Abstract
Maltodextrins show increasing industrial applications depending on the extent of the starch hydrolysis. This paper reports a relationship between morphological and physico-chemical characteristics of different maltodextrins (A, B, and C) in order to establish appropriate uses. The moisture content of the maltodextrins varied from 2.82 to 6.47% and true and bulk density, average particle size, and porosity of the maltodextrins ranged from 1.14–1.44 g/mL, 0.33–0.49 g/mL, 39.44–289.17 μm, and 59.70–67.58%, respectively. Spherical, irregular, and filamentous shapes conduct to low values of wetting times. The increase in the DE level of maltodextrins from the same botanical source caused a decrease in the dissolution time. Results demonstrated that products obtained by supplier B showed the best performance and regularity in the dissolution/dispersion characteristics in water meanwhile maltodextrins manufactured by supplier A would be favored by an agglomeration process.
INTRODUCTION
Maltodextrins are, by definition, hydrolyzed starch build up by units of α-D-glucose bound together, mainly, by glycosidical (1→4) linkages with a general formula [(C6H10O5)nH2O].[Citation1] As hydrolyzed products of starch, they consist of a mixture of saccharides, mainly D-glucose, maltose and a series of oligosaccharides and polysaccharides, such as maltotriose and mixtures of maltotetraose and therefore present a wide distribution of molecular mass. Commercially, they are produced from native starch through partial hydrolysis, purification, and spray-drying. These physical and enzymatic treatments lead to the loss of the granular starch structure. Unlike natural starches, maltodextrins are soluble in water, a convenience that assisted their appreciation by the food industry, where they are used as texture modifier, gelling agent, fat replacer, volume enhancers, crioprotectors, and to extend the product shelf-life, mainly as encapsulation matrix.[Citation2] Maltodextrins are less expensive than other edible hydrocolloids and their aqueous solutions have more subtle flavor and mouthfeel.
Maltodextrins are usually classified by their values of dextrose equivalent, ranging up to 20. Dextrose Equivalent (DE) expresses the number of reducing ends aldehyde groups relative to pure glucose at the same concentration, so that high DE indicates high hydrolytic conversion and lower average molecular mass. The source of the starch (corn, oatmeal, manioc, rice or potato) also affects the molecular segments distribution of maltodextrin. The ratio between the molecules of linear amylose chain and the branched-chain of amylopectin varies according to the nature of the starch. Most starches contain between 15 and 35% of amylose.[Citation1]
The functional characteristics of maltodextrins are assumed to be correlated to the degree of DE and empirically serve as a guide to determine their applications. For example, maltodextrins with DE10 are usually applied to flavor vehicle, aroma encapsulation, instant sauces and diet and light products, DE 15 maltodextrins are employed to isotonic beverages, dehydrated soups and DE 20 maltodextrins are used for chocolate powder, dairy desserts, pannings, beverage powder, and industrial bakery pre-mixes. Variations in DE values result in maltodextrins with different physico-chemical properties, such as hygroscopicity, osmolality, viscosity, and cohesivity. Commercial maltodextrins, therefore, usually inform their DE value as the only reference.
It is possible to produce maltodextrin that have similar DE values but with different proportions of saccharides of high and of low molecular mass by altering hydrolysis conditions. These combinations produce maltodextrins with different physico-chemical characteristics. In particular, the stability and solubility are affected by the high molecular mass components, whereas viscosity, crystallinity, and sweetness depend on the predominance of low molecular mass components.[Citation3] As a consequence studies have demonstrated that the dextrose equivalent of maltodextrin alone is not appropriate to predict the perfomance of the product in several applications[Citation4,Citation5] such as, spray-drying products, coatings and extruded encapsulation. Technological advances allow the control of the hydrolysis pathway, mainly through enzymatic processes, in order to produce maltodextrin with better defined saccharide composition.
The distribution of molecular mass has been considered to be a more accurate parameter to predict the properties of maltodextrin, but only few specific applications are known.[Citation4] The relation between molecular mass distribution and the intrinsic properties of the product (crystalinity and molecular mobility) help to rationalize the understanding of technological events as, for instance, the wet agglomeration. The agglomeration process occurs when the glass transition temperature (Tg ) of the material is reduced, under the influence of water plasticization. As a result, the material becomes sticky on the surface favoring the particles cohesion. The temperature of glass transition is strongly dependent on the molecular weight of the material.
