Abstract
Water loss (WL), solid gain (SG), weight reduction (WR) and shrinkage were quantitatively investigated during osmotic dehydration of plum using response surface methodology with the sucrose concentration (30–60g/100 g sample), temperature of sucrose solution (40–60°C) and immersion time (60–240 min). Experiments were designed according to Central Composite Rotatable Design with these three factors. For each response, second order polynomial models were developed using multiple linear regression analysis. With respect to water loss, solid gain, weight reduction and shrinkage, both linear and quadratic effects of four variables were found to be significant. In most cases, an increase of sucrose concentration, temperature and immersion time increased WL, SG, WR and shrinkage, except the increasing of immersion time for osmotic treatment has no effect on shrinkage. It was found that immersion time and temperature were the most significant factors affecting the WL during osmotic dehydration of plum followed by concentration of sucrose solution. This was also true for WR. Effect of temperature and time were more pronounced for SG than the concentration of sucrose solution.
INTRODUCTION
Fruits and vegetables play a very important role in our diet and nutrition, since they are a source of not only raw fiber but also essential nutrients, vitamins, and minerals.[Citation1] Plum is an important temperate zone fruit crop. They come in a wide variety of colours and sizes. Some are much firmer fleshed than others are, and some have yellow, white, green or red flesh, with equally varying skin colour. Plums are rich in sugars and carotenes, low in fat and low in calorie. They are an excellent source of vitamin A, calcium, magnesium, iron, potassium and fiber. Like all fruits, plums contain a substantial amount of vitamin C. The plum and the food processed from it have been widely consumed due to its possible health benefits. In addition, the plum is believed to have a natural remedy against various diseases.[Citation2]
The moisture content of fresh harvested plums is about 78–90% wet basis (w.b.) which makes them highly perishable and hence drying and storage are considered important.[Citation2] Plum fruits can be used as fresh dessert fruit, prunes (the dried form of plums) or cooked.[Citation3,Citation4] They are also used for its juice and often jam or thick syrup is made out of it. The prune juice is widely used as flavouring in the food industry in areas such as biscuit making.[Citation5]
Plums are native in China, United States and Europe.[Citation5] Worldwide productions plums are about 10.1 million tones in 2003. Approximately, half of the world production is received from China that is producing about 4.2 million tones. Romania, USA, Serbia Montenegro, Germany, France, Chile and Turkey are the other main producer of plum in the world, respectively.[Citation6] The epicuticular wax is the limiting factor for the plums' moisture loss; it consists of an underlying amorphous wax layer adjacent to the cuticle proper, together with crystalline granules of wax protruding from the surface.[Citation5,Citation7]
Dehydrated products can be used in many processed or ready-to-eat foods in place of fresh foods and have several advantages such as convenience in transportation, storage, preparation and use.[Citation8,Citation9] Osmotic dehydration as a partial dehydration process involves direct contact of plant or animal tissue with a hypertonic solution leading to removal of water through the semi-permeable membrane of the food material and transferring of solute from the osmotic solution into the food. This osmotic dehydration process can reduce the water content of vegetables and fruits by 50%.[Citation10] The driving force for the diffusion of water from the tissue into the solution is provided by the higher osmotic pressure of the hypertonic solution. During osmotic dehydration step, an impregnation process with the osmotic solute from the solution to food material takes place simultaneously at countercurrent with the water flow from the food material (accompanied with the leaching of soluble compounds of the food material).[Citation11,Citation12]
Complex cellular structure of food could be considered as semi-permeable membrane and the difference in the chemical potential of water between the food and osmotic medium is the driving force for dehydration. Osmotic dehydration has been widely used as a pretreatment step in food drying processes to improve the quality of food by maintaining its integrity.[Citation13] This process is one of the energy efficient means of removing moisture from a food product,[Citation13,Citation14,Citation15] as the water does not have to go through a phase change to be released from the product. It is stated that some of the advantages of direct osmosis in comparison with other drying processes include minimized heat damage to colour and flavour, and less decolourisation of fruit by enzymic oxidative browning.[Citation12,Citation16,Citation17]
Several factors are known to affect the osmotic dehydration. These are the type of osmotic agent used, concentration and temperature of osmotic solution, the solution to material mass ratio, physicochemical properties of food materials, and the level of agitation of the solution.[Citation13,Citation18,Citation19] Sucrose has been recommended for osmotic dehydration of fruits because of its effectiveness, convenience and desired flavour.[Citation20] Besides the chemical changes, osmotic dehydration causes alteration of physical properties of plant tissue. Shrinkage, decreased water holding capacity, changes in porosity and resistance to deformation is usually observed during osmotic dehydration.
Response surface methodology has been reported to be an effective tool for optimising a process when the independent variables have a combined effect on the desired response. RSM is a collection of statistical and mathematical technique that has been successfully used for developing, improving and optimizing processes.[Citation21] It is a designed regression analysis meant to predict the value of a dependent variable based on the controlled values of the independent variables.[Citation22,Citation23] RSM reduces the number of experimental trials needed to evaluate multiple parameters and their interactions; therefore, it is less laborious and time consuming than other approaches. RSM has been widely applied for optimizing processes in food science.[Citation24–27]
Although several studies have been carried out to optimize the osmotic dehydration of fruits and vegetables by RSM,[Citation12,Citation15,Citation28,Citation29,Citation30,Citation31] negligible work has been done on osmotic dehydration of plum. Thus, the objective of the present study was to determine the effect of sucrose concentration (30–60 g/100g sample), temperature of sucrose solution (40–60°C) and immersion time (60–240 min) on the water loss, solid gain, weight reduction, and shrinkage of plum during osmotic dehydration using response surface methodology.
