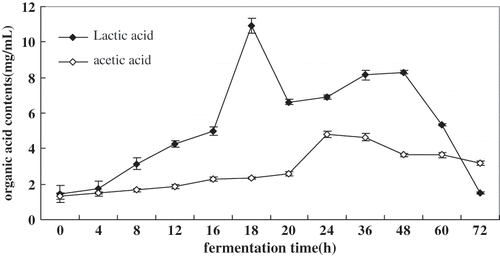
The highest level of L (+)-lactic acid was produced in tofu whey after 18 h fermentation, attaining 10.97 mg/mL significantly(p < 0.05) higher than that of acetic acid (4.82 mg/mL), possibly because of a significantly greater supply of available reducing sugar () and the greatest increase in the viable population of lactic acid bacteria (). The level of L (+)-lactic acid was found to decrease rapidly after 48–72 h fermentation, to 1.50 mg/mL, presumably due to the decrease of the total soluble sugar and available reducing sugar (). The highest level of acetic acid was produced in the tofu whey after 24 h fermentation, attaining 4.82 mg/mL, corresponding to the greatest increase in the population of acetibacteria (). As a result, the pH in the tofu whey was lower than that of the tofu whey fermented before 20 h (). The level of acetic acid then decreased slowly to 3.18 mg/mL after 72 h fermentation. This level is higher than that of L-lactic acid in the tofu-whey, which is not suitable for making tofu as coagulant because of its undesirable “vinegary” taste. A higher proportion of lactic acid by-product is preferred than acetic acid as it provides a sweeter acidic taste which is, generally, more favourable. The acidic whey fermented for 18 h is more suitable as the tofu-coagulant.
Instrumental Textural properties
The instrumental textural properties of tofu play an important role in terms of quality and consumer acceptability. As shown in , the tofu texture varied depending on the pH of the acidic whey. The hardness of acidic whey tofu gradually increased with decreasing pH of the acidic whey. The hardness of the acidic whey tofu (AWT 5 pH 3.5) is significantly higher than that of other acidic whey tofu. These results indicate that decreasing pH of acidic whey with extending fermentation time is useful in preparing a tofu with harder textural properties. Cohesiveness is a measure of the degree of difficulty to break down the internal structure of the tofu. The cohesiveness of AWT1, AWT4, and AWT5 was lower than that of other acid whey tofu. AWT1 made with acidic whey pH4.2 forming a softer structure containing more water was easy to break down. AWT4 and AWT5 made with acid whey (pH 3.6 and 3.5, respectively) had a large amount of free acids, which caused solidification to take place quickly, forming ragged texture that is easy to break down. It implies that the pH of the acidic whey plays an important role on the textural properties of the acidic whey tofu. Springiness represents the extent of recovery of tofu height after pressing and sometimes is referred to as an “elasticity.”[Citation33] The springiness of the acidic whey tofu samples differ significantly among the samples. The springiness of AWT 3 is significantly higher than that of the other tofu. The gumminess and chewiness of the acidic whey tofu increased with decreasing pH of acidic whey. These results suggest that the decreasing water-holding of tofu triggers the increasing hardness. This is because the decreasing pH of the acidic whey causes soy proteins to quickly form the ragged gel texture. The texture properties of AWT3 made with acid whey pH 3.8 was similar to that of the tofu made with Magnesium Chloride, which is a traditional salt-coagulant of tofu (Data not shown).
Table 1 Texture profile analysis of acidic whey tofu (AWT)
Sensory Characteristics
The sensory panels were convened to assess the effects of the pH of the acidic whey on the flavour, sour taste and overall acceptability of the resulted acidic whey tofu (). The results were expressed on a 5-point hedonic scale. Good quality tofu is white or light yellow in colour. All of the tofu samples prepared in this experiment were light yellow in colour. The hunter colorimetric readings also indicate no difference in the L*, a* and b* values. This means that the pH of acidic whey had little effect on the colour of tofu. The sour taste of tofu was found to have increasing scores (very strong) with the decreasing pH of the acid whey, corresponding to the decreasing pH of the acidic whey tofu. This result was due to the increasing acetic acid content of the tofu whey after 24 h fermentation. The flavour and overall acceptability of AWT5 have the lowest scores (not good), while the highest scores (excellent) were observed in AWT3 because of the high level of L-lactic acid and better gel properties. As with the case of textural properties, AWT3 showed overall better sensory properties among the acidic whey tofu samples in this study.
Table 2 Effect of pH of acidic whey on sensory characteristics of acidic whey tofu
CONCLUSION
Based on the findings obtained from the present study, it can be concluded that the various species of micro organism occurred in acidic whey during natural fermentation, and the lactic acid bacterial (LAB) was the dominating strains. The LAB attained maximum populations of about 109cfu/mL after 18 h fermentation. The acidic whey with pH 3.8 and higher level of lactic acid after 18 h fermentation makes an important contribution as the tofu coagulant in order to make good quality acidic whey tofu. However, it was found the number of bacterial spores as 4.07 log10 cfu/g in acidic whey after 18 h fermentation. Moreover, most of them could not be killed during processing of tofu because of heat-resistant properties, and would result in the short shelf life of acidic whey tofu. These results suggested that the sources of acidic whey fermented by inoculating with starter cultures selected from acidic whey after 18 h fermentation have been evaluated as tofu-coagulant to make tofu with the good quality and longer shelf life.
ACKNOWLEDGMENTS
This study was conducted within the framework of the collaborative research project between Japan and China titled “Development of sustainable production and utilization of major food resources in China” supported by Japan International Research Centre for Agricultural Sciences (JIRCAS).
REFERENCES
- Baik , O.D. and Mittal , G.S. 2003 . Determination and Modeling of Thermal Properties of Tofu . International Journal of Food Properties , : 9 – 24 .
- Koury , S.D. and Hodges , R.E. 1968 . Soybean proteins for human diets . Journal of the American Dietetic Association , 52 : 480 – 483 .
- Prabhakaran , M.P. , Perera , C.O. and Valiyaveettil , S. 2005 . Quantification of isofalvones in soymilk and tofu from South East Asia . International Journal of Food Properties , 8 : 113 – 123 .
- Molamma , P.P. , Conrad , O.P. and Suresh , V. 2006 . Effect of different coagulants on the isoflavone levels and physical properties of prepared firm tofu . Food Chemistry , 99 : 492 – 499 .
- Cheng , Y.Q. , Naoto , S. and Toshinori , K. 2005 . The viscoelastic properties of soybean curd (tofu) as affected by soymilk concentration and type of coagulant . International Journal of Food Science and Technology , 40 : 385 – 390 .
- Utsumi , S. and Kinsella , J.E. 1985 . Forces involved in soy protein gelation: effects of various reagents on the formation, hardness and solubility of heat-induced gels from 7S, 11S and soy isolate . Journal of Food Science , 50 : 1278 – 1282 .
- No , H.K. and Meyers , S.P. 2004 . Preparation of tofu using chitosan as a coagulant for improved shelf-life . International Journal of Food Science and Technology , 39 : 133 – 141 .
- Chen , T. S. 1989 . Past, present and future of Chinese fermented food products . Food Reviews International , 52 : 177 – 208 .
- Watanabe , T. , ed. 1997 . “ Science of Tofu ” . 126 – 127 . Kyoto , , Japan : Food Journal Co., Ltd .
- Mengkebilige; Chen , P. , Wang , L.Y. , Li , S.Y. and Bao , S.J. 2000 . Studies on Biological properties of Lactic acid bacterial from acidic whey . Journal of Inner Mongolia Agricultural University , 3 : 90 – 93 . (in Chinese)
- Kohyama , T. , Sano , Y. and Doi , E. 1995 . Rheological characterization and gelation mechanism of tofu (soybean curd) . Journal of Agricultural and Food Chemistry , 43 : 1808 – 1812 .
- Chai , X.J. , Mi , Y.L. , Yue , P.L. and Chen , G.H. 1999 . Bean curd wastewater treatment by membrane separation . Separation and Purification Technology , 15 : 175 – 180 .
- Zhao , D.M. , Liu , L. and Zhang , J.J. 2006 . Detection and analysis of the concentrated waste water from soybean processes . Journal of Food Fermentation Industry , 1 : 68 – 71 . (in Chinese)
- Lě , N.T. , Claude , P.C. , Byong , H.L. and Jacques , G. 2003 . Growth of Lactobacillus paracasei ssp. paracasei on tofu whey . International Journal of Food Microbiology , 89 : 67 – 75 .
- Abdul , G. , Shingo , O. and Takao , K. 2005 . Production of L-lactic acid from fresh cassava roots slurried with tofu liquid waste by Streptococcus bovis . Journal of Bioscience and Bioengineering , 6 : 606 – 612 .
- Wang , G.L. , Song , J.M. and Qu , J.R. 2006 . Identification for a strain of acid-production bacteria isolated from acidic whey . Journal of Henan University of Technology (Natural Science Edition) , 1 : 77 – 81 . (in Chinese)
- Zhang , S.X. , Song , J.M. and Qu , J.R. 2006 . Analysis of the flavor components produced by an aroma producing strain isolated from acid tofu-whey . Food Science and Technology , 1 : 22 – 23 . (in Chinese)
- Li , F.D. , Li , L.T. , Li , Z.G. and Tatsumi , E. 2004 . Determination of starch gelatinization temperature by ohmic heating . Journal of Food Engineering , 62 : 113 – 120 .
- Nout , M.J.R. , Beernink , G. and Bonants-van Laarhoven , T.M.G. 1987 . Growth of Bacillus cereus in soybean tempeh . International Journal of Food Microbiology , 4 : 293 – 301 .
- Hatzikamari , M. , Litopoulou-Tzanetaki , E. and Tzanetakis , N. 1999 . Microbiological characteristics of Anevato: a traditional Greek cheese . Journal of Applied Microbiology , 87 : 595 – 601 .
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.L. and Randall , R.L. 1951 . Protein measurement with the Folin Phenol reagent . Journal of Biology and Chemistry , 193 : 265 – 275 .
- Nielsen , S.S. and Yang , Y.J. , eds. 2002 . “ Analysis of Food ” . 32 – 33 . Beijing , , China : China Light Industry Press . (in Chinese)
- Somogyi , M. 1952 . Notes on sugar determination . Journal of Biology and Chemistry , 195 : 19 – 23 .
- Nelson , N.A. 1944 . Photometric adaptation of the Somogi method for the determination of glucose . Journal of Biology and Chemistry , 153 : 375 – 378 .
- Bourne , M.C. 1978 . Texture profile analysis . Food Technology , 32 : 62 – 66 . 72
- Lim , B.T. , DeMan , J.M. , DeMan , L. and Buzzell , R.I. 1990 . Yield and quality of tofu as affected by soybean and soymilk characteristics: Calcium sulphate coagulant . Journal of Food Science , 55 : 1088 – 1092 .
- Zhang , S. H. , Xu , S. L. and Wang , Y.H. , eds. 2006 . “ Sensory evaluation and experiment of food ” . 34 – 52 . Beijing : Chemistry Industry press . (In Chinese)
- KeShun , L. 1997 . “ Soybeans, Chemistry, Technology and Utilization ” . 25 – 26 . New York , NY : Chapman & Hall .
- Zhu , Y. P. , Cheng , Y. Q. , Wang , L. J. , Fan , J. F. and Li , L. T. 2008 . Enhanced antioxidative activity of Chinese traditionally fermented okara (Meitauza) prepared with various microorganism . International Journal of Food Properties , 3 : 519 – 529 .
- Dimitri , T. and Nagendra , P. S. 2004 . Metabolism of oligosaccharides and aldehydes and production of organic acids in soymilk by probiotic bifid bacteria . International Journal of Food Science and Technology , 39 : 541 – 554 .
- Cruez , R. and Park , Y.K. 1982 . Production of fungal a-galactosidase and its application to the hydrolysis of galactooligosaccharides in soybean milk . Journal of Food Science , 47 : 1973 – 1975 .
- Cogan , T. M. and Daly , C. 1987 . “ Cheese starter cultures. In Cheese ” . In Chemistry, Physics and Microbiology, Vol. 1 General Aspects; Fox, P.F , 179 – 249 . London : Elsevier Applied Science Publishers .
- Yang , H. S. , Choi , S. G. , Jeon , J. T. , Park , G. B. and Joo , S. T. 2007 . Textural and sensory properties of low fat pork sausages with added hydrated oatmeal and tofu as texture-modifying agents . Meat science , 2 : 283 – 289 .