In the present study, the physical and morphological characteristic of different maltodextrins commercialized in the Brazilian market were evaluated in order to identity features that provide support for specific applications and also select maltodextrins that require additional agglomeration process to improve instant properties.
MATERIALS AND METHODS
Maltodextrins
Twelve commercial samples of maltodextrins with different DE, manufactured in Brazil by three different suppliers (A, B, C) were evaluated: A1 (DE 05) (hydrolyzed manioc starch) and maltodextrins A2 (DE 10), A3 (DE 15), and A4 (DE 20) (hydrolyzed corn starch); maltodextrins B1 (DE 10) and B2 (DE 20) (hydrolyzed manioc starch) and B3 (DE 10) and B4 (DE 20) (hydrolyzed corn starch); maltodextrins C1 (DE 07), C2 (DE 21), C3 (DE 11), and C4 (DE 30), (hydrolyzed manioc starch).
Characterization
Morphology
Morphological observations of the particles of maltodextrins were made by a Scanning Electronic Microscope (SEM) (model LEO 440i, LEICA Electron Microscope Ltd., Germany). The samples were previously dried in a vacuum oven (model TE-395, TECNAL, Brazil) at a temperature of 70°C and under vacuum of 15 inHg for 48 hours and then metalized with a gold/palladium alloy in a Sputter Coater (mod. SC7620, POLARON, UK) at a coating rate of 0.51 Å /s, for 180 seconds, at 3 mA, 1 volt and 2 × 10−1 Pa. The image acquisition was performed by LEO software, 3.01 version.
Moisture content
The moisture content (Xw , in% wet basis) was determined gravimetrically with a vacuum-oven at 70°C and 15 inHg until constant weight according to AACC method n° 44–5A.[Citation6]
True and bulk density
True density, ρ true , of the maltodextrin particles was determined by an Automatic Gas Pycnometer (mod. AccuPyc 1330, Micromeritics, USA), with samples previously dried in a vacuum oven at 70°C and 30 inHg for 48 hours. The bulk density of the maltodextrin powders, ρ bulk , was obtained gravimetrically, weighing a powder sample poured into a 25 mL graduated cylinder at 20°C under defined standard conditions.
Molecular mass
The Average Molecular Mass Number (Mn) of the maltodextrins was determined by Gel Permeation Chromatography (GPC) using a GPC-Millenium Chromatograph (Waters, USA). The 30 cm long and 0.75 cm diameter columns were connected in series in a pore decreasing sequence (G 3000 PW, G 4000 PW and G 6000 PW). The following dextranes standards were used: DXT 11K; DKT 43K; DXT 79K; DXT 165K, DXT 685K; DXT 1750K; DXT 5000K (American Polymer Standards Corporation, USA). Samples were initially diluted in Mili-Q Plus water at a concentration of 1% (w/v), submitted to an ultrasonic bath and filtered through a Millipore HV membrane of 0.43 μm. The injection conditions were: oven temperature (40°C), detector temperature (40°C), injected volume (98 μ L), eluent flow (1 mL/min) and running time (40 minutes). The data were treated using Millenium Chromatograph Manager – GPC (version 2.10) software (Waters Corporation, USA). The weighted average molecular mass, was calculated by EquationEq. (1):
Particle size distribution
The size distribution of the particles and the particle diameter, D4,3 (volume or mass moment mean), was determined by a Laser Granulometer MasterSizer (mod. S-MAM 5005, MALVERN, UK). Ethanol 96% was used as dispersion media and the analysis of the results was performed with a MasterSizer software, 2.18 version.
Powder void fraction
Intergranular porosity, ϵ, of the powder was estimated through EquationEq. (2) according to YU et al.[Citation7]:
Wetting time
The wettability of the raw materials was evaluated as suggested by Kyaw Hla and Hogekamp[Citation8] using a device made of Plexiglas® tube (4-cm diameter, 8-cm long) divided in two chambers by a guillotine type slide. The powder (3.5 g) was deposited over the guillotine and the lower chamber contained water at room temperature (25°C) up to the top, just underneath the guillotine. When the guillotine slips open the powder is gentle deposited over the water surface and immerses after being wetted by the liquid. The wetting time is considered as the time when no powder material remains on the surface of the liquid.
Dissolution time
The determination of the dissolution time of the sample was performed according to Omobuwajo et al.[Citation9] It corresponds to the time necessary for the complete dissolution of 1 g of the powder in 100 mL of distilled water in a 250 mL recipient, under a defined level of agitation at a temperature of 20°C. A stirring plate with the magnetic stirring level at position 5 was used (model 753, FISATOM, Brazil).
Degree of crystallinity
The determination of the crystallinity of the samples was performed by X-Ray diffraction, in a diffractometer (PHILLIPS, model X'Pert, USA) with a CuKα radiation of wavelength λ = 1.54 Å, voltage of 40 kV, current of 40 mA and scanning angle interval 5°< 2θ <60°, under a scanning speed of 0.02°/s. Samples were previously dried in a vacuum oven at 70° C for 24 hours and deposited as a flat surface on a sample board which was rotated during readings. The index of crystallinity (IC) was obtained according to the following expression[Citation10]:
RESULTS AND DISCUSSION
Morphology
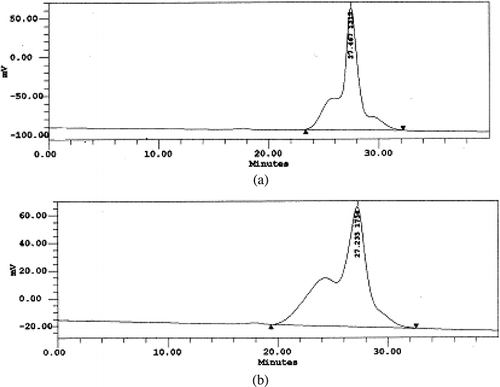
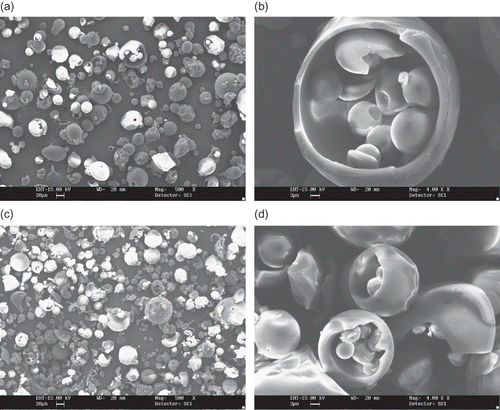
Samples of microphotographs of the commercial maltodextrins powders are shown in Figs. . and correspond to maltodextrins produced by supplier A. The micrographs shown in indicate that two maltodextrins produced by supplier A (A1 (DE 05) and A2 (DE 10)) although made from two different type of starch and have distinctive DE values, present similar rounded shaped particles. They consist of a mixture of spherical, cylindrical and filamentous particles. A1 (DE 05) maltodextrin particles are more voluminous and show smoother surfaces than A2 (DE 10), but both products present tubular open structures, as can be seen in and . has micrographs of A3 (DE 15) and A4 (DE 20) maltodextrins and consist predominantly of globular shaped and partially encapsulated particles (, ). The sample of A4 (DE 20) () is more fractured than the formers.
Figure 1 SEM microphotographs of different maltodextrins from supplier A: (a) A1 (DE 05 manioc maltodextrin, 500X); (b) A1 (DE 05 manioc maltodextrin, 4000x); (c) A2 (DE 10 corn maltodextrin, 500X); (d) A2 (DE 10 corn maltodextrin, 4000x).
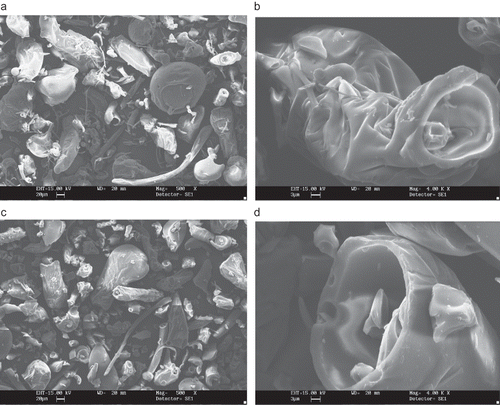
Figure 2 SEM microphotographs of different maltodextrins from supplier A: (a) A3 (DE 15 corn maltodextrin, 500X); (b) A3 (DE 15 corn maltodextrin, 4000x); (c) A4 (DE 20 corn maltodextrin, 500X); (d) A4 (DE 20 corn maltodextrin, 4000x).
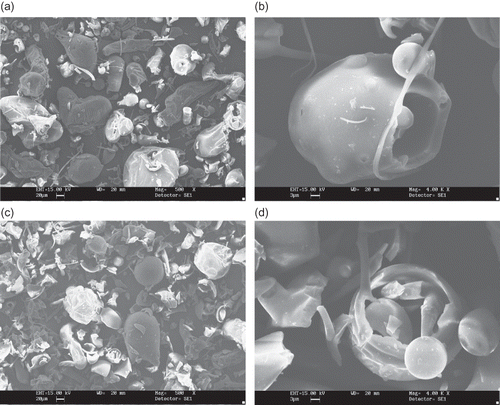
Figure 3 SEM microphotographs of different maltodextrins from supplier B: (a) B1 (DE 10 manioc maltodextrin, 500X); (b) B1 (DE 10 manioc maltodextrin, 1000x); (c) B2 (DE 20 manioc maltodextrin, 500X); (d) B2 (DE 20 manioc maltodextrin, 1000x).
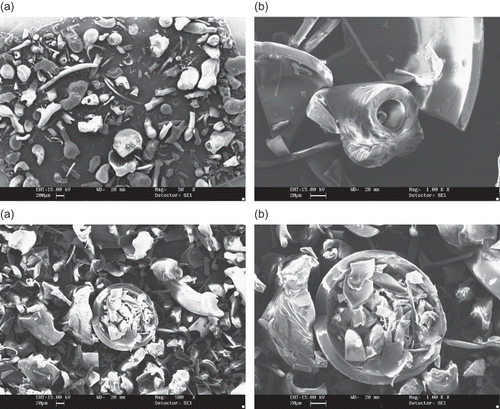
Figure 4 SEM microphotographs of different maltodextrins from supplier B: (a) B3 (DE 10 corn maltodextrin, 500X); (b) B3 (DE 10 corn maltodextrin, 4000x); (c) B4 (DE 20 corn maltodextrin, 500X); (d) B4 (DE 20 corn maltodextrin, 4000x).
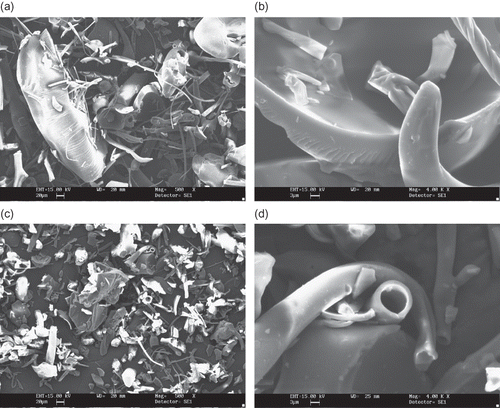
Figure 5 SEM microphotographs of different maltodextrins from supplier C: (a) C1 (DE 07 manioc maltodextrin, 500X); (b) C1 (DE 07 manioc maltodextrin, 4000x); (c) C2 (DE 21 manioc maltodextrin, 500X); (d) C2 (DE 21 manioc maltodextrin, 4000x).
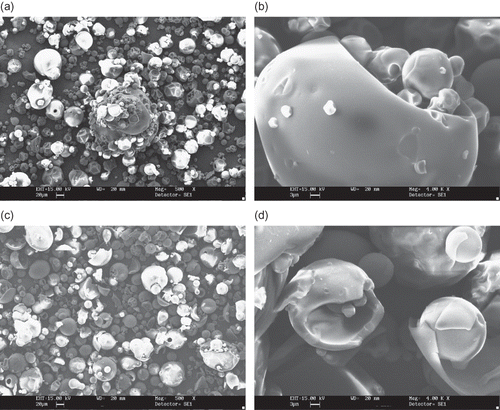
Figure 6 SEM microphotographs of different maltodextrins from supplier C: (a) C3 (DE 11 manioc maltodextrin, 500X); (b) C3 (DE 11 manioc maltodextrin, 4000x); (c) C4 (DE 30 manioc maltodextrin, 500X); (D) C4 (DE 30 manioc maltodextrin, 4000x).
The powders produced by supplier B are shown in (manioc starch maltodextrin) and (corn starch maltodextrin) and show distinct features between themselves. The manioc starch maltodextrin () differed from all other samples for having larger sizes, so that lower magnification had to be used. They tend to have irregular rounded shaped particles, without the thin filaments found with B3 (DE 10) and B4 (DE 20) maltodextrins (). The particles of corn starch maltodextrins consist mainly of large irregular bodies entangled by thin filaments. No encapsulated structures could be observed (). Boutboul et al.[Citation11] also observed strongly fragmented structures for a sample derived from corn starch with DE between 11–14.
Micrographs of maltodextrins produced by supplier C are shown in and and exhibit distinct features compared to the previous powders. All particles are globular, a few of them encapsulating smaller ones. C1 (DE 07), () and C2 (DE 21), () maltodextrins have wrinkled surfaces and small agglomerated structures can be found. Maltodextrins C3 (DE 11), and C4 (DE 30), () present even more regular shaped particles, but of smaller size, and with some aggregates with denticulate surfaces (, ). No differences in shape of the particles were observed even at a magnification of 4000 times, despite the different molecular masses between them.
The morphological differences found in this study can be attributed to spray-drying conditions, such as feed viscosity and surface tension, type of atomizer used, fines reinjection and, mainly, inlet/outlet air temperature. Alamilla-Béltran et al.[Citation12] described the changes in the morphology of maltodextrin particles along a vertical cross-section of a spray-dryer. They reported that the final morphology of the particle could be correlated to the drying temperature in the chamber. Low process temperatures (110/74°C, inlet/outlet temperature) promote a higher degree of surface wrinkling if compared to the particles obtained at higher temperatures (200/173°C). The morphology of the particles shown in and are typical of a low temperature drying. Moreover, there is a greater tendency to produce particles with larger diameter when operating at high temperatures. The authors also reported that inflating and collapsing structures were obtained at low and intermediate temperatures (as observed in and ), whereas a mixture of both fractured and integral spheres (similar to the particles in and ) was observed when high process temperature was used.
Average Molecular Mass
shows the values of number average molecular mass (Mn), weighted average molecular mass (Mw), polydispersity index (PDI), and the degree of dextrose-equivalent (DE) determined for the different maltodextrins. These indexes were calculated from the molecular mass distributions curves obtained by GPC, like those given in
Table 1 Values of number average molecular mass (Mn), weighted average molecular mass (Mw), dextrose-equivalent (DE), polydispersity index (PDI) of maltodextrins analyzed by GPC
The results indicate that the samples A2 (DE 10) and A3 (DE 15) presented DE values considerably higher and outside the range of DE informed by the manufacturer. The decrease in molecular mass can be attributed to over processing of the hydrolysis during the production of the maltodextrins. Avaltroni et al.[Citation4] also reported DE values higher than producer's specification. These authors reported that the starch had a distribution of large molecular weights from which small molecules are absent. Upon hydrolysis, the large molecules disappeared and very small ones appeared which could explain the higher DE found.
The largest deviation between the twelve analysed samples was found for C1 (DE 07). The DE calculated using the Mn value determined by chromatography was more than three times higher than the average DE informed by the manufacture. However, the value of polydispersity index (PDI) was 1.6, the lowest of all samples. The PDI values of the supplier B were slightly higher than A and C maltodextrins. B3 maltodextrin showed the largest value (8.4) and the molecular mass distribution curve suggests a bimodal distribution ().