MATERIAL AND METHODS
Material
Samples of plums (Prunus spinosa L. Var. Bukharensis) were purchased from Golmakan Research Institute, Iran. The fruits were transported in plastic bags and sorted visually for colour (maturity) size (mass and diameter) and physical damage once in laboratory. Since the epicuticular wax is the limiting factor for the plums' moisture loss, samples were washed and then dipped into sodium hydroxide (1.5%, v/v) for 1 minute at room temperature in order to increase the permeability of the skin. Then the plums were neutralized with 1% ascorbic acid. Plums were stored in a cold chamber at 4°C until use, and their initial moisture content was 70% expressed in wet basis. Moisture content was determined by heating in a drying oven at 105°C for 48 h according to AOAC method 931.04.[Citation32]
OSMOTIC DEHYDRATION
Osmotic solutions were prepared by mixing food grade sucrose with the required amount of pure water. Plums were immersed in the osmotic solutions of given concentration (30–60 g/100 g sample) and temperature (40-60°C) during a given immersion time (60–240 min). A ratio of plum:sugar solution (1:4, by weight) was chosen in order to avoid significant dilution during osmotic treatment[Citation33]. The use of highly concentrated viscous sugar solutions creates major problems such as floating of food pieces, hindering the contact between the food material and the osmotic solution, causing a reduction in the mass transfer rates. Mavroudis, Gekas, and Sjoholm[Citation34] explain that the increase of agitation level could be a good alternative for this case.
The osmotic dehydration was carried out in a 1 L beaker fitted with a screen sieve. The osmotic solution was stirred with a magnetic stirrer at 200 rpm and the temperature was controlled by placing the beaker in a water bath. In each of the experiments, fresh osmotic solutions were used. After removal from the solution, the dehydrated samples from each group were drained and blotted with absorbent paper to remove the excess solution. The weight and moisture content data of each sample were utilized in order to calculate the response variables weight reduction (WR), water loss (WL) and sugar gain (SG), according to the following equations[Citation29,Citation31]:
where Mi = moisture content of fresh sample (g); Mo = moisture content of osmotically treated sample (g); Si = solids content of osmotically treated sample (g); So = solids content of fresh sample (g); and Wi = total weight of fresh sample (g), respectively. In order to determine the shrinkage of each sample, the plums volume was determined using the liquid displacement method.[Citation35] Toluene (C7H8) was used in instead of water, because it is absorbed by fruit to a lesser extent. In addition, its surface tension is low, so that it fills even shallow dips in a seed and its dissolution power is low.[Citation36] A standard pycnometric method was used to determine the volume of weighed samples. The volume (V, m3) calculated by the following relationship[Citation35]:
where, Mt is mass of pycnometer is filled by toluene; Mp, mass of pycnometer; Mpts, mass of pycnometer filled with toluene & sample; Mps, mass of pycnometer & sample; and ρtol, density of toluene. Then, shrinkage indices during osmotic treatment of each sample were measured using the following equation:
where, V is volume of fresh plum and V0 is volume of plum after osmotic treatment.
Experimental Design and Statistical Analysis
Response surface methodology (RSM) was used to estimate the main effects of osmotic dehydration process on shrinkage, weight reduction (WR), water loss (WL), and sugar gain (SG) in plum. A rotatable central composite design was used with sugar concentration (30–60 g/100 g sample), temperature (40–60°C) and immersion time (60–240 min) being the independent process variables. The design variables selected in this study with actual and coded levels along with response variables are shown in .
Table 1 Central Composite Rotatable Design for the independent variables (actual and coded levels)
The RSM was applied to the experimental data using a commercial statistical package, Design-Expert version 6.0.4 (Statease Inc., Minneapolis, USA). The experimental design included star points, and six centre points (0, 0, 0). The response functions (Y) were shrinkage, WR, WL, and SG. These values were related to the coded variables (xi , i = 1, 2 and 3) by a second-degree polynomial using the equation below:
The coefficients of the polynomial were represented by b0 (constant term), b1, b2 and b3 (linear effects), b11, b22 and b33 (quadratic effects), and b12, b13 and b23 (interaction effects). Statistical significance of the terms in the regression equations was examined. Regression analysis and analysis of variance (ANOVA) were conducted for fitting the models represented by EquationEq. (6) and to examine the statistical significance of the model terms. The adequacy of the models were determined using model analysis, lack-of fit test and R2 (coefficient of determination) analysis. The lack of fit is a measure of the failure of a model to represent data in the experimental domain at which points were not included in the regression or variations in the models cannot be accounted for by random error[Citation37]. If there is a significant lack of fit, as indicated by a low probability value, the response predictor is discarded. Coefficient of variation (CV) indