Abstract
A confectionery gel (CG) consists of high sugar components of sucrose and glucose syrup, combined with gelling components such as starch, gelatin, or pectin, along with food acid, flavourings and colourings. Common CG products include “jelly snakes,” “jelly babies,” “jelly beans,” and form a portion of the lucrative confectionery market; however, there are continual consumer demands for more interesting and innovative products that have new and exciting textures, flavors and appearances. Improving or modifying CG textures can meet these demands, but first an understanding of how the behaviour and structure of the gel is developed must be achieved. Companies that will gain a competitive advantage in the confectionery market will be those able to actively manipulate and control sensory properties to meet exacting customer demands. This paper is a review of literature available on confectionery gels, their components, and factors that may affect their microstructure, texture, and rheology.
INTRODUCTION
Gels exist in many material systems including diverse media such as polymers, plant and animal tissues, and food. The majority of foods are, or consist of gels[Citation1] and gels found in foods provide easily recognized, although widely varying textures.[Citation2] National tastes can often dictate which are the gelling ingredients of choice to provide pleasing textures to consumer markets.[Citation3] There are several definitions of a gel within literature. Hermans[Citation4] stated that a gel must be: (1) a coherent two component system formed by a solid substance finely dispersed, or dissolved in, a liquid phase that also (2) exhibits solid-like behaviour under mechanical forces. Another view put forward is that all gels are solid, as they are self-supporting and can recover elastically after deformation, however some gels may have some unrecovered stresses that can be present after deformation, and gels may deform in a brittle manner, such as in agar or kappa-carrageenan gels.[Citation5] Flory[Citation6] suggested that gels consist of polymeric molecules crosslinked to form a tangled interconnected network immersed in a liquid medium. Djabourov and LeBlond[Citation7] mentioned that in a gel both the dispersed component and the solvent should extend continuously throughout the whole system, each phase being interconnected, which is similar to the theory proposed by Flory.[Citation6] A gel has also been described as a giant polymer made up of molecules that are branched in three dimensions and form a lattice.[Citation8]
Obviously there are several types of gels and they are commonly classified into two main groups – chemical and physical gels, which are distinguished by the differences in nature of the bonds linking the gels' molecules together.[Citation8] Chemical gels usually describe covalently crosslinked gels, whilst physical gels consist of chains which are “physically” (non-covalently) crosslinked into networks.[Citation9,Citation10]
The crosslinks in physical gels are of small but finite energy and/or finite lifetime, and can be found in biological and synthetic polymers. In biopolymer gels the crosslinks are formed by physical gel mechanisms such as Coulombic, dipole-dipole, van der Waal's, charge transfer, hydrophobic, and hydrogen bonding interactions. The term physical gel is often assumed to imply thermoreversibility in the gel, such as in the case of gelatin, although this is not the case in every physical gel system (e.g. casein gels).[Citation9]
COMPOSITE GELS
Most food gel products in the market today are in fact composite gels, containing two or more gelling components. Most components contribute to structure and physical properties of food, with the two main structural materials being proteins and polysaccharides.[Citation11] Biopolymer mixtures are used in many industries, including food, to impart specific flow behaviours, textures, appearances, and where required, tactile and mouthfeel properties, to products.[Citation12,Citation13] As a consequence of the wide use of biopolymer mixtures in food, these types of systems have been greatly researched, but it is only recently that biopolymer phase separation in composite gels and the effects of this on composite gel properties have been studied.[Citation12–15] Most food components have limited miscibility on a molecular level and tend to form multicomponent heterophase and non-equilibrium dispersed systems.[Citation11]
For polymers dissimilar in shape or structure, segregation leads to a reduction of the polymer concentration near the other type of polymer particle. Once a critical polymer concentration is exceeded, this leads to phase separation.[Citation12,Citation13] Interactions of proteins and polysaccharides range from complete segregation to complexation (). These interactions can greatly affect mechanical gel properties whilst chain structure, molecular weight and composition are important for structure.[Citation16] Often, linear polysaccharides are more incompatible with proteins than branched polysaccharides[Citation17] due to rigidity and the presence of less independently moving macromolecular segments and lower mixing entropy than in flexible branched polymers.[Citation11] The differences in hydrophobicity in proteins and polysaccharides are of great importance for phase equilibrium in protein-polysaccharide-water systems.[Citation17] The level of phase separation, and shape and appearance of phase components, in these systems depends on polymer concentration and the compatibility of the biopolymers.[Citation12,Citation13]
Figure 1 Main trends in the behaviour of protein/polysaccharide mixtures (modified from de Kruif and Tuinier[Citation13]).
![Figure 1 Main trends in the behaviour of protein/polysaccharide mixtures (modified from de Kruif and Tuinier[Citation13]).](/cms/asset/7ba197dd-c496-4481-943f-7a91dc947313/ljfp_a_322507_o_f0001g.gif)
Phase separation in composite gels is a result of thermodynamic incompatibility of gel components[Citation16,Citation17]. Phase separation is entropically unfavourable, but enthalpically advantageous as molecules prefer like molecules.[Citation12,Citation17] Temperature changes can also affect biopolymer phase separation, as well as pH and shear.[Citation12,Citation16,Citation18–20] Interfacial tension between gel phases is important in determining biopolymer composite behaviour, whilst thermodynamic incompatibility and complex formation by food hydrocolloids can greatly affect mechanical and other physicochemical gel properties.[Citation12,Citation16] It has been possible to apply synthetic polymer blending laws to food gel composites, in order to predict and quantify mechanical behaviour of such composites.[Citation21] This theory is described by the Isostrain and Isostress models, where the Isostrain model sets an upper bound for composite modulus, whilst the Isostress model sets a lower bound. The equations for each are shown below where Equationequation 1 is applicable for the Isostrain condition and Equationequation 2 applies to the Isostress situation:
CONFECTIONERY GELS
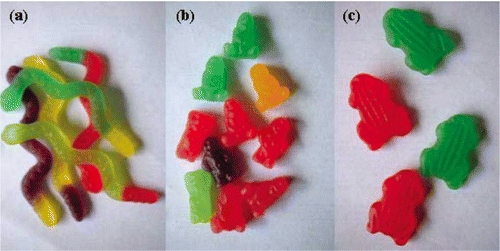
Confectionery gels (CGs) are often high sugar systems, with one or more gelling components, which are chosen for their textural attributes to give firmer or softer textures to the CGs. The ingredients are formed into a molten material that can be moulded into many different shapes (). The aforementioned definitions of gels and composite gels refer more to the conventional ideas of a biopolymer gel system made in an aqueous environment. Recent studies show that on the addition of sugars to such a system, the morphology of the high solid network is distinctly different from that of the aqueous system. This means that the standard concepts of gelation, which apply in aqueous systems are no longer valid in CG systems.[Citation23] However, the aqueous gel system model could be valid if a non-nutritive sweetener is used in a sugar-free CG formulation. This type of product could have a viable market as obesity in children is an increasing problem worldwide.
In the gelling of aqueous biopolymer systems, network formation is governed by some important characteristics of the system which are: 1. the minimum critical gelling concentration (C0), which is the minimum concentration of biopolymer that allows gel networks to form; and 2. the concentration of coil overlap and entanglement of biopolymer chains (C*), which affects density of the network. These characteristics are no longer the main influence on biopolymer gel network formation once sugars are introduced into the system, as is the case in confectionery gels. Sugars change morphology of single biopolymer gel systems, as well as affecting phase separation characteristics in mixed biopolymer gel systems. The major textural properties of CGs are imparted by the gelling components used, such as starch or gelatin. The sugar co-solutes are not part of the polymer network in the confectionery gels but can greatly contribute to formation and behaviour of CGs.[Citation24]
Studies of single biopolymer systems containing sugar have shown that in polysaccharides, such as starch that as sugar concentration approaches levels found in confectionery gels, chain-chain association is reduced. This leads to a less aggregated structure than in the case of the aqueous system, meaning that C0 must be increased as the sugar is inhibiting gel network aggregation. Gelatin gels behave quite differently to starch gel systems, with chain association being increased in the presence of sugar, rather than reduced. Sugars tend to destabilize polysaccharide gel networks at levels of 40–60% sugars, but increase gelatin gel networks at these levels of sugar.[Citation25]
In aqueous systems, gelatin gels appear featureless, but upon addition of sugar they separate into sugar-rich and gelatin-rich phases, showing high biopolymer aggregation. In aqueous systems, polysaccharides show fibrillar network structures, but upon addition of sugar some of these features are lost. This is due to the threshold of thermodynamic stability of structure formation for polysaccharides being exceeded at these levels, and considerable parts of the network “dissolving” in the saturated sugar environment. Thermodynamic stability of gelatin gel network formation is increased at 40–60% sugar and continues to increase at levels above this.[Citation23]
In mixed biopolymer systems phase separation is governed by critical polymer concentration, polymer shape, and compatibility of the biopolymers present. The extent of mixing is governed by the “Flory-Huggins interaction parameter,” which is dependent on solvent quality and volume as well as polymer concentration and size.[Citation26,Citation27] Altering the quality of the solvent (e.g., adding sugar to water), results in changes to the extent of mixing in the mixed biopolymer system. This leads to mixed biopolymer systems that contain sugars having a different morphology to those formed in an aqueous environment.[Citation25]
Some work has been carried out into investigating the structure of confectionery gel systems [Citation28,Citation29], as well as thermomechanical behaviour of such systems.[Citation25,Citation29,Citation30] Moisture content and water activity can also have an effect on the behaviour of these gels, with the equilibrium relative humidity of confectionery gels being 65–75%. Due to the complexity of these systems, and the interactions of confectionery gel system components, limited information is available on quantifiable structure-property interactions of these gels.
CONFECTIONERY GEL FORMULATIONS
A range of gelling agents can be used in the manufacture of confectionery gels, which include: agar, starch, pectin, alginates, and gums.[Citation31] However, the majority of gel confectionery consists primarily of sucrose, glucose syrup, starch, gelatin and water, with a number of minor components including food acids, flavourings, and colourings. Pectin may also be used as a gelling agent.
Sucrose and Glucose Syrup
Sucrose used in food is often in granule form and is used as a sweetener. It is often used in concert with glucose syrup, as the syrup can enhance sucrose solubility and retard sucrose crystallization in food products.[Citation3,Citation32] It serves to contribute to the texture and sensory properties of the gels and may be used to increase product bulk or weight, giving body or mouthfeel to the product. The form of sucrose used in jelly and gum confectionery manufacture is non-crystalline.[Citation33,Citation34]
Glucose syrups refer to products with a dextrose equivalent (DE) of between 20 and 80 (where 100 indicates pure glucose and 0 indicates no glucose). Products with a DE of less than 20 are termed maltodextrins, and those with a DE of greater than 80 are called hydrolysates or hydrols. Glucose syrup is specified by its DE, carbohydrate composition, solids, and sulphur dioxide content in order to allow manufacturers to produce a product exacting to customer requirements.[Citation31] Traditionally, 42DE glucose syrup is used in confectionery to prevent sucrose crystallization [Citation31,Citation35] to meet the requirement of confectionery that it must not undergo any change in physical properties during storage. Use of higher viscosity glucose syrup (lower moisture or higher DE) will slow sucrose molecule migration and inhibit graining.[Citation31,Citation36]
Glucose syrup is essentially shelf-stable and no preservatives need to be added to prevent microbial growth, due to the high dissolved solids content which reduces the water activity to below the level required for microbial growth.[Citation31] Because confectionery must not undergo fermentation, mould growth or other microbiological spoilage during a long storage life, glucose syrup also aids in promoting a lower water activity in confectionery gels, thus preventing microbial growth.[Citation35] Glucose syrup is used in the production of jelly and gum confectioneries, where its principal role is as a sweetener, although it also contributes to texture and microbial stability, as well as stabilizing other ingredients, e.g. sucrose or gelatin.[Citation32,Citation37] The gelling agents (e.g., starch, gelatin) in CGs usually make up approximately 10% of the food gel, and the rest consists of glucose syrup and sucrose. Confectionery products can often contain more glucose syrup than sucrose.[Citation3]
Water
Water is a major constituent in many foods, supporting chemical reactions and acting as a reactant in hydrolytic processes,[Citation38] so removing water or binding it by increasing the concentration of common salt or sugar in foodstuffs inhibits many reactions and retards micro-organism growth, aiding to improve shelf life of the food product.[Citation32] The physical interaction of water with proteins, polysaccharides, lipids, and salts can contribute significantly to food texture, as foods tend to become plastic when their hydrophilic components are hydrated. The water content can affect glass transition temperature of a food (the temperature where the material goes from glassy to rubbery behaviour).[Citation32] In confectionery gels, water often acts as a plasticizer to aid gel formation.
Studies by Cornillon, Andrieu, Duplan, and Laurent[Citation39] showed bound water values in sucrose/starch/gelatin systems which were: sucrose, 0.05 g/gDM; starch, 0.26 g/gDM; gelatin, 0.44 g/gDM, where g/gDM denotes grams of water per gram of dry material. The study shows that gelatin has the highest affinity for water when combined with sucrose and starch, and hence requires it to hold its integrity.[Citation32] The physical state of metastable foods can depend on composition, temperature, and storage time. Water can affect their properties, causing them to be glassy, rubbery, or viscous by altering glass transition temperature. Variations in moisture content can lead to quality variations such as premature crystallization, stickiness, accelerated rancidity, lack of body, difference of chew, hardness, poor handling on forming and cutting machines, and product flaws in surface texture.[Citation31]
Starch
Starch is present in most plant tissues, laid down in granular form in defined cells,[Citation35] as a storage carbohydrate. The major botanical sources of industrial starch are tubers, grains and pulses,[Citation40] with the composition and place of storage of the granules, as well as granule shape and size being specific to the plant source.[Citation41] Starches from various origins have characteristic properties that are related back to granule size distribution.[Citation32]
In addition to its role in plant physiology, starch is the most important source of carbohydrates in human nutrition[Citation42] and is widely used, alone or in conjunction with other gelling agents, to thicken and bind foods,[Citation32] and to provide a wide range of jelly and gum products. Native and modified starches are important ingredients of many fabricated foods [Citation40] and are often added to semisolid food products to contribute to their structure, and thus improve fat and water-holding properties.[Citation43]
Composition and structure of starch
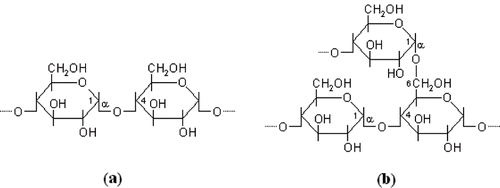
Starch granules consist of starch, moisture and small amounts of lipids and protein. The starch consists of two main components: amylose and amylopectin.[Citation32,Citation41] Amylose is a predominantly linear α(1–4)-glucan (), whereas amylopectin is a highly-branched molecule consisting of an α(1–4)-glucan chains with α(1–6) branch points ().[Citation44] The proportions of amylose and amylopectin in the starch granules, as well as structure of the molecules (e.g. average molecular weight, frequency of branching in amylopectin, naturally occurring level of phosphorylation) also depends on plant source.[Citation41]
The role of starch in confectionery gels is to provide the base of the gel structure, and hence many of the gels' textural characteristics. Confectionery gels often contain “thin boiling starches.”[Citation32,Citation33] These starches are formed by adding a small amount of acid to a starch suspension and heating at a temperature below the starch gelatinization temperature producing starch hydrolysis.[Citation31] When the required degree of chain concision is achieved, the mix is neutralized and the starch filtered off and dried.
The acid hydrolyses some bonds within the starch, leading to a less linked structure and a reduction in molecular weight of some of the chains. This causes the starch granules to be readily soluble in boiling water [Citation32] and disintegrate when cooked to give a lower hot paste viscosity and higher gel viscosity than non-acid modified starches.[Citation43–45] This type of chemical modification is done to alter the nature of the interactions between polysaccharide chains in the starch.[Citation43,Citation44] The differences between native and acid modified starches are varied depending on the acid concentration used as well as time for acid hydrolysis.[Citation46,Citation47] Acid-thinning can “roughen” the surface of starch granules, as well as decrease granule amylose content, as amylose is more easily cleaved by acid hydrolysis than amylopectin.[Citation48] This causes a drop in crystallinity of the starch, and hence a more amorphous material, which causes starch processing for food applications to be easier.
Starch gelatinization
Starch gelatinization is often a major step in starch processing and is loosely termed as a loss of starch crystallinity, coupled with granule swelling.[Citation43,Citation49,Citation50] Starch gelatinization occurs when aqueous suspensions of starch granules are heated to above their gelatinization temperature Tgel, then cooled to form a rubbery gel ().[Citation51,Citation52] When suspended in cold water, air-dried starch granules swell increasing in diameter by 30–40%. When heating is applied to these swollen granules, irreversible changes occur at Tgel,[Citation32] for native starches, often 60–70°C at 30% moisture.[Citation53] At Tgel starch granules swell further, and amylose begins to leach out of the granules. On cooling, the amylose forms a gel network, whilst amylopectin remains in granules during moderate cooking.[Citation35,Citation54] Starch gels can be composite gels as the matrix and granules have distinct, different properties. Swollen granules are remarkably elastic and give textural body to the gel.[Citation55] Depending on severity of processing and the starch source, starch gels can have varying structures, including: 1. amylose with water entrapped in a three dimensional network; 2. highly swollen and fragmented granules present in a gel matrix; 3. highly swollen and intact granules in a gel matrix; and 4. unswollen and intact granules.[Citation56]
Figure 4 The starch gelatinization mechanism. a) Starch granules; b) Swelling of granules upon application of heat and moisture; c) Leaching of amylose; and d) Creation of starch gel matrix (modified from Remsen and Clark[Citation52]).
![Figure 4 The starch gelatinization mechanism. a) Starch granules; b) Swelling of granules upon application of heat and moisture; c) Leaching of amylose; and d) Creation of starch gel matrix (modified from Remsen and Clark[Citation52]).](/cms/asset/84db35b0-ae26-49e6-8343-05c1bcb3ebec/ljfp_a_322507_o_f0004g.gif)
On cooling and re-melting of starch gels, they have been found to consist of a crystalline phase and two immiscible liquid phases.[Citation57] However, this may be dependent on moisture content; Yuryev et al.[Citation1] stated that at high water contents (> 70%) starch gels are biphasic, whilst at low water contents (15–40%) they are monophasic. Tolstoguzov[Citation58] found that amylose and amylopectin can phase separate in starch gels. In acid-thinned starch the gelatinization behaviour is altered depending on the native starch source. In the case of barley and maize starches, acid modification can weaken gel formation if heating is to 90°C, with the weakness due to increased amounts of amylopectin in the continuous phase of hydrolyzed starch pastes [Citation47]; heating hydrolyzed barley starch to 98°C can overcome this problem as the amylose in the granules is liberated more easily.
The gelatinization temperature and the breadth of the gelatinization endotherm (range of temperatures over which starch is gelatinizing) have been shown to increase on acid hydrolysis of starch.[Citation59] Acid modification has been found to increase solubility and gel strength and decrease viscosity of starches.[Citation60, Citation61] The viscoelastic properties of starches are also affected by acid hydrolysis. Virtanen et al.[Citation47] reported that the gel of acid modified oat starch, although less rigid, was more elastic than the corresponding native starch gel.
Composition and structure of starch gelatinization in high sugar systems
Many factors affect the process of starch gelatinization and gel formation and so starch gelatinization must be carefully controlled to give a product with preferred consumer attributes, such as texture.[Citation62] Water concentration affects the heat treatment required to gelatinize starch. Starch requires approximately 30% moisture to fully gelatinize, any lower and the gelatinization extent reduces whilst the gelatinization temperature, Tgel, will rise.[Citation53] Some studies have shown that the temperature profile of the starch gelatinization process, and the moisture present, can be used to control the degree of gelatinization, with little effect on final mechanical properties.[Citation63] The presence of sugar can also affect starch gelatinization. Sugar type and concentration can have several effects, with the most important being to raise starch Tgel. In limited water (<30%), starch Tgel increases with decreasing moisture content. When sugar is dissolved in the water being used for gelatinization, part of the water is bound by the sugar, effectively decreasing the moisture content, leading to an increased starch Tgel.[Citation53,Citation64]
It has been proposed that upon reduction of available water, a point is reached at which the limited extent of starch granule swelling is insufficient to disrupt the starch granule completely.[Citation65] Adding 12% or 24% sucrose has been found to delay swelling of wheat starch granules at up to 50°C; above 50°C amylose leakage and granule fragmentation is still delayed, but swelling is accelerated.[Citation55] There are several theories as to why sugars inhibit starch gelatinization, and how and where the sugar acts to inhibit starch gelatinization. One theory is that sugar molecules interact with starch granule amorphous regions and increases energy required to melt them, while another theory is that sugar displaces water inside the starch granules, therefore inhibiting one of the promoters of starch gelatinization. Sugars are also thought to stabilize crystalline regions in the starch and hence immobilize water molecules, hindering starch gelatinization.[Citation53]
Starch retrogradation
Upon completing gelatinization, the starch now has a gel structure; however this is a metastable structure, and is subject to changes over time, depending on its molecular configuration. Starch molecules are known to associate on ageing, and crystallites form.[Citation40] This is a form of retrogradation, and syneresis (separation of liquid and solid) can occur in conjunction.[Citation66] While acid-thinned starches have shown slower retrogradation rates than native starch [Citation32], they can display an increase in retrogradation rate with increased hydrolysis.[Citation46]
Retrogradation affects quality, acceptability, and shelf life of starch gelled products.[Citation66] It is often detected during storage where the starch gels become firmer, which is associated to the retrogradation and syneresis,[Citation35] often in the form of amylopectin recrystallization.[Citation67,Citation68] Retrogradation can usually be quantified through differential scanning calorimetry (DSC) studies where the movement of the glass transition temperature Tg (the temperature where a material changes from an amorphous to a crystalline structure or vice versa) can indicate the growth of crystalline areas in the starch gel.[Citation21,Citation69]
In gelled starch, sugars tend to help stabilize the structure, inhibiting chain reorganization (also known as recrystallization or retrogradation), where starch molecules reorganize into their crystalline form, though they do not achieve the structure found in the native starch granule.[Citation70] Waxy maize and wheat starches have been found to be the more sensitive to this effect. The order of stabilizing effect provided by different sugars is sucrose>glucose>fructose.[Citation71]
Gelatin
Gelatin is a thermoreversible gel formed in aqueous solvents through lowering the temperature.[Citation72] It is derived from collagen via controlled acid or alkaline hydrolysis. Collagen may come from hide, bone, or other collagenous material.[Citation32] Commonly, the collagen used is of bovine (cow), porcine (pig), or piscine (fish) origins.[Citation73] The properties of the gelatins derived are affected by the source, age and type of collagen.[Citation45] Gelatin has a role as a gelling agent, providing texture and water binding properties to food materials. Like starch, it is one of the most widely studied functional biopolymers.[Citation74] The functional properties of gelatin depend on the collagen source, and the extraction process used, both of which affect molecular weight of the gelatin product.[Citation32]
Composition and structure of gelatin
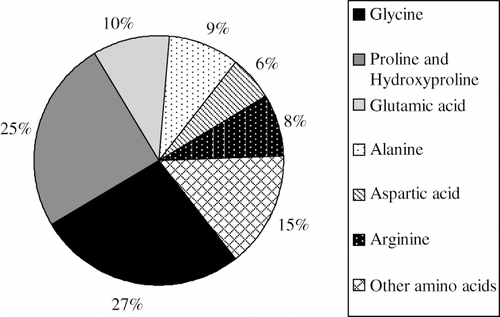
A typical gelatin consists of 14% moisture, 84% protein and 2% ash.[Citation45] The protein portion consists of several different amino acids and an approximate composition of gelatin is shown in . The major amino acids present are glycine, proline and hydroxyproline.[Citation18,Citation73]
These amino acids are arranged in gelatin gels to form long molecular chains, similar to the collagen source from whence they came. These chains then form structures, which then interact to lead to the overall gelatin gel network structure. Gelatin molecules contain repeating sequences of glycine-X-Y triplets, where X and Y are frequently proline and hydroxyproline amino acids. The glycine is said to be responsible for chain flexibility.[Citation75] These sequences lead to a triple helical structure in gelatin and its ability to form gels where helical regions form in the gelatin protein chains immobilizing water. This triple helix structure is synonymous with many proteins. The basic macromolecular unit of collagen in which the individual chains are wound in a gentle superhelix around a common molecular axis,[Citation72] which to some extent carries through to the gelatin structure. These helices are stabilized by hydrogen bonds perpendicular to their axes.[Citation76] The triple helices interact via secondary forces to create a three-dimensional network. The arrangement and appearance of this network is dependent on processing methods by which the gelatin becomes a gel.
Gelation of gelatin
Gelatin can undergo thermoreversible gelation at protein concentrations > 2–3%. The sol-gel transition of gelatin has been studied extensively and has been shown to be affected by many different factors.[Citation77–80] A gelatin solution, which can eventually produce a gelatin gel, is normally formed through either an indirect solution or a direct solution. To form an indirect solution, gelatin particles are added to cold water just ensuring that particle wetting and that the granules swell until a soft friable mass is formed. A gelatin solution is formed when this mass is heated to 50–60°C. Constant stirring of the solution aids dissolution.[Citation73]
Forming a direct solution is the more common method, since it eliminates the cold soaking stage in the indirect solution method, thereby reducing production time. This method requires higher temperatures (60–80°C) and high speed agitation to prevent clumping when gelatin is added to the water. Water is heated, and stirred vigorously enough to create a vortex. Gelatin powder or granules are then slowly added, with stopping and stirring to ensure even dispersion and dissolution. After all the requisite gelatin is added, extra stirring is carried out to ensure complete dispersion of the gelatin in the water.[Citation73]
There are several theories about how formation of gelatin gels occurs. Many of these may be valid for the types and concentrations of gelatin, and processing methods, used in confectionery gels. Above 40°C gelatin in solution behaves like a typical synthetic polymer with the individual macromolecules each assuming random-coil configurations [Citation79] with typical molecular weights of 2 × 105 Daltons.[Citation77] These random coils consist of single polypeptide chains, termed α–chains that may be entangled. Upon cooling these coils undergo a random coil-to-helix transition, leading to gelation.[Citation79] These solutions may then be cooled to form a gelatin gel. This occurs through a sol-gel transition.
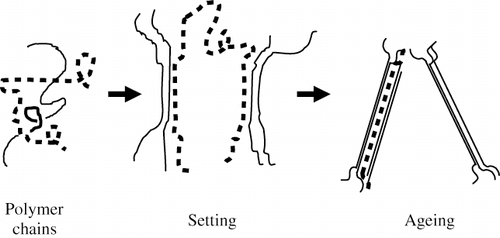
The two important steps in gelatin gelation which are often termed setting and ageing. Setting involves the linking together of irregular regions on the triple helices to form a network throughout the whole gelatin solution. Ageing is the step where gel strength is developed -it appears to go on indefinitely, however the rate of gelation decreases with time at a constant temperature ().[Citation79] Ageing involves two mechanisms: first, there is continuous adjustment of the molecular network, through motions of the chains between links; and second, by the dynamic nature of hydrogen bonding. The original interchain links formed in the setting stage are strengthened in this way, as only the strongest bonds of these can survive at constant temperature. These links are extended by incorporating adjacent parts of chains.[Citation76] Occurring simultaneously is the linking together of adjacent collagen-like regions, the network thickens and becomes more fibrillar. The setting process of gelatin is naturally slow and problems of too rapid aggregation are not normally encountered.
The ageing step shown in takes a long period of time, and in confectionery gels, it is believed that interactions between gelatin and other components would slow this process — as sucrose and glucose syrup tend to stabilize gelatin structure. Sugars are good at enhancing the stability of conformationally ordered junctions in gelatin gels.[Citation23] Finer, Franks, Phillips, and Sugget[Citation79] studied pure α-chain gelation and found that random coils in the gelatin sol would take some time to become nucleated random coils, but after this stage would rapidly gel into a rigid state molecule. The gelation process is connected to conformation transitions of protein chains at <36°C. The triple helix structure of the collagen can be partially recovered, but can depend on local thermal history.[Citation7]
It has been suggested that the most suitable technique to investigate the sol-gel transition of gelatin is rheological analysis, particularly small angle oscillatory measurements, as viscosity and elasticity can be measured without disturbing the gelation process if carried out carefully. Some rheological results on gelatin gelation have shown various phases or steps in the gelatin gelation process and displayed that with the growth of G′ (storage or elastic modulus) the gelatin system has become more solid-like.[Citation74]
Factors affecting gelation of gelatin
Many of the factors affecting starch gelatinization also affect gelatin gelation. With regards to temperature, since gelatin is a thermoreversible gel, gelling temperature, and temperature gradient during cooling from the sol-state and temperature fluctuations during the gelation process all affect product properties.[Citation81] The lower the ageing temperature, the faster the helix content within the gel will increase, but the lower the stability of the helices will be.[Citation80] A small increase in temperature leads to melting of some junction zones, along with formation at new ones.
The presence of water aids gelatin gelation, however the higher the solids (which could be gelatin granules, powder or leaf) content of the gelatin solution precursor, the faster the gelatin gel will form. The initial change in modulus with time tends to become steeper as gelatin concentration increases, and the modulus plateaus sooner with higher concentrations.[Citation82] Shear can also have an effect on gelatin gelation, and some studies have shown that viscosity of the sol-gel can depend on a combination of shear rate and the probability of bonding occurring.[Citation78,Citation81]
The presence of other ingredients during gelatin gel formation can affect the gelation process as well as final properties of the gel. When mixing gelatin with gelatinized starch, there is the possibility that amylose leaches into the gelatin phase — perhaps forming a composite and affecting crosslink formation in the gelatin gel. Polymer blending laws indicate that if the concentration of leached amylose is significant in a starch-gelatin-composite system, then the strength of the system may be weaker than a pure gelatin gel due to crosslink inhibition in the gelatin phase.[Citation21] Many factors can affect gelatin gel structure and strength, which in turn affects gel stability.
The final gel, after gelation is complete, is a clear, orange-tinged gel with elastic properties. The specific properties of the gel should indicate what use it is good for. One of the measured properties of a gelatin gel is Bloom. Bloom is determined by measuring the mass required to press a 4 mm diameter probe to a depth of 12.5 mm into a 6.666 w/w% gelatin gel at 10°C. It is the weight that mass, expressed in grams, that is the Bloom number. The Bloom number will determine what application the gelatin is used for, and is set by the method used for extraction of gelatin from collagen, and the conditions under which this extraction is carried out.[Citation73]
As with starch, sugars also serve to stabilize the gelatin gel configuration. Sucrose can help with gelatin dissolution, and stabilize the final product.[Citation23] This is because sucrose/glucose syrup blends can establish a continuous liquid phase with gelatin.[Citation23,Citation83] The presence of sugar co-solutes can increase the strength of gelatin gels up to a peak co-solute concentration, above which the gel weakens due to a lack of water available to maintain gel integrity.[Citation23,Citation83] When combined with typical confectionery gel ingredients of starch, sucrose, and water, gelatin tends to retain bound water more easily than the other ingredients.[Citation39]
Ionic force and pH can also have an effect on gelatin gel properties,[Citation84] where pH can affect turbidity (transparency) of gelatin, with the transmittance of gelatins dependent on the isoelectric point of the gelatin.[Citation73] The isoelectric point is the pH at which no net migration occurs within the gelatin when placed in an electric field, and it is dependent on the process for forming the gelatin from collagen, as well as collagen source. A low pH can lower viscosity of gelatin solution during processing due to degradation effects. This pH degradation effect is amplified at high temperature and hence food acids are usually the last ingredient to be added to confectionery gel products during manufacture, usually during cooling.[Citation45,Citation73]
Pectin
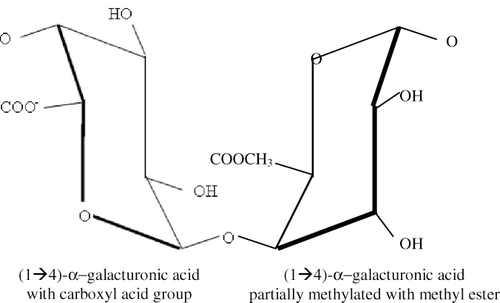
Pectin is found in virtually all land-based plants and is a structural polymer, the intercellular “glue” that helps to reinforce the basic cellulose structure of plant cell walls.[Citation85,Citation86] Commercial pectin is extracted under mildly acidic conditions from citrus peel or apple pomace (dried pulp) and sometimes from sugar beet residues or sunflower heads.[Citation86,Citation87] The chemical structure of pectin consists of a linear chain of galacturonic acid units and can have a molecular weight of up to 150,000 ().[Citation85,Citation86]
While still in the fruit, there is, on average, one free acid group (COO-) to every three to four methyl esters of galacturonic acid; although, there is no repeating sequence within the polymer chain. This corresponds to a degree of esterification of 70–80%. Esterification can be controlled during the extraction process, so that the degree of esterification of the final pectin product can range from 0–75%. It is the degree of esterification and the arrangement of methyl esters along the pectin molecule that controls how the pectin behaves as a gelling agent or protein-stabilizing agent.[Citation87]
Pectin with a degree of esterification of < 50% is termed low ester or low methoxy pectin and does not require either sugar or acid to gel,[Citation31] but does require calcium to aid gel formation.[Citation87] Pectin with a degree of esterification of > 50% is termed high ester or high methoxy pectin, and requires the presence of sugar and acid to set to a firm gel. Grade strength of this type of pectin is determined by the ratio of sucrose:pectin required to form a gel of a particular strength.[Citation31] It is the high methoxy form of pectin that is widely used in the manufacture of jam and gel confectionery.[Citation28,Citation88]
Commercial pectin used in food production often has sugar added to standardize viscosity and to prevent clumping when the pectin is added to water. Gel formation is carried out by addition of the pectin, with added sugar, in a thin stream to well-agitated water. As pectin absorbs water, the mixture thickens and gelation depends on acidification to the correct level of pH, as well as on the concentration of dissolved sugar. Gelation of pectin occurs very rapidly, so pectin gel confectionery is able to be produced in a continuous manner.[Citation31,Citation33] Recent advances in the study of pectin have uncovered a wide range of physical pectin structures that may be used for a variety of applications, including confectionery, and this is discussed in greater detail in an excellent recent review by Willats, Knox, and Mikkelsen.[Citation89]
Other Confectionery Gelling Agents
Recently there has been growing interest in the use of bacterial polysaccharides for gel formation as procedures for forming these polysaccharides on a larger scale are developed. Gellan and curdlan are examples of such materials and they have been proposed as useful gelling agents in confectionery.[Citation3,Citation35,Citation90–92] Gellan is a linear, anionic heteropolysaccharide, with a molecular weigh of approximately 5 × 105 Da. The structure contains of tetrasaccharide repeat units consisting of 1,3 – β-D-glucose, 1,4-β-D-glucuronic acid, 1,4 – β-D-glucose and 1,4,-α-L-rhamnose, along with acyl groups on the 3-linked glucose. If left acylated, gellan forms soft, elastic, transparent and flexible gels but once de-acylated it forms hard, non-elastic brittle gels.[Citation3,Citation91] Gels formed from gellan are usually very stable over a wide pH range, while an increase in sugar or ion concentration can greatly increase gellan gel strength.[Citation3,Citation35,Citation91]
Curdlan is a moderate molecular weight unbranched linear 1,3-β-D glucan with a molecular weight of approximately 100,000 with no side-chains. An advantage of using curdlan as a gelling agent is that gels of differing strength are formed depending on the heating temperature, time of heat-treatment and curdlan concentration.[Citation90] It is commonly used in Japan to improve texture of tofu, bean jelly and fish paste and may have other applications in confectionery jelly.[Citation92]
Food Acids
Food acids are added to confectionery gels primarily to give a tart tangy taste, and in the case of pectin, to aid gel formation. The choice of food acid is related to the desirable level of sharpness and the likely effect of the acid on rather raw materials present. Common organic food acids used are citric acid, malic acid, tartaric acid, adipic acid and fumaric acid. Lactic acid may also be used, particularly when gelatin is added to fermented milk.[Citation73] The most commonly used acid is citric acid as it causes the least degradation in other food materials used in food products, and it is the food acid being utilized in the gels being studied. The other acids are used in foods to achieve a different flavour profile to citric acid.
Citric acid occurs naturally in lemon juice. It is produced by fermentation through the action of certain moulds on sugar syrups. It is commercially available in anhydrous form and as a monohydrate, is odourless and colourless and dissolves readily in water. In confectionery manufacture, a 50% citric acid solution is commonly added near the end of confectionery gel processing to avoid the harsh combination of acid and high temperature affecting the other ingredients.[Citation36, Citation45] All fruit acids have the ability to break down sucrose to invert sugar, which is a mixture of dextrose (glucose) and laevulose (fructose).[Citation31] Food acids also lower viscosity in gelatin,[Citation73] as well as cause coacervation of gelatin in mixed gels. The food acids alter the pH of the confectionery gels, and this acidity has some preservative action which helps shelf stability of the gels.[Citation36]
Colourings
Colourings make CGs more attractive to the consumer. There are three main categories of food colourings.
| • | Synthetic — no similar natural colour. | ||||
| • | Nature identical — synthetic material but identical to a natural colour. | ||||
| • | Natural — obtained from plants or animals.[Citation36] | ||||
It is assumed that colourings have little effect on the mouthfeel/texture of the gels, only affecting appearance properties and perhaps flavour perception.[Citation3] Most colourings are water soluble and are added in very small amounts <1%) during confectionery gel processing. The choice of colour depends on many factors, which can include the effect of highly acid conditions on colour stability, and the presence of protein and various other ingredients which can affect colour intensity.[Citation31]
Food standards of the relevant jurisdiction will affect the choice and concentration of colouring agents.[Citation3] Other factors that influence colour choice include those that affect colour stability and appearance, including: excessive heat and light exposure; use of strong acid; reducing ingredients such as sugars; proteins that bind some types of colours; strong light, preservatives, background colour of raw materials, trace metallic contaminants, and micro-organisms. Colour defects that can arise from non-conducive conditions include discolouration, fading, loss of sparkle, which can be affected by supply variations, inadequate mixing, and poor storage.[Citation31]
Flavourings
Chemically, flavourings are very complex substances, and like colourings they are divided into the three main groups of synthetic, nature-identical, and natural.[Citation3] Flavourings can be essential oils obtained from basic fruit or spice, or a wholly synthetic mixture produced by blending approved chemical materials. Flavouring agents are added at the last moment of production, as they are volatile and can be affected by high temperature.[Citation31] Flavourings must be dosed into the product accurately and blended well to ensure even flavour dispersion. The flavour compounds can be intense, and so are diluted before incorporating into the food product. Natural flavour compounds are more volatile, less stable, than synthetic flavour compounds, but are perceived as better, healthier, and safer.[Citation3]
CONFECTIONERY GEL FORMING PROCESSES
Currently most CG products are prepared in the food industry via a multi-step batch process. This process consists of several mixing and heating stages, and a prolonged drying period ().[Citation3,Citation93] Confectionery gels processes serve to gelatinize the starch in the product and blend the sugars, starch gel and gelatin gel together. The ‘cooking’ step can be attained through a range of manufacturing methods using different apparatus.
Figure 8 Schematic of confectionery gel process (modifed from Edwards[Citation3]).
![Figure 8 Schematic of confectionery gel process (modifed from Edwards[Citation3]).](/cms/asset/96591011-8f1c-42ed-9c56-380cfcaec54f/ljfp_a_322507_o_f0008g.gif)
Batch Processes
If starch is being used as a gelling agent, this step is often used to form the starch gel, as well as dissolving and concentrating the sugar ingredients. Common cooking methods used in industry include jet cooking,[Citation3,Citation94] coiled heater cooking,[Citation95] and the more traditional, and now less common, open-pan boiling.[Citation36] Jet cooking involves the injection of high temperature steam at pressure to cook the confectionery gel ingredients. The mix of ungelatinized starch suspended in the sugar solution is often preheated to 70–80°C, prior to being fed into the jet cooker. The jet cooker is often a small vessel, in the order of litres, with a short residence time. Alteration of pump rates and steam valve back pressures can alter the amount of starch gelatinized, and hence, the degree of cooking. This process can be very efficient as both sensible heat and latent heat of the steam can be used to cook the gel ingredients. The main disadvantage is that the steam becomes an ingredient and so care must be taken with boiler water, so it can be of sufficient food grade.[Citation3]
Coil cooking involves passing the carbohydrate slurry through a coiled length of pipe, which is often heated by high temperature steam. Studies have shown that heating and the amount of starch gelatinization is highest in tightly wound coils versus being lowest in straight tubes. At low coil diameters, the increase in relative viscosity of the carbohydrate solution is the highest.[Citation95] The increase in starch gelatinization could be due to the introduction of increased shear, via a more convoluted flow path, and hence higher destruction of starch granules.
These processes can stand alone as continuous processes, but because of the presence of the stoving step and limited resources (ovens) and time associated with this step, the overall process must be carried out in batches. For the Australian confectionery gels mentioned here, there is a “blending” step prior to the ‘depositinG′ step, whereby a gelatin solution is mixed with the cooked carbohydrate ingredients, which can be done via large mixing tanks or complex inline mixing apparatus. Additives are also added prior to depositing.’
Depositing often involves moulding of the cooked gel into starch moulds. The stoving step is necessary to reduce the moisture content of the final gel products down to an acceptable level, whereby microbial activity will not occur. Stoving may involve drying at room temperature for 4–6 days, or oven drying for 2–3 days. This step within the process is time and resource-intensive, with high wastage, and there is a need for reducing the time taken to carry this out. The rate of stoving is determined by: size of the jelly product; viscosity of the deposited gel; degradation temperature; and moisture diffusion limitations.[Citation3] Moisture content of the moulding starch will also have an effect, as the moisture gradient between the gel and the starch will determine diffusion rate, as well as moisture diffusivities in these materials.
Continuous Processes
A trend in recent times has been to develop continuous processes for making food products. Gel confectionery can be formed using a scraped-surface heat exchanger,[Citation33] however, in recent times research into confectionery gel formation has turned to some of the more traditional synthetic polymer processing techniques for inspiration. Two of these techniques are injection moulding and extrusion processing.
Extrusion of confectionery gels
Extrusion processing is becoming increasingly important in food processing and is characterized by diverse applications. The technology is more available than it was in the past, but the science base is difficult to generate because of complex applications.[Citation96] Extrusion is a series of unit operations [Citation97] including: solids and liquid conveying, melting, pumping (mixing), forming, and cooling. Extrusion processing is not completely new to the food industry and has been utilized to produce pasta for more than 60 years.[Citation98] It is widely used in the food industry for products such as pastas and breakfast cereals, due to its versatility, high productivity, low cost and energy efficiency, resulting in the potential for improved cost-effectiveness when compared to traditional processing methods.[Citation99,Citation100]
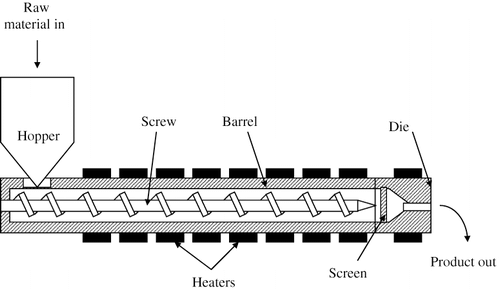
A screw extruder consists of several main features-a barrel, a screw or screws, heating source, feed hopper and feed ports, a drive, and a die where the extruded product emerges (). The process begins with feeding, whereby granules or powder are placed in the feed hopper and metered down into the screw or screws to be conveyed; liquid may also be injected to promote plasticization. The extruder screw/s has three main zones which are the feed zone, the transition zone and the metering zone. The appearance and physical configuration in each of these zones may differ, to carry out the relevant operations.[Citation101]
The screws can be shaped as a solid piece, or they can be modular with interchangeable screw elements. Modular screws are particularly common in twin screw extrusion. Depending on what type of material manipulation is required a screw configuration can be “built” to achieve this. Elements that just have a basic screw profile are used for conveying, whilst more intricate screw elements such the angled paddles, are used for mixing, with reverse elements providing some of the most intense mixing.[Citation102]
Extrusion cooking allows starchy and proteinaceous food materials to be cooked and formed in a single continuous high temperature short time (HTST) process.[Citation103] It has been studied as a processing technique to process starch,[Citation98,Citation104–106] starch-gelatin composites,[Citation107] pectin-starch composites,[Citation63] fruit-starch gels,[Citation108] and due to its flexible nature as a processing technique, it has the potential to extrude other systems containing biopolymers.
Injection moulding of confectionery gels
Injection moulding involves a similar setup to the extrusion process, but with the use of a die capable of being closed and pressurized to form shapes, rather than a continuous extrudate. It has been studied as a viable process for moulding 85% solids gelatin fruit gums containing 40–60% gelatin. In the study, the parameters of die cylinder temperature, mould time, and plunger dwell time were optimized to give suitable gum products. The main difficulty in the process seemed to be the slow gelling of gelatin, and its low susceptibility to frozen in stress. The gums had strong relaxation on demoulding, indicating that processing parameters had a lesser effect on product properties and all the products formed under different circumstances had similar characteristics.[Citation109] Current processing methods require controlled time and energy. For injection moulded gums, one minute in the mould seemed appropriate to give the requisite gum textural characteristics. However, this amount of time would also be a hindrance unless several hundred products could be moulded with each die cycle. For this reason, extrusion processing may be a better option as a continuous process for confectionery gels containing gelatin. The product continuously emerges in extrusion processing, and there is no waiting for process cycle times.[Citation109]
The rational development of food extrusion processing is dependent on understanding the relationship between microstructural changes of the cooking material, equipment design, and processing conditions. Knowledge of melt rheological characteristics is a prerequisite to performing design calculations on extruder screws and dies and modeling the overall extrusion process.[Citation103] Parameters such as barrel temperature, screw speed, water content and feed rate can be optimized to obtain the required product.[Citation98,Citation108]
In extruders, starch material is modified all along the screw shown by an intrinsic viscosity decrease, and increase in degree of gelatinization. In single screw extrusion this happens progressively, but in twin screw extrusion there is a characteristic very short melting zone,[Citation105] powder is compacted and dissolved, from which a polymer + melt mix is evolved, then a homogenous molten phase is obtained in a very short time (3–10 s) at high shear rate (approximately 100/s).[Citation110] Transformations occurring here are difficult to analyse experimentally with respect to instrumentation and sampling, as melting is initiated as soon as the first restrictive screw element (die, reverse screw, kneading block) encountered after feeding.[Citation110]
GEL CHARACTERIZATION TECHNIQUES
In order to understand gels; how they form; what properties they have; and what causes the behaviour of these properties; it is necessary to characterize gel properties. The relevant properties of jellies for processing and eating quality are: rheology which can affect pumping and transport during processing, molecular structure, molecular weight, and texture, which gives an indication of gel eating quality.[Citation3]
MICROSTRUCTURE
Foods have microstructure that is imparted to them by nature or through processing. Structure is important, as almost all relevant food properties are structure sensitive, reflecting interactions of components and structural elements occurring at different levels in food.[Citation111] Microscopy can be used as a technique to better explain published physical properties, such as rheological or texture measurements.[Citation112–114] Microstructure has been practically ignored by food engineers in the past for several reasons, particularly:
| • | The emphasis of the food industry in the last century has been on designing safe and reliable processes for large volumes of traditional products. | ||||
| • | Innovation and quality has not been a major driving force in the food market until recently. | ||||
| • | Food engineers knew little about the underlying sciences linking food structure to product properties. | ||||
| • | The techniques aiding the study of microstructure were not developed to the point that they could provide useful data (e.g. quantitative information) to engineers.[Citation111] | ||||
Because of this, attempts to observe food microstructure and link food properties to this have been made only recently. Advances in computer technology and development of more sophisticated structure analysis programs have allowed the rapid analysis of microstructural images, greatly expanding their use. Now it is possible to perform several measurements on the structures, which include:
| • | feature specific measurements (area, length, perimeter, volume, surface area, grey level, etc.); | ||||
| • | shapes (form factor, aspect ratio, fractal dimension, number of holes, convexity, and solidity); and | ||||
| • | global measurements (area fraction, line length and curvature etc).[Citation115] | ||||
Light microscopy (LM) may be useful in observing phases in CGs; however, it does not have the resolution of scanning electron microscopy (SEM) and transmission electron microscopy (TEM). It is, nevertheless, a quick and simple method to check what is observable within the gel microstructure before a more complex method is utilized to examine it further. Light microscopy has proved useful in observing the microstructure of agar gels,[Citation116] as well as confectionery gel systems (), which helped in determining the structural composition of the gels.[Citation28]
Figure 10 Structure models of three jelly pastille products (modified from Groves 2003 [Citation28]).
![Figure 10 Structure models of three jelly pastille products (modified from Groves 2003 [Citation28]).](/cms/asset/5a0118d7-140a-488f-b4a5-37481ec9957c/ljfp_a_322507_o_f0010g.gif)
Bright field and fluorescence LM are frequently used because they allow selective staining of different chemical components. Cryo-sectioning involves freezing, and ice crystals may damage the structure. Plastic embedding involving dehydration can cause shrinkage. Confocal Scanning Laser Microscopy (CSLM) offers more advantages in that there is minimal sample processing, and it can yield three-dimensional images. Electron microscopy is required to investigate fine details.[Citation117]
Scanning Electron Microscopy (SEM) is a useful tool for examining the surface of microstructural components.[Citation118] Samples are mounted on stubs, sometimes fractured, and coated with a metal compound.[Citation119] Environmental Scanning Electron Microscopy (ESEM) allows viewing of the surface of a hydrated sample without the need for coating; this is possible by observing the samples under vacuum.[Citation118]
Transmission Electron Microscopy (TEM) is a method that may be used to view the microstructure of a thin sample by passing electrons through it. In the case of food gels, the section must be fixed and stained with a heavy metal compound to provide contrast between the various components.[Citation115] A successful method of carbohydrate/gelatin food gel sample preparation for microscopic examination involves fixing in glutaraldehyde to stabilize the gel by crosslinking the gelatin protein.[Citation115,Citation120] Following this step, the sample is then post-fixed in osmium tetraoxide in to assist the staining contrast.[Citation121] Finally the samples are stained with uranyl acetate, dehydrated in a gradient series of acetone, and embedded in a hard medium, often resin.[Citation14]
TEM studies of gelatin-pectin-sugar gummy confection ultrastructure showed phase-separation. Most of the combinations of pectin and gelatin gave gels that formed a pectin-rich continuous phase with a gelatin-rich dispersed phase.[Citation14] Due to the sample preparation method gelatin-rich phases showed up as dark, while pectin-rich phases showed up lightly coloured. Phase separation also occurred when sucrose and glucose syrup were added to a pectin-gelatin mixture.[Citation83]
DeMars and Ziegler[Citation14] found that in gelatin-pectin-sugar confections that a gelatin:pectin ratio of 3:1, an extensively coalesced dispersed phase was found while in confections with a gelatin:pectin ratio of 4.5:1 a more homogeneous structure was observed. This was explained by the competing effects of coalescence (affected by phase viscosity) and rate of gelation (affected by gel concentration). Lower total polymer (gelatin and pectin) concentrations caused increased rate of coalescence and decreased gelation rates, leading to larger dispersed phase microstructures, whereas the opposite is true of higher total polymer concentrations. Recent research into the structure of gelatin-starch-sugar confections has shown a range of CG microstructures ranging from a gelatin-rich matrix enclosing starch/sugar-rich inclusions to bicontinuous structures, to a starch/sugar-rich matrix enclosing gelatin-rich inclusions ().[Citation122]
Figure 11 TEM micrographs of four different commercial confectionery gels showing a) a starch/sugar-rich matrix enclosing gelatin-rich inclusions; b-c) a bicontinuous structure d) a gelatin-rich matrix enclosing starch/sugar-rich inclusions (scale bars all 2μm) from Burey[Citation122].
![Figure 11 TEM micrographs of four different commercial confectionery gels showing a) a starch/sugar-rich matrix enclosing gelatin-rich inclusions; b-c) a bicontinuous structure d) a gelatin-rich matrix enclosing starch/sugar-rich inclusions (scale bars all 2μm) from Burey[Citation122].](/cms/asset/e83c804a-17de-4633-8e16-e34b07f6528f/ljfp_a_322507_o_f0011g.gif)
Confocal Laser Scanning Microscopy (CLSM) and TEM combined with stereological image analysis have been used to investigate gelatin/maltodextrin gel systems. For gel compositions ranging from 4% gelatin/2.25% maltodextrin to 5% gelatin/5% maltodextrin, there was a continuous gelatin phase with maltodextrin inclusions. The size of the inclusions increased with maltodextrin content. At high temperature the gel appeared relatively homogeneous and upon cooling over time, phase separation occurred.[Citation19] The system goes from a fine dispersion at 60°C to a greatly phase-separated gel at 30°C.[Citation123]
In gelatin/maltodextrin gels, it is possible to cause an inversion from gelatin-continuous to maltodextrin-continuous gels by decreasing the gelatin chain length and/or increasing maltodextrin chain length.[Citation124] This allows the buildup of long chain links throughout the gel by the polysaccharide component. Gelatin/maltodextrin is one of the widely studied composite gel systems and Butler[Citation15] found when looking at gelatin/maltodextrin systems that in the system there was a loss of molecular mobility caused by gelation, although over time the gelled system continued to evolve. He found that in this system droplet coalescence was the dominant structure coarsening mechanism in the liquid system, which agrees with Lorén, Altskär, and Hermansson.[Citation123] Nucleation also occurs, leading to phase separation.
In addition to observation of food gel phases, it has been possible to observe the network structure of food gels using a surface replication technique and then viewing under an electron microscope with a goniometric (moveable) stage. The images of gelatin showed a dense filamentous network, the features of which were dependent on the type of gelatin being investigated and method for forming it.[Citation125] The addition of sugar to gelatin or polysaccharide systems can result in changes in gel network morphology, when compared to aqueous gels, and it has been shown by phase separation in gelatin gels, and in a less fibrillar network in gellan gels upon the addition of sugar.[Citation23] These changes show that polysaccharide gel networks are weakened by the addition of sugar, whilst gelatin gel networks are reinforced by self-attraction of the gelatin chains and repulsion from the sugar phase. These changes in morphology will obviously affect other gel properties such as mechanical properties and sensory properties.
Atomic Force Microscopy (AFM) is a very recent technique which can observe structures at much higher resolution than other techniques such as LM, SEM, TEM, and CLSM. Typically, a cantilever with a sharp tip is scanned over the surface of a sample; a laser is aimed at the top of the cantilever above the tip, and the deflections of the cantilever are measured by a detector which converts the signal received into a topographical image of the sample. Traditionally AFM has been used to observe surface structures of materials, and is an attractive technique to utilize for this purpose as it has the ability of performing three-dimensional measurements with a resolution on the order of nanometres.[Citation126] Some other advantages of this technique is the very simple sample preparation, hence, avoiding damaging or altering the sample prior to measurement,[Citation127] and also the ability to observe samples in air or under solution. The use of AFM for observing fine gel network structures has developed only recently and hence the capabilities are still being explored. It has been used to investigate structure in pectin, gelatin and starch structures.[Citation128–130] AFM also has the capability of carrying out mechanical measurements, providing further information on sample characteristics.[Citation131]
MECHANICAL BEHAVIOUR
There are several empirical mechanical tests that can be carried out in order to characterize a gel, depending on how rigid it is. Some tests that depend on linear motion include: shearing, puncture, extrusion, bending, compression, tension, and cutting.[Citation132] The results obtained using these methods are strongly dependent on sample mass and geometry, sample surface, cell geometry, test speed, and soon.[Citation133,Citation134] The irregular shape of CG product samples can also cause problems in texture analysis, so it is important to prepare uniform samples.[Citation135] Large deformation mechanical testing is used to investigate failure and texture, whilst small deformation testing is used to investigate network gel structure.[Citation136] The tests differ on how their loading affects the sample dimensions during and after the test ().[Citation137]
Figure 12 The three stages during deformation testing. Stage 1. Deformation during small deformation testing; Stage 2. deformation during intermediate deformation testing; Stage 3. Deformation during large deformation testing (modified from Olkku and Sherman[Citation137]).
![Figure 12 The three stages during deformation testing. Stage 1. Deformation during small deformation testing; Stage 2. deformation during intermediate deformation testing; Stage 3. Deformation during large deformation testing (modified from Olkku and Sherman[Citation137]).](/cms/asset/d89e3625-a2b7-41e1-8143-cc5d3709b6df/ljfp_a_322507_o_f0012g.gif)
Large Deformation Testing — Texture Profile Analysis
In an industrial context, the ultimate judge of food texture is the consumer.[Citation138] The term texture is often misunderstood, and hence, there is a requirement for a standard and consistent use of terminology when investigating texture.[Citation139] Texture as applied to foods in general has been defined in many different ways. One official definition provided by the International Organization for Standardization is that texture encompasses all rheological and structural (geometrical and surface) attributes of a food product, which are perceptible by means of mechanical, tactile and when appropriate, visual and auditory receptors.[Citation138] Often these attributes are evaluated in relation to deformation under an applied force and measured objectively in terms of force applied, distance the force is applied through and time of deformation.[Citation139] The first implication of this definition is that texture is related to sensory perception of food product attributes. Examples of sensory descriptors in the case of CGs are chewiness and gumminess.[Citation138] Jellies can be termed “chewy,” and this behaviour is a result of their ductile nature.[Citation140]
Texture arises from the arrangement of various chemical species by physical forces into distinct macro-and microstructures. Texture is an external manifestation of these structures.[Citation111] Texture awareness is often at a subconscious level, if texture is as people have come to expect it to be, it may go un-noticed; however if the texture is not as expected, it may become a point of criticism.[Citation138,Citation141] For this reason, food manufacturers are often reluctant to move from an existing food production process to a more economic one, for fear that they will lose the very textural attributes that attract consumers in the first place.
The relationship between objective texture measurements and sensory properties are often poorly characterized or misunderstood. Only through application of a number of objective methods, based on different principles (e.g., compression, shear) and property measurement on differing scales (e.g., large versus small deformation) can a food material's texture be fully characterized. However, complete texture is rather ambitious, therefore, only a few highly relevant tests are often carried out.[Citation139] Large deformation studies are relevant to food texture as large deformations occur in the mouth.[Citation138] The selection of instrumental method for texture analysis is important, and it is necessary to consider the purpose of the test, ensuring that it accurately measures quality characteristic of choice, with instrument choice being influenced by ruggedness and versatility within cost constraints.[Citation132]
A puncture test measures the force required to push a probe or punch into food to a depth that causes irreversible crushing of flow of food. This is the simplest and most widely used texture testing technique, and it is very useful, as the test can measure compression or shear or a combination of the two.[Citation142] The choice of testing speed is important, and it is necessary to select one that correlates well with sensory perception.[Citation143] In compression testing, it is possible for the finish of the compression surfaces to influence the results obtained under certain conditions. This is because surface finish has a great bearing on contact stresses developed. Samples may be tested under constant area conditions (bonded) or lubricated (sample is free to move).[Citation144]
The instrumental texture profile analysis (TPA) method was developed at General Foods in the early 1960s [Citation145] and involves imitating the first two bites of the food mastication process with an instrument, and relating the data obtained to textural descriptors.[Citation138,Citation146] The test involves a two-cycle penetration test into the food sample, with the force developed observed over time. The output obtained shows a two-peak force curve, and calculations are carried out to determine the magnitude of textural parameters.[Citation146] The magnitudes of the textural parameters are dependent on testing geometries, so it is important to be consistent with sample size when testing products.
Gellan gum is a gel-forming polysaccharide and gellan gels have been studied using a texture analyzer.[Citation147] The samples were 21 mm in diameter and height and compressed at a rate of 0.3 mm/s until failure. Failure strains ranged from 0.5–1.6% and failure stresses ranged from 10–70 kPa based on polymer concentration and calcium presence.[Citation147] Studies were also carried out on the effects of gellan gum, citric acid and sweetener on lemon jellies. The main findings were that jellies were harder with low citric acid and high gum presence and that the influence of citric acid varied with gum contents.[Citation148]
Texture of gelatin-pectin confectionery gels has also been investigated. The gels were made up of 33.4% sucrose, 29.8% 42DE corn syrup solids and varying gelatin and high-methoxyl pectin contents. Using a tensile test on a texture analyzer it was observed that these gels had a fracture strain on order of 0.5–1.2 and a fracture stress of 290–300 kPa based on gelatin and pectin concentrations.[Citation14] TPA is a technique commonly used in industry for the evaluation of food textural behaviour,[Citation146,Citation149,Citation150] as it can give an indication of sensory eating properties.[Citation151] An interesting engineering approach is to use TPA as a quality control tool, with engineering specifications of textural parameters required to be met, in order for food materials to be deemed suitable for the consumer market.[Citation140] This approach can aid in setting up a model for texture-sensory relationships.
Small Deformation Testing — Rheological Behaviour
Small deformation studies allow the study of effects of composition and processing variables to lead to a greater understanding of mechanical properties of food.[Citation132] Rheology is the study of deformation (solid) and flow (liquid) of matter. The rheological properties of interest are elasticity (solid behaviour) and viscosity (liquid behaviour) and foods generally tend to have both.[Citation138] Rheological information of a food gel can give an indication of some of the sensory and textural properties it will have when it reaches the consumer,[Citation152] as well as provide useful information on sol-gel transitions and gel characteristics.[Citation153]
Constant strain dynamic (oscillatory) rheological tests can provide valuable rheological information on the viscoelastic nature of foods. In dynamic oscillatory rheometry a specimen is subjected to a small amplitude strain where the strain is applied at a given frequency (Hz or angular frequency). If the material is viscoelastic, it will exhibit solid-like and liquid-like behaviour.[Citation154] If the material is linearly viscoelastic, stress will vary sinusoidally with strain, but will be out of phase with it. In an ideal elastic solid, stress and strain are in phase, while in a true Newtonian fluid, stress is 90° out of phase with strain. Thus when a sinusoidal deformation is applied and the resultant stresses observed, real materials are found to be intermediate between the two elastic and viscous extremes.[Citation21,Citation155] Modulus is defined as stress/strain in any constant deformation experiment. For a dynamic sinusoidal experiment it follows that two moduli can be defined: 1. in-phase stress/strain or storage modulus (G′); and 2. out-of-phase stress/strain or loss modulus (G″) G is the nomenclature used for shear tests, and E is used when strain (tension or compression) tests are performed. G′ is a measure of the energy stored and recovered from a material during each cycle whilst G′ is a measure of energy dissipated or lost (as heat) during each cycle of sinusoidal deformation.[Citation21,Citation55] A complex modulus, G*, which takes into account both viscous and elastic behaviour, can be developed from the two moduli G′ and G″, where:
There are several dynamic oscillatory tests that can be carried out. Strain sweeps need to be carried out to determine appropriate conditions for non-destructive testing and to determine the linear viscoelastic range — where moduli are virtually independent of strain.[Citation21,Citation155] Following on from the dynamic strain sweep test there are three other rheological tests that can be carried out which are:
| • | frequency sweeps, where frequency of oscillation is varied, and the temperature and strain are kept constant. The viscosity profile, moduli, and other parameters are measured as a function of frequency; | ||||
| • | temperature sweeps where temperature is varied, and the frequency of oscillation and strain are kept constant. Viscometric parameters, moduli, and other parameters are measured as a function of temperature; and | ||||
| • | time sweeps where temperature, frequency and strain are kept constant and the test is run for a period of time. The viscometric and moduli time profile is determined.[Citation153] | ||||
Because gels are viscoelastic, dynamic tests are well suited for studying characteristics of gels as well as of gelation and melting.[Citation153] Starch pastes or gels can have viscous and elastic properties.[Citation40] Rheological tests have been carried out on several food gels and provided information of interest. Investigations carried out on maize starch of 20% moisture at 110°C found that viscosity was 1000–2000 Pa.s.[Citation103] An 11% wheat starch dispersion has shown a complex modulus G* on cooling over time of 2000–3500 Pa.[Citation43]
Time sweeps carried out on wheat starch solutions containing up to 40% starch, showed that the 40% solution took the longest time to plateau when tests were carried out at 0.2 Hz and 2.0% strain for 24 hours.[Citation40] Other time sweep investigations carried out on starch gels showed a plateau for most concentrations after pasting for 45 minutes, however the heating method used was not stated.[Citation156] Jet-cooked starch has shown a crossover from solid behaviour dominance to viscous behaviour dominance during frequency response tests.[Citation94] This behaviour is dependent on the presence of stirring during gel formation, and time.
Penetration tests may be utilized to obtain rheological information. Gelatin gels show a shear modulus which is independent of penetration depth, whilst polysaccharide gels are initially independent of depth as long as the ratio of sample height:sample radius is > 0.2.[Citation157] It was found that results for gelatin were similar to those obtained in a rotational rheometer, whereas this was not so for polysaccharide gels.
Studies of the effects of sucrose/glucose syrup on gelatin gels have yielded some interesting rheological results. A combination of 5% gelatin and 66% sucrose/glucose syrup shows predominantly elastic behaviour over the range of frequencies 0.01–10Hz while 3% gelatin in 70% co-solute exhibits a cross-over frequency of 3–5 Hz.[Citation83] Temperature sweeps carried out on amylopectin-gelatin mixtures show two different thermal transitions occurring when carried out at 1 and 5 Hz at a rate of 2°C/min.[Citation158] This provides some inkling of how confectionery gel systems may behave. Investigating rheology of gels online during processing could prove useful in providing a control measure.[Citation159]
SIGNIFICANCE OF GLASS TRANSITION
Glass transition of an amorphous polymer system is the change in system behaviour from rubbery to hard and relatively brittle. In recent times the importance of this transition has gained wider appreciation of its use in understanding and quality control of such systems. Confectionery gels are amorphous polymer systems and hence the rubber to glass transition is a significant factor in analysis of the gels. A common method to find when glass transition occurs is to determine glass transition temperature (Tg), but other techniques such as dynamic mechanical analysis may be used to observe where the changes in behaviour from rubber to glass occur. shows a master curve detailing the four regions of mechanical behaviour for amorphous polymer systems.[Citation25,Citation29]
Figure 13 Variation of G′, G″, and tan δ as a function of temperature, frequency, molecular weight, and concentration for amorphous polymers (modified from Kasapis, Mitchell, Abeysekera and MacNaughtan[Citation23]).
![Figure 13 Variation of G′, G″, and tan δ as a function of temperature, frequency, molecular weight, and concentration for amorphous polymers (modified from Kasapis, Mitchell, Abeysekera and MacNaughtan[Citation23]).](/cms/asset/72a840c1-ae68-407a-83ff-9454b6cdaeb0/ljfp_a_322507_o_f0013g.gif)
Section I of the curve denotes where molecular flow is predominant in protein or polysaccharide solutions and concentrated preparations of glucose syrup, leading to G′ being greater than G′. In section II of the curves, the samples form elastic networks of stable physical associations (G′ > G″) which can be seen in gummy candies at room temperature.[Citation123] In the absence of crystallization, cooling of rubbery foods ushers in the glass transition region shown in section III of the curve at which the viscous response becomes again dominant (G″ > G′). Finally, the moduli crossover for a third time and the system enters the glassy state in section IV at which the rates of chemical, enzymatic and microbial processes decelerate dramatically.[Citation25]
The appearance of the master curve gives some indication of molecular movements within the material during changes in frequency or temperature. In the glass transition region (section III) backbone adjustments of polymer chains (elastic contribution) is limited, but short-range pendant group, or molecular movements capable of dissipating energy are present. Upon entering the glassy zone (section IV), extremely high frequencies prevent configurational rearrangement of chains; only stretching and bending of bonds are permitted leading to an elastic, rather than a viscous, character.[Citation29]
The appearance of this curve, and the temperatures and frequencies at which the boundaries of the curve sections occur are important for understanding of confectionery gel behaviour. Frequency dependence curves obtained from dynamic mechanical thermal analysis (DMTA) can be compared to such a master curve, and the structural behaviour analyzed.
STRUCTURE-PROPERTY RELATIONSHIPS IN FOOD GELS
In an attempt to investigate how properties of food gels are developed, a few recent studies have looked at the relationships between microstructure and properties of food gel type systems. Many studies have begun to look at composite gels and related properties, while taking into account the effects of microstructural characteristics on these properties.[Citation113,Citation114,Citation160–165] Composite gels often consist of protein and polysaccharide components, and hence have a tendency to phase separate.[Citation11–13,Citation166,Citation167] The studies state some reasons why the molecular arrangement of the composite gel systems affects such properties. New techniques have even looked at combining microstructural analysis with rheological analysis, in one machine measurement,[Citation168–170] whilst models are now being developed to predict biopolymer gel properties based on their structural characteristics.[Citation141,Citation171]
In some studies, polymer blending models have been used to explain the properties of composite food gels. Studies by Abdulmola, Hember, Richardson, and Morris[Citation21] were done on gelatin-starch composite gels where X in Equationequations 1 and Equation2 was considered as gelatin, the “stronger” gelling component. In the study, there was little change in composite moduli with starch concentration when starch granules were present in a gelatin matrix, however at higher gelatin concentrations the composite modulus went closer to the upper bound at lower temperature (5°C). At low gelatin concentration, the lower bound fitted.
The focus in the past has been on the effects of additives or processing changes on individual gel characteristics, without considering the relationships of gel characteristics to each other.[Citation172–174] Very few studies have looked at complete confectionery gel systems, containing carbohydrate, protein and high sugar co-solutes, and the relationships between gel properties and structure.[Citation14,Citation28,Citation175] Some studies have been carried out looking at gel composites with co-solutes of sucrose and glucose syrup, as these systems are representative of true food gel products.[Citation25,Citation67,Citation83]
It has been simpler in the past to investigate structure-property relationships in gels containing only one biopolymer, as the system is not so complex.[Citation94,Citation176] Now that a greater understanding is required of complex food systems, in order to develop models for processing-property relationships and quality control, there is a need for further study into structure-property-processing interactions.
SUMMARY
Whilst much is known about confectionery gel ingredients, and interactions between some of these ingredients, there is limited knowledge available about how the entire system is developed, due to the complexity of ingredient interactions and processing-induced changes. Although there is a lack of knowledge about the development of confectionery gel microstructure, and texture development in typical industrial processes, gel formation of individual biopolymers (e.g., starch, gelatin) has been studied separately; and, the fundamental phenomenon of phase separation in similar biopolymer mixtures has also been investigated. Previous food gel studies have focused on looking at individual gel characteristics, such as microstructure, texture and rheological behaviour, as well as behaviour of single biopolymer gel systems. It is proposed by further studies that textural behaviour and rheological behaviour of food systems is dependent on the structural characteristics of the food, and this principle can be applied to a confectionery gel system. It is necessary to acknowledge the importance of creating consumer-acceptable textures, and understanding how these textures come about, through processing and structure manipulation.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Australian Research Council (ARC Linkage projects LP LP0211915 and LP0453529), and the industrial partners on these projects. PB would also like to thank The University of Queensland for providing her scholarship.
REFERENCES
- Yuryev , V.P. , Nemirovskaya , I.E. and Maslova , T.D. 1995 . Phase state of starch gels at different water contents . Carbohyd. Polym. , 26 ( 1 ) : 43 – 46 .
- Edwards , S. , Lillford , P. and Blanshard , J. 1987 . “ Gels networks in practice and theory ” . In Food Structure and Behaviour , Edited by: Blanshard , J. and Lillford , P. 1 – 12 . London : Academic Press Ltd .
- Edwards , W.P. 2000 . The Science of Sugar Confectionery , 166 Cambridge : The Royal Society of Chemistry .
- Hermans , P.H. 1949 . “ Gels ” . In Colloid Science , Edited by: Kruyt , H. Vol. II , 483 – 651 . Amsterdam : Elsevier Science .
- Stainsby , G. Gelation and Gelling Agents . Proceedings of the UK Symposium on Gelation and Gelling Agents . Oct 20th 1971 , London , UK . London : The British Food Manufacturing Industries Research Association .
- Flory , P.J. 1953 . Principles of Polymer Chemistry , 672 Ithaca : Cornell University Press .
- Djabourov , M. and LeBlond , J. 1987 . Thermally reversible gelation of the gelatin-water system . ACS Sym. Ser. , 350 : 211 – 223 .
- Peyrelasse , J. , Lamarque , M. , Habas , J.P. and El Bounia , N. 1996 . Rheology of gelatin solutions at the sol-gel transition . Phys. Rev. E. , 53 ( 6 ) : 6126 – 6133 .
- Kavanagh , G.M. and Ross-Murphy , S.B. 1998 . Rheological characterisation of polymer gels . Prog. Polym. Sci. , 23 ( 3 ) : 533 – 562 .
- Burchard , W. and Ross-Murphy , S.B. 1990 . “ Introduction: Physical gels from synthetic and biological macromolecules ” . In Physical Networks — Polymers and Gels , Edited by: Burchard , W. and Ross-Murphy , S. 1 – 14 . Essex : Elsevier Science .
- Tolstoguzov , V. 2000 . Phase behaviour of macromolecular components in biological and food systems . Food. , 44 ( 5 ) : 299 – 308 .
- Norton , I.T. and Frith , W.J. 2001 . Microstructure design in mixed biopolymer composites . Food Hydrocolloid. , 15 ( 4–6 ) : 543 – 553 .
- de Kruif , D.G. and Tuinier , R. 2001 . Polysaccharide protein interactions . Food Hydrocolloid. , 15 ( 4–6 ) : 555 – 563 .
- DeMars , L.L. and Ziegler , G.R. 2001 . Texture and structure of gelatin/pectin-based gummy confections . Food Hydrocolloid. , 15 ( 4–6 ) : 643 – 653 .
- Butler , M.F. 2002 . Mechanism and Kinetics of Phase Separation in a Gelatin/Maltodextrin Mixture Studied by Small-Angle Light Scattering . Biomacromol. , 3 ( 4 ) : 676 – 683 .
- Zasypkin , D.V. , Braudo , E.E. and Tolstoguzov , V.B. 1997 . Multicomponent biopolymer gels . Food Hydrocolloid. , 11 ( 2 ) : 159 – 170 .
- Grinberg , V.Y. and Tolstoguzov , V.B. 1997 . Thermodynamic incompatibility of proteins and polysaccharides in solutions . Food Hydrocolloid. , 11 ( 2 ) : 145 – 158 .
- Cuppo , F. , Venuti , M. and Cesàro , A. 2001 . Kinetics of gelatin transitions with phase separation: T-jump and step-wise DSC study . Int. J. Bio. Macromolecules. , 28 ( 4 ) : 331 – 341 .
- Lorén , N. and Hermansson , A.-M. 2000 . Phase separation and gel formation in kinetically trapped gelatin/maltodextrin gels . Int. J. Bio. Macromol. , 27 ( 4 ) : 249 – 262 .
- Wolf , B. , Scirocco , R. , Frith , W.J. and Norton , I.T. 2000 . Shear-induced anisotropic microstructure in phase-separated biopolymer mixtures . Food Hydrocolloid. , 14 ( 3 ) : 217 – 225 .
- Abdulmola , N.A. , Hember , M.W.N. , Richardson , R.K. and Morris , E.R. 1996 . Application of polymer blending laws to starch-gelatin composites . Carbohyd. Polym. , 31 ( 1–2 ) : 53 – 63 .
- Clark , A.H. 1987 . “ The application of network theory to food systems ” . In Food Structure and Behaviour , Edited by: Blanshard , J. and Lillford , P. 13 – 34 . London : Food Science and Technology, Academic Press Limited .
- Kasapis , S. , Al-Marhoobi , I.M. , Deszczynski , M. , Mitchell , J.R. and Abeysekera , R. 2003 . Gelatin vs Polysaccharide in Mixture with Sugar Biomacromolecules. , 4 ( 1 ) : 1142 – 1149 .
- Morris , V.J. 1985 . Food gels – roles played by polysaccharides . Chem. Ind. (London). , : 159 – 164 .
- Kasapis , S. , Mitchell , J. , Abeysekera , R. and MacNaughtan , W. 2004 . Rubber-to-glass transitions in high sugar-biopolymer mixtures . Trends Food Sci. Tech. , 15 ( 6 ) : 293 – 304 .
- Clark , A. 1995 . “ Kinetics of demixing ” . In Biopolymer Mixtures , Edited by: Harding , S. , Hill , S. and Mitchell , J. 37 – 64 . Nottingham : Nottingham University Press .
- Danner , R. 1993 . Handbook of polymer solution thermodynamics , 184 New York : American Institute of Chemical Engineers .
- Groves , K. 2003 . Microscopy Techniques for Confectionery Gels . Manuf. Confect. , 83 ( 9 ) : 110 – 114 .
- Ong , M.H. , Whitehouse , A.S. , Abeysekera , R. , Al-Ruqaie , I.M. and Kasapis , S. 1998 . Glass transition-related or crystalline forms in the structural properties of gelatin/oxidised starch/glucose syrup mixtures . Food Hydrocoll. , 12 ( 3 ) : 273 – 281 .
- Nickerson , M. , Paulson , A. and Speers , R. 2004 . Time-temperature studies of gellan polysaccharide gelation in the presence of low, intermediate and hgh levels of co-solutes . Food Hydrocolloid. , 18 ( 5 ) : 783 – 794 .
- Lees , R. 1980 . Faults, causes and remedies: in sweet and chocolate manufacture , 384 Surbiton , , England : Specialised Publications .
- Belitz , H.-D. and Grosch , W. 1999 . Food Chemistry , 774 Berlin, New York : Springer Verlag .
- Cakebread , S. 1975 . Sugar and Chocolate Confectionery , 60 London : Oxford University Press .
- Richards , G.N. 1986 . Initial steps in thermal degradation of sucrose . Int. Sugar J. , 88 ( 1052 ) : 145 – 145 .
- Jackson , E.B. , ed. 1995 . Sugar Confectionery Manufacture , 2nd , 400 London : Blackie Academic and Professional .
- Minifie , B. 1989 . Chocolate, Cocoa and Confectionery , 904 New York : Van Nostrand Reinhold .
- Gillies , M.T. 1979 . Candies and Other Confections , 354 New Jersey : Park Ridge .
- Slade , L. and Levine , H. 1991 . Beyond Water Activity — Recent Advances Based on an Alternative Approach to the Assessment of Food Quality and Safety . Crit. Rev. Food Sci. , 30 ( 2–3 ) : 115 – 360 .
- Cornillon , P. , Andrieu , J. , Duplan , J.-C. and Laurent , M. 1995 . Use of Nuclear Magnetic Resonance to Model Thermophysical Properties of Frozen and Unfrozen Model Food Gels . J. Food Eng. , 25 ( 1 ) : 1 – 19 .
- Abd Karim , A. , Norziah , M.H. and Seow , C.C. 2000 . Methods for the study of starch retrogradation . Food Chem. , 71 ( 1 ) : 9 – 36 .
- Kearsley , M.W. and Dziedzic , S.Z. , eds. 1995 . Handbook of Starch Hydrolysis Products and their Derivatives , 1st , 275 Glasgow : Blackie Academic and Professional .
- Veiga , V. , Ryan , D.H. , Sourty , E. , Llanes , F. and Marchessault , R.H. 2000 . Formation and characterization of superparamagnetic cross-linked high amylose starch . Carbohyd. Polym. , 42 ( 4 ) : 353 – 357 .
- Hermansson , A.-M. and Svegmark , K. 1996 . Developments in the understanding of starch functionality . Trends Food Sci. Tech. , 7 ( 11 ) : 343 – 353 .
- Gaillard , T. 1987 . Starch: Properties and Potential , 151 Chichester : Wiley .
- Rix , A. 1990 . “ Gelling and Whipping Agents ” . In Sugar Confectionery Manufacture , Edited by: Jackson , E.B. 57 – 76 . Glasgow; Blackie; New York : Van Nostrand Reinhold .
- Wang , Y.-J. , Truong , V.-D. and Wang , L. 2003 . Structures and rheological properties of corn starch as affected by acid hydrolysis . Carbohyd. Polym. , 52 ( 3 ) : 327 – 333 .
- Virtanen , T. , Autio , K. , Suortti , T. and Poutanen , K. 1993 . Heat-induced changes in native and acid-modified oat starch pastes . J. Cereal Sci. , 17 ( 2 ) : 137 – 145 .
- Atichokudomchai , N. , Shobsngobb , S. and Varavinita , S. 2000 . Morphological properties of acid-modified tapioca starch . Starch. , 52 ( 8–9 ) : 283 – 289 .
- Tester , R.F. and Morrison , W.R. 1990 . Swelling and gelatinization of Cereal Starches. I. Effects of Amylopectin, Amylose and Lipids . Cereal Chem. , 67 ( 6 ) : 551 – 557 .
- Atkin , N.J. , Abeysekera , R.M. , Cheng , S.L. and Robards , A.W. 1998 . An experimentally-based predictive model for the separation of amylopectin subunits during starch gelatinization . Carbohyd. Polym. , 36 ( 2–3 ) : 173 – 192 .
- Carnali , J.O. and Zhou , Y. 1996 . An examination of the composite model for starch gels . J. Rheol. , 40 ( 2 ) : 221 – 234 .
- Remsen , C.H. and Clark , J.P. 1978 . A viscosity model for cooking dough . J. Food Process Eng. , 2 ( 1 ) : 39 – 64 .
- Beleia , A. , Miller , R.A. and R.C , H. 1996 . Starch Gelatinization in Sugar Solutions . Starch. , 48 ( 7–8 ) : 259 – 262 .
- Miles , M. , Morris , V. , Orford , P. and Ring , S. 1985 . The roles of amylose and amylopectin in the gelation and retrogradation of starch . Carbohyd. Res. , 135 ( 2 ) : 247 – 269 .
- Richardson , G. , Langton , M. , Bark , A. and Hermansson , A.-M. 2003 . Wheat starch gelatinization – the effects of sucrose, emulsifier and the physical state of the emulsifier . Starch. , 55 ( 3–4 ) : 150 – 161 .
- Appelqvist , I.A.M. and Debet , M.R.M. 1997 . Starch-biopolymer interactions – a review . Food Rev. Int. , 13 ( 2 ) : 163 – 224 .
- Takahashi , A. and Yamada , T. 1998 . Crystalline Copolymer Approach for Melting Behaviour of Starch . Starch. , 50 ( 9 ) : 386 – 389 .
- Tolstoguzov , V. 2003 . Thermodynamic considerations of starch functionality in foods . Carbohyd. Polym. , 51 ( 1 ) : 99 – 111 .
- Shi , Y.-C. and Seib , P.A. 1992 . The structure of four waxy starches related to gelatinization and retrogradation . Carbohyd. Res. , 227 : 131 – 145 .
- Osunsami , A.T. , Akingbala , J.O. and Oguntimein , G.B. 1989 . Effect of storage on starch content and modification of cassava starch . Starch. , 41 ( 2 ) : 54 – 57 .
- Kim , R.E. and Ahn , S.Y. 1996 . Gelling properties of acid-modified red bean starch gels . Agric. Chem. Biotech. , 39 : 49 – 53 .
- Iizuka , K. and Aishima , T. 1999 . Starch Gelation Process Observed by FT-IR/ATR Spectrometry with Multivariate Data Analysis . J. Food Sci. , 64 ( 4 ) : 653 – 658 .
- Fishman , M.L. , Coffin , D.R. , Konstance , R.P. and Onwulata , C.I. 2000 . Extrusion of pectin/starch blends plasticized with glycerol . Carbohyd. Polym. , 41 ( 4 ) : 317 – 325 .
- Perry , P.A. and Donald , A.M. 2002 . The effect of sugars on the gelatinisation of starch . Carbohyd. Polym. , 49 ( 2 ) : 155 – 165 .
- Donovan , J.W. 1979 . Phase transitions of the starch water system . Biopolymers. , 18 ( 2 ) : 263 – 275 .
- Biliaderis , C. 1991 . The structure and interactions of starch with food constituents . Can J. Physiol. Pharm. , 69 ( 1 ) : 60 – 78 .
- Farhat , I.A. , Mousia , Z. and Mitchell , J.R. 2001 . Water redistribution during the recrystallisation of amylopectin in amylopectin/gelatin blends . Polymer. , 42 ( 10 ) : 4763 – 4766 .
- Keetels , C. , Oostergetel , G. and van Vliet , T. 1996 . Recrystallization of amylopectin in concentrated starch gels . Carbohyd. Polym. , 30 ( 1 ) : 61 – 64 .
- Jang , J.K. and Pyun , Y.R. 1996 . Effect of Moisture Content on the Melting Point of Wheat Starch . Starch. , 48 ( 2 ) : 48 – 51 .
- Evageliou , V. , Richardson , R.K. and Morris , E.R. 2000 . Effect of sucrose, glucose and fructose on gelation of oxidised starch. Carbohyd . Polym. , 42 ( 3 ) : 261 – 272 .
- Prokopowich , D.J. and Biliaderis , C.G. 1995 . A comparative study of the effect of sugars on the thermal and mechanical properties of concentrated waxy maize, wheat, potato and pea starch gels . Food Chem. , 52 ( 3 ) : 255 – 262 .
- Djabourov , M. 1991 . Gelation – A Review . Polym. Int. , 25 ( 3 ) : 135 – 143 .
- Johnston-Banks , F.A. 1990 . “ Gelatine ” . In Food Gels , Edited by: Harris , P. 233 – 289 . Elsevier Science Publishers Ltd .
- Normand , V. , Muller , S. , Ravey , J.-C. and Parker , A. 2000 . Gelation Kinetics of Gelatin: A Master Curve and Network Modeling . Macromolecules. , 33 ( 3 ) : 1063 – 1071 .
- Clark , A. and Ross-Murphy , S. 1990 . “ Shear modulus – concentration relationships for biopolymer gels. comparison of independent and cooperative crosslink descriptions ” . In Physical Networks — Polymers and Gels , Edited by: Burchard , W. and Ross-Murphy , S. 209 – 230 . London : Elsevier Science .
- Veis , A. 1964 . The Macromolecular Chemistry of Gelatin , 433 New York : Academic Press Ltd .
- Bohidar , H.B. and Jena , S.S. 1993 . Kinetics of sol-gel transition in thermoreversible gelation of gelatin . J. Chem. Phys. , 98 ( 11 ) : 8970 – 8977 .
- de Carvalho , W. and Djabourov , M. 1997 . Physical gelation under shear for gelatin gels . Rheol. Acta. , 36 ( 6 ) : 591 – 609 .
- Finer , E.G. , Franks , F. , Phillips , M.C. and Sugget , A. 1975 . Gel formation from solutions of single chain gelatin . Biopolymers. , 14 ( 10 ) : 1995 – 2005 .
- Michon , C. , Cuvelier , G. , Relkin , P. and Launay , B. 1997 . Influence of thermal history on the stability of gelatin gels . Int. J. Bio. Macromolecules. , 20 ( 4 ) : 259 – 264 .
- Huang , H. and Sorenson , C.M. 1996 . Shear effects during the gelation of aqueous gelatin . Phys. Rev. E. , 53 ( 5 ) : 5075 – 5078 .
- Tosh , S. , Marangoni , A. , Hallet , F. and Britt , I. 2003 . Aging dynamics in gelatin gel microstructure . Food Hydrocolloid. , 17 ( 4 ) : 503 – 513 .
- Al-Ruqaie , I.M. , Kasapis , S. and Abeysekera , R. 1997 . Structural properties of pectin-gelatin gels Part II: Effect of sucrose/glucose syrup . Carbohyd. Polym. , 34 ( 4 ) : 309 – 321 .
- Djabourov , M. , Maquet , J. , Theveneau , H. , Leblond , J. and Papon , P. 1985 . Kinetics of Gelation of Aqueous Gelatin Solutions . Brit. Polym. J. , 17 ( 2 ) : 169 – 174 .
- Christensen , S.H. 1983 . “ Pectins ” . In Food Hydrocolloids , Edited by: Glicksman , M. 205 – 229 . Boca Raton : CRC Press Inc .
- May , C.D. 2000 . “ Pectins ” . In Handbook of Hydrocolloids , Edited by: Phillips , G.O. and Williams , P.A. 169 – 188 . Boca Raton : CRC Press .
- Hoefler , A.C. 2004 . Hydrocolloids , 111 Minnesota : Eagen Press .
- Nussinovitch , A. 1997 . Hydrocolloid Applications , 354 London : Chapman and Hall .
- Willats , W.G.T. , Knox , P. and Mikkelsen , J.D. 2006 . Pectin: new insights into an old polymer are starting to gel . Trends Food Sci. Tech. , 17 ( 3 ) : 97 – 104 .
- Hino , T. , Ishimoto , H. and Shimabayashi , S. 2003 . Thermal gelation of aqueous curdlan suspension: preparation of curdlan jelly . J. Pharm. Pharmacol. , 55 ( 4 ) : 435 – 441(7) .
- Gibson , W. and Sanderson , G.R. 1997 . “ Gellan Gum ” . In Thickening and gelling agents for food , Edited by: Imeson , A. 119 – 143 . London, New York : Blackie Academic & Professional .
- Laroche , C. and Michaud , P. 2007 . New Developments and Prospective Applications for β (1,3) Glucans . Recent Pat. Biotechnol. , 1 : 59 – 73 .
- Williams , C.T. 1964 . Chocolate and Confectionery , 221 London : Leonard Hill .
- Byars , J.A. , Fanta , G.F. and Felker , F.C. 2003 . The effect of cooling conditions on jet-cooked normal corn starch dispersions . Carbohyd. Polym. , 54 ( 3 ) : 321 – 326 .
- Kelder , J.D.H. , Ptasinski , K.J. and Kerkhof , P.J.A.M. 2004 . Starch gelatinization in coiled heaters . Biotechnol. Progr. , 20 ( 3 ) : 921 – 929 .
- Cayot , N. , Bounie , D. and Baussart , H. 1995 . Dynamic Modelling for a Twin Screw Food Extruder: Analysis of the Dynamic Behaviour Through Process Variables . J. Food Eng. , 25 ( 2 ) : 245 – 260 .
- Rohn , C. 1995 . Analytical Polymer Rheology – Structure-Processing-Property Relationships , 314 Munich : Hanser .
- Akdogan , H. 1999 . High moisture food extrusion . Int. J. Food Sci. Tech. , 34 ( 3 ) : 195 – 207 .
- Elsey , J. , Riepenhausen , J. , McKay , B. , Barton , G.W. and Willis , M. 1997 . Modeling and Control of a Food Extrusion Process . Comput. Chem. Eng. , 21 ( Suppl ) : s361 – s366 .
- Harper , J. 1981 . Extrusion of Foods , Vol. 1 , 212 Boca Raton : CRC Press Inc .
- Berins , M.L. , ed. 1991 . SPI Plastics Engineering handbook of the Society of the Plastics Industry Inc , 5th , 78 – 132 . New York : Chapman & Hall .
- van Zuilichem , D.J. , Kuiper , E. , Stolp , W. and Jager , T. 1999 . Mixing effects of constituting elements of mixing screws in single and twin screw extruder . Powder Technol. , 106 ( 3 ) : 147 – 159 .
- Parker , R. , Ollett , A.-L. , Lai-Fook , R.A. and Smith , A.C. 1990 . “ The rheology of food ‘melts’ and its application to Extrusion Processing ” . In Rheology of food, pharmaceutical and biological materials with general rheology , Edited by: Carter , R.E. 57 – 73 . London, New York : Elsevier Applied Science .
- van de Velde , F. , van Riel , J. and Tromp , R.H. 2002 . Visualisation of starch granule morphologies using confocal scanning laser microscopy (CLSM) . J. Sci. Food Agr. , 82 ( 1–4 ) : 1528 – 1536 .
- Barron , C. , Buleon , A. , Colonna , P. and Della Valle , G. 2000 . Structural modifications of low hydrated pea starch subjected to high thermomechanical processing . Carbohyd. Polym. , 43 ( 2 ) : 171 – 181 .
- Lai , L.S. and Kokini , J.L. 1991 . Physicochemical changes and rheological properties of starch during extrusion . Biotechnol. Progr. , 7 ( 3 ) : 251 – 266 .
- Wulansari , R. , Mitchell , J.R. and Blanshard , J.M.V. 1999 . Starch conversion during extrusion as affected by added gelatin . J. Food Sci. , 64 ( 6 ) : 1055 – 1058 .
- McHugh , T.H. and Huxsoll , C.C. 1999 . Extrusion Processing of Restructured Peach and Peach/Starch Gels . Food Sci. Technol.-Leb. , 32 ( 8 ) : 513 – 520 .
- Collyer , A.A. , Bennett , M.J. , Clegg , D.W. , Elson , C.R. and Jones , S.A. 1990 . “ A Feasibility Study on the Injection Moulding of Fruit Gums at 85% Solids ” . In Rheology of Food, Pharmaceuticals and Biological Materials with General Rheology , Edited by: Carter , R.E. 13 – 26 . London, New York : Elsevier Applied Science .
- Barres , C. , Vergnes , B. , Tayeb , J. and Della valle , G. 1990 . Transformation of wheat flour by extrusion cooking – influence of screw configuration and operating conditions . Cereal Chem. , 67 ( 5 ) : 427 – 433 .
- Aguilera , J.M. 2000 . Microstructure and Food Product Engineering . Food Technol. , 54 ( 11 ) : 56 – 65 .
- Langton , M. , Aström , A. and Hermansson , A.-M. 1996 . Texture as a reflection of microstructure . Food Qual. Prefer. , 7 ( 3–4 ) : 185 – 191 .
- Lofgren , C. , Walkenström , P. and Hermansson , A.-M. 2002 . Microstructure and rheological behaviour of pure and mixed pectin gels . Biomacromol. , 3 ( 5 ) : 1144 – 1153 .
- Olsson , C. , Langton , M. and Hermansson , A.-M. 2002 . Microstructures of beta-lactoglobulin/amylopectin gels on different length scales and their significance for rheological properties . Food Hydrocolloid. , 16 ( 2 ) : 111 – 126 .
- Kaláb , M. , Allan-Wojtas , P. and Miller , S.S. 1995 . Microscopy and other imaging techniques in food structure analysis . Trends Food Sci. Tech. , 6 ( 6 ) : 177 – 186 .
- Sun , M. , Griess , G.A. and Serwer , P. 1994 . Light Microscopy of the Structure of a Gel . J. Struct. Biol. , 113 ( 1 ) : 56 – 63 .
- Autio , K. and Laurikainen , T. 1997 . Relationships between flour/dough microstructure and dough handling and baking properties . Trends Food Sci. Technol. , 8 ( 6 ) : 181 – 185 .
- Stokes , D.J. and Donald , A.M. 2000 . In-situ mechanical testing of dry and hydrated breadcrumb in the environmental scanning electron microscope (ESEM) . J. Mater. Sci. , 35 ( 3 ) : 599 – 607 .
- Bhatnagar , S. and Hanna , M.A. 1997 . Modification of Microstructure of Starch Extruded with Selected Lipids . Starch. , 49 ( 1 ) : 12 – 20 .
- Barron , C. , Bouchet , B. , Della Valle , G. , Gallant , D.G. and Planchot , V. 2001 . Microscopical Study of the Destructuring of Waxy Maize and Smooth Pea Starches by Shear and Heat at Low Hydration . J. Cereal Sci. , 33 ( 3 ) : 289 – 300 .
- Hayat , M.A. 1993 . Stains and Cytochemical Methods , 455 New York : Plenum Press .
- Burey (nee Ramesh Sukha) , P. Characterisation and Processing of Starch / Sugar / Gelatin Confectionery Gels . School of Engineering . Brisbane : The University of Queensland .
- Lorén , N. , Altskär , A. and Hermansson , A.-M. 2001 . Structure Evolution during Gelation at Later Stages of Spinodal Decomposition in Gelatin/Maltodextrin Mixtures . Macromolecules. , 34 ( 23 ) : 8117 – 8128 .
- Alevisopoulos , S. and Kasapis , S. 1999 . Molecular weight effects on the gelatin/maltodextrin gel . Carbohyd. Polym. , 40 ( 1 ) : 83 – 87 .
- Djabourov , M. , Bonnet , N. , Kaplan , H. , Favard , N. , Favard , P. , Lechaire , J.P. and Maillard , M. 1993 . 3D Analysis of gelatin gel networks from transmission electron microscopy imaging . J. Phys. II. , 3 ( 5 ) : 611 – 624 .
- Bonell , D. , ed. Scanning tunneling microscopy and spectroscopy : theory, techniques, and applications , 1993 New York : Wiley-VCH .
- Starostina , N. and West , P. 2006 . Part II: Sample Preparation for AFM Particle Characterization , Santa Clara : Pacific Nanotechnology .
- Fishman , M.L. , Cooke , P.H. , Chau , H.K. , Coffin , D.R. and Hotchkiss , A.T. 2007 . Global structures of high methoxyl pectin from solution and in gels . Biomacomol. , 8 ( 2 ) : 573 – 578 .
- Morris , V.J. , Gunning , A.P. , Faulds , C.B. , Williamson , G. and Svensson , B. 2005 . AFM images of complexes between amylose and Aspergillus niger glucoamylase mutants, native, and mutant starch binding domains: A model for the action of glucoamylase . Starch. , 57 ( 1 ) : 1 – 7 .
- Uricanu , V.I. , Duits , M.H.G. , Nelissen , R.M.F. , Bennink , M.L. and Mellema , J. 2003 . Local structure and elasticity of soft gelatin gels studied with atomic force microscopy . Langmuir. , 19 ( 20 ) : 8182 – 8194 .
- Benmouna , F. and Johannsmann , D. 2004 . Viscoelasticity of gelatin surfaces probed by AFM noise analysis . Langmuir. , 20 ( 1 ) : 188 – 193 .
- Stanley , D. , Stone , A.P. and Tung , M.A. 1996 . “ Mechanical Properties of Food ” . In Handbook of Food Analysis: Physical Characterisation and Nutrient Analysis , Edited by: Mollet , L.M.L. 93 – 113 . New York : Marcel Dekker .
- Voisey , P. 1975 . “ Modernisation of texture instrumentation ” . In Theory, Determination and Control of Physical Properties of Food Materials , Edited by: Rha , C. 63 – 130 . Dordrecht : Reidel .
- Anand , A. and Scanlon , M.G. 2002 . Dimensional effects on the prediction of texture-related mechanical properties of foods by indentation . T. ASAE. , 45 ( 4 ) : 1045 – 1050 .
- Teratsubo , M. , Tanaka , Y. and Saeki , S. 2002 . Measurement of stress and strain during tensile testing of gellan gum gels: effect of deformation speed . Carbohyd. Polym. , 47 ( 1 ) : 1 – 5 .
- Ross-Murphy , S.B. 1995 . Rheological characterisation of gels . Rheological characterisation of gels. , 26 ( 4 ) : 391 – 400 .
- Olkku , J.E. and Sherman , P. 1982 . “ Compression Testing of Cylindrical Samples with an INSTRON Universal Testing Machine ” . In Food texture and viscosity : concept and measurement , Edited by: Bourne , M.C. 157 – 175 . New York : Academic Press .
- Borwankar , R.P. 1992 . Food Texture and Rheology: A Tutorial Review . J. Food Eng. , 16 ( 1–2 ) : 1 – 16 .
- Jackman , R.L. and Stanley , D.W. 1995 . Perspectives in the textural evaluation of plant foods . Trends Food Sci. Tech. , 6 ( 6 ) : 187 – 194 .
- Jowitt , R. 1982 . “ An engineering approach to some aspects of food texture ” . In Food Texture and Viscosity: Concept and Measurement , Edited by: Bourne , M. 142 – 155 . New York : Academic Press Inc .
- Wilkinson , C. , Dijksterhuis , G.B. and Minekus , M. 2000 . From food structure to texture . Trends Food Sci. Tech. , 11 ( 12 ) : 442 – 450 .
- Bourne , M.C. 2002 . Food Texture and Viscosity: Concept and Measurement , 427 New York : Academic Press Inc .
- Bourne , M.C. 1992 . Calibration of Rheological Techniques Used for Foods . J. Food Eng. , 16 ( 1–2 ) : 151 – 163 .
- Bagley , E.B. , Christianson , D.D. and Wolf , W.J. 1985 . Friction Effects in Compressional Deformation of Gelatin and Starch Gels and Comparison of Material Response in Simple Shear, Torsion and Lubricated Uniaxial Compression . J. Rheol. , 29 ( 1 ) : 103 – 108 .
- Friedman , H.H. , Whitney , J.E. and Sczczesniak , A.S. 1963 . The Texturometer — A new instrument for objective texture measurement . J. Food Sci. , 28 ( 4 ) : 390 – 396 .
- Antoniou , K.D. , Petridis , D. , Raphaelides , S. , Ben Omar , Z. and Kesteloot , R. 2000 . Texture Assessment of French Cheeses . J. Food Sci. , 65 ( 1 ) : 168 – 173 .
- Mao , R. , Tang , J. and Swanson , B.G. 2000 . Texture properties of high and low acyl mixed gellan gels . Carboyhyd. Polym. , 41 ( 4 ) : 331 – 338 .
- Moritaka , H. , Naito , S. , Nishinari , K. , Ishihara , M. and Fukuba , H. 1999 . Effects of gellan gum, citric acid and sweetener on the texture of lemon jelly . J. Texture Stud. , 30 ( 1 ) : 29 – 41 .
- Bourne , M.C. 1986 . Effect of Water Activity on Texture Profile Parameters of Apple Flesh . J. Textur. Stud. , 17 : 331 – 340 .
- Olkku , J. and Rha , C.K. 1975 . Textural Parameters of Candy Licorice . J. Food Sci. , 40 ( 5 ) : 1050 – 1054 .
- Hough , G. , Califano , A.N. , Bertola , N.C. , Bevilacqua , A.E. , Martinez , E. , Jose Vega , M. and Zaritzky , N.E. 1996 . Partial least squares correlations between sensory and instrumental measurements of flavor and texture for reggianito grating cheese . Food Qual. Pref. , 7 ( 1 ) : 47 – 53 .
- Ronn , B.B. , Hyldig , G. , Wienberg , L. , Qvist , K.B. and Laustsen , A.M. 1998 . Predicting sensory properties from rheological measurements of low-fat spreads . Food Qual. Pref. , 9 ( 4 ) : 187 – 196 .
- Rao , M.A. , ed. 1999 . Rheology of Fluid and Semisolid Foods — Principles and applications , 433 Maryland : Aspen Publishers. Inc .
- Larson , R.G. 1999 . The Structure and Rheology of Complex Fluids , 663 New York : Oxford University Press, Inc .
- Menard , K.P. 1999 . Dynamic Mechanical Analysis: A Practical Introduction , 208 Boca Raton : CRC Press .
- Rezler , R. , Baranowska , H.M. , Surma , S. and Poliszko , S. 2000 . Investigation of starch gels by means of the relaxation method . Zywnosc. , 2 ( 23 Supp ) : 194 – 203 .
- Gregson , C.M. , Hill , S.E. , Mitchell , J.R. and Smewing , J. 1999 . Measurement of the Rheology of Polysaccharide Gels by Penetration . Carbohyd. Polym. , 38 ( 3 ) : 255 – 259 .
- Mousia , Z.F., I.A. , Blachot , J.F. and Mitchell , J.R. 2000 . Effect of water partitioning on the glass-transition behaviour of phase separated amylopectin-gelatin mixtures . Polym. , 41 ( 5 ) : 1841 – 1848 .
- Steffe , J.F. and Morgan , R.G. 1987 . On-line measurement of dynamic rheological properties during food extrusion . J. Food. Proc. Eng. , 10 ( 1 ) : 21 – 26 .
- Langley , K.R. , Green , M.L. and Brooker , B.E. 1991 . “ Mechanical properties and structure of model composite foods ” . In Food Polymers, Gels and Colloids , Edited by: Dickinson , E. 383 – 391 . Cambridge : The Royal Society of Chemistry .
- Maurer , K. and Hardy , J. 1996 . Rheological and sensory characterization of viscous and pasty food products: application of fractal concepts and fourier analysis . J. Text. Stud. , 27 ( 1 ) : 41 – 59 .
- Morikawa , K. and Nishinari , K. 2000 . Rheological and DSC studies of gelatinization of chemically modified starch heated at various temperatures . Carbohyd. Polym. , 43 ( 3 ) : 241 – 247 .
- Noël , Y. , Zannoni , M. and Hunter , E.A. 1996 . Texture of Parmigiano Reggiano cheese: Statistical relationships between rheological and sensory variates . Lait. , 76 ( 3 ) : 243 – 254 .
- Taneya , S. , Izutsu , T. , Kimura , T. , Shioya , T. , Buchheim , W. , Kindstedt , P.S. and Pechak , D.G. 1992 . Structure and Rheology of String Cheese . Food Struct. , 11 ( 1 ) : 61 – 71 .
- Vega , C. and Goff , H. 2005 . Phase separation in soft-serve ice cream mixes: rheology and microstructure . Int. Dairy J. , 15 ( 33 ) : 249 – 254 .
- Tolstoguzov , F.B. 2000 . The importance of glassy biopolymer components in food . Food. , 44 ( 2 ) : 76 – 84 .
- de Bont , P.W. , Hendriks , C.L.L. , van Kempen , G.M.P. and Vreeker , R. 2004 . Time evolution of phase separating milk protein and amylopectin mixtures . Food Hydrocolloid. , 8 ( 6 ) : 1023 – 1031 .
- Mackley , M.R. , Marshall , R.T.J. and Smeulders , J.B.A.F. 1995 . The multipass rheometer . J.Rheol. , 39 ( 6 ) : 1293 – 1309 .
- Páques , M. , Tromp , R.H. and van Aken , G.A. 2000 . Microrheology, a New Approach in the Study of Structure-/Function-relationships in Food Systems . Scanning. , 22 ( 2 ) : 114 – 115 .
- Nicolas , Y. , Paques , M. , van den Ende , D. , Dhont , J.K.G. , van Polanen , R. , Knaebel , A. , Steyer , A. and Munch , J.-P. 2003 . Microrheology: new methods to approach the functional properties of food . Food Hydrocolloid. , 17 ( 6 ) : 907 – 913 .
- Stokes , J.R. , Wolf , B. and Frith , W.J. 2001 . Phase-separated biopolymer mixture rheology: Prediction using a viscoelastic emulsion model . J. Rheol. , 45 ( 5 ) : 1173 – 1191 .
- Marcotte , M. , Hoshahili , A.R.T. and Ramaswamy , H.R. 2001 . Rheological properties of selected hydrocolloids as a function of concentration and temperature . Food Res. Int. , 34 ( 8 ) : 695 – 703 .
- Williams , P.A. and Ahmad , F.B. 2000 . “ Effect of dextran on the thermal and rheological properties of starch ” . In Hydrocollids Part. 2. Fundamentals and applications in food, biology, and medicine , Edited by: Nishinari , K. 165 – 176 . Amsterdam : Elsevier Science .
- Walkenström , P. and Hermansson , A.-M. 1998 . Effects of shear on pure and mixed gels of gelatin and particulate whey protein . Food Hydrocolloid. , 12 ( 1 ) : 77 – 87 .
- Marrs , W.M. 1982 . Gelatin carbohydrate interactions and their effect on the structure and texture of confectionery gels . Progr. Food Nutr. Sci. , 6 ( 1–6 ) : 259 – 268 .
- te Nijenhuis , K.T. 1997 . Thermoreversible Networks — Viscoelastic properties and structure of gels . Adv. Polym. Sci. , 130 : 1 – 12 .