Physical and Instant Properties
The values describing the physical characterization of the maltodextrins are presented in . The moisture content (Xw ) of the maltodextrins varied from 2.09% (C4 DE 30 maltodextrin) to 6.47% (B1 DE 10 maltodextrin). Che Man et al.[Citation13] found moisture content of 5.02% for a maltodextrin derived from corn starch with DE in the range of 12–15, which agrees with the values found in this study. The moisture content reflects the drying conditions (mainly outlet air temperature) and the packaging protection and is less influenced by the molecular mass. Alamilla-Béltran et al.[Citation12] also reported that it was possible to explore relations of morphology and moisture content of particles with drying temperature operation, especially those related to breakage and inflation (intermediate and high drying temperatures) and with collapse (low temperature drying)
Table 2 Physical properties of the maltodextrins with different degrees of DE
The D4,3 values indicated that B1 (DE 10 maltodextrin) has the largest particle diameter (289.2 μm) and C4 (DE 30 maltodextrin) has the lowest (39.4 μm) and these observations are in accordance with the morphological features. The A and B maltodextrins show a decreasing tendency of particle diameter (D4,3) with the increase of DE, meaning that larger particles were obtained when more viscous feed solution was used into the spray-dryer, as expected.[Citation14]
The true density (ρ true ) and the bulk density (ρ bulk ) of the C samples are inferior to the others and presenting statistical difference (p ≤ 0.05). The bulk densities of the maltodextrins produced by A and by B have similar ranges of values (0.42–0.49 and 0.42–0.47 g/mL, respectively), however, the true densities of the maltodextrins from supplier B are higher (1.35–1.47 g/mL). Turchiuli et al.[Citation15] reported similar value (1.37 g/mL) for true density of DE 12 maltodextrin powders. The void fraction of a powdered material has a large influence in wettability characteristics. The void fraction (ϵ) of the B1 , B2, C2 , C3, and C4 powders show a decrease in their value with the increase in the degree of DE. However, this behavior was not observed among the A2 , A3 , and A4 samples, which did not present significant difference (p ≤ 0.05) except for A2 (DE 10 maltodextrin). C1 (DE 07 maltodextrin) showed the highest value of porosity among all samples but poor instant properties. This could be resulted from the morphological characteristics since they are small spherical denteated particles, more resistant to capillary wetting. The C samples did not show this tendency. Comparing maltodextrins of the same DE, but from different sources, it can be seen that the sample B1 (manioc) presents D4,3 superior to B3 (corn). The same is observed for B2 , which is 30% superior to B4 . These differences are possibly due to the morphological characteristics of the samples influenced by the drying process conditions.
Results for dissolution and wetting time for the maltodextrin samples are also presented in . The WT of the B1 , B2, B3 , and B4 samples did not present significant differences between themselves (p ≤ 0.05), being the lowest values of all the evaluated samples. This behavior can be explained by a combination of adequate morphological features, which favors capillarity. A tendency of a decrease in wetting time (WT) with the increase in the degree of hydrolysis for the A2 , A3 and A4 samples and also for the B samples was observed. These samples showed adequate WT values ranging from 4 to 8 sec in contrast to A maltodextrins that needed 29 and 2127 sec to sink. C1 (DE 07 maltodextrin) needed over one hour of WT since the low porosity favored local dissolution and the viscous concentrated solution envolved a core of still dry powder hindering its wetting by capilarity. The clumps could be visually observed.
The WT of maltodextrins from different sources but with the same DE, tend to favor the manioc products. B1 (DE 10 manioc maltodextrin) for instance, presents WT 25% superior to B3 (DE 10 corn maltodextrin) and B2 (DE 20 manioc maltodextrin) is 20% superior to B4 (DE 20 corn maltodextrin). However, DT for B1 is 62% lower than for B3 and B2 is 41% lower than B4 . These product manufactured by supplier B presented the fastest dissolution behavior of all analyzed samples although, differently from the WT values, their DT are significantly different (p ≤ 0.05). It is also observed that the increase in DE and the decrease in D4,3 for B3 and B4 samples decreased the dissolution time by a factor of 6 (178.0 to 29.0 sec). The same performance was observed for B1 and B2 samples, with a reduction by a factor of four.
According to Shittu and Lawal,[Citation16] the relationship between the instant characteristics and the physical and chemical properties is not linear and could involve some cross interactions of these properties. These instant characteristics are related to wetting and dissolution time. The first, refers to the ability of a bulk powder to imbibe a liquid under the influence of capillary forces and the latter include the dispersion of insoluble powders in liquids due to mainly a two-step process: submersion of the powder and destruction of aggregates in the liquid under external agitation.[Citation17] Shittu & Lawal[Citation16] concluded that solubility of the powder cocoa beverages depended mainly on the chemical constituents (sugar and fat contents), and that the wetting time was more significantly affected by the physical properties (bulk density), while the interaction between the angle of repose and sugar content also affected the wettability of the powder.
Crystallinity Pattern
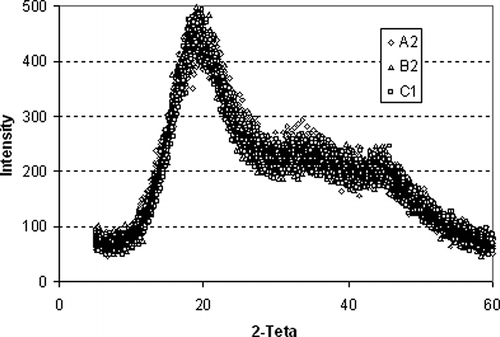
The X-ray diffractograms of the maltodextrins are shown in for samples manufactured by suppliers A, B, and C, respectively. The diffractometric profiles of the curves for all the samples show similarities between themselves and exhibit the same pattern of crystallinity, since all of them present three peaks at the angles of 2θ equal to (i) 19.1; 33.3 and 43.9°; (ii) 20.0; 34.8 and 43.3° and (iii) 19.2; 34.5 and 44.0°, respectively. Samples that are totally amorphous do not present peaks in the range of the angle scanned. All samples present peaks that have similar intensity, approximately 450 units for the first, 250 for the second and 200 for the third.
Figure 8 – X-Ray diffractograms for A2 (corn maltodextrin), B2 (cassava maltodextrin), C1 (cassava maltodextrin) with DEs = 10; 20 and 30, respectively.
The calculated values for the maltodextrin crystallinity index (IC), which were obtained according to Alexander,[Citation10] are shown in . These results express the “true” crystallinity of the samples, since no reference materials (amorphous and crystalline) were used. The results show that the crystallinity index of all maltodextrins is very similar between them (0.66–0.72) except for maltodextrin C4 , which presented higher values. The values of IC found are unexpectedly high but could not be correlated to instant properties, which are affected mainly by their drying conditions.
Table 3 Crystallinity index (IC) of maltodextrins
CONCLUSIONS
The morphology of the particle influences to a great extent the rate of wetting and dissolution, important quality factor for instant powder products. A mixture of fractured spheres, broken shells, non-broken particles, and cylindrical shapes, forms all type of maltodextrins powder. Inflated as well as collapsed particles can also be noted. The higher the DE degree of the same source of maltodextrin (A2 ; A3 and A4 or B1 ; B2 or B3 ; B4 ) the better their instant characteristics. The products obtained by supplier B showed the best performance and regularity in the dissolution/dispersion characteristics in water. The maltodextrins manufactured by supplier A would be favored by an agglomeration process due to the homogeneity of their physico-chemical characteristics, as well as wetting and dissolution times which should be improved.
NOMENCLATURE
| CI | = |
Crystallinity index | |||||||||||||||||
| DE | = |
Dextrose-equivalent | |||||||||||||||||
| DT | = |
Dissolution time (s) | |||||||||||||||||
| D4, 3 | = |
Volume or mass moment mean (μm) | |||||||||||||||||
| Mn | = |
Number-average molecular mass (Da) | |||||||||||||||||
| Mw | = |
Weighted average molecular mass (Da) | |||||||||||||||||
| PDI | = |
Polydispersity index | |||||||||||||||||
| Xdb | = |
Moisture content (dry basis) | |||||||||||||||||
| Xw | = |
Moisture content (wet basis) | |||||||||||||||||
| WT | = |
Wetting time (s)
| |||||||||||||||||
ACKNOWLEDGMENTS
We acknowledge the Fundação de Amparo à Pesquisa de São Paulo (FAPESP) for the research grant (proc. n° 03/08377-4) and for the doctorate scholarship (proc. n° 03/001105-9) granted to Cristina Yoshie Takeiti.
REFERENCES
- Kennedy , J.F. , Knill , C.J. and Taylor , D.W. 1995 . “ Maltodextrins ” . In Handbook of starch hydrolysis products and their derivatives , Edited by: Kearsley , M.W. and DZIEDZIC , S.Z. 65 – 82 . Glasgow : Blackie Academic & Professional .
- Anandaraman , S. and Reineccius , G.A. 1986 . Stability of encapsulated orange peel oil . Food Technology , 40 ( 11 ) : 88 – 93 .
- Griffin , V.K. and Brooks , J.R. 1989 . Production and size distribution of rice maltodextrins hydrolyzed from milled rice flour using heat-stable alpha-amylase . Journal of Food Science , 54 ( 1 ) : 190 – 193 .
- Avaltroni , F. , Bouquerand , P.E. and Normand , V. 2004 . Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions . Carbohydrate Polymers , 58 : 323 – 334 .
- Chronakis , I.S. 1998 . On the molecular characteristics, compositional properties, and structural-functional mechanisms of maltodextrins: A review . Critical Reviews in Food Science , 38 ( 7 ) : 599 – 637 .
- AACC . 1995 . Approved Methods of the American Association of Cereal Chemists , 9th , St. Paul, MN : Method 44-5A. v.2 .
- Yu , A.B. , Standich , N. and Lu , L. 1995 . Coal agglomeration and its effect on bulk density . Powder Technology , 82 : 177 – 189 .
- Kyaw Hla , P. and Hogekamp , S. 1999 . Wetting behavior of instantized cocoa beverage powders . International Journal of Food Science and Technology , 34 : 335 – 342 .
- Omobuwajo , T.O. , Busari , O.T. and Osemwegie , A.A. 2000 . Thermal agglomeration of chocolate drink powder . Journal of Food Engineering , 46 : 73 – 81 .
- Alexander , L.E. 1969 . “ Degree of crystallinity of polymers ” . In X-Ray Diffraction Methods in Polymer Science , 582 New York : John Wiley & Sons, Inc .
- Boutboul , A. , Giampaoli , P. , Feigenbaum , A. and Ducruet , V. 2002 . Influence of the nature and treatment of starch on aroma retention . Carbohydrate Polymers , 47 : 73 – 82 .
- Alamilla-Béltran , L. , Chanona-Pérez , J.J. , Jiménez-Aparicio , A.R. and Gutiérrez-López , G.F. 2005 . Description of morphological changes of particles along spray-drying . Journal of Food Engineering , 67 : 179 – 184 .
- Che Man , Y.B. , Irwandi , J. and Abdullah , W.J.W. 1999 . Effect of different types of maltodextrin and drying methods on physico-chemical and sensory properties of encapsulated durian flavour . Journal of the Science of Food and Agriculture , 79 : 1075 – 1080 .
- Masters , K. and Stolze , A. 1973 . Agglomeration Advances: Range of instantized powderer foods increases and new designs have been developed . Food Engineering , 33 : 64 – 67 .
- Turchiuli , C. , Eloualia , Z. , El-Mansouri , N. and Dumoulin , E. 2005 . Fluidized bed agglomeration: Agglomerates shape and end-use properties . Powder Technology , 157 : 168 – 175 .
- Shittu , T.A. and Lawal , M.O. 2007 . Factors affecting instant properties of powdered cocoa beverages . Food Chemistry , 100 : 91 – 98 .
- Freudig , B. , Hogekamp , S. and Schubert , H. 1999 . Dispersion of powders in liquids in a stirred vessel . Chemical Engineering and Processing , 38 : 525 – 532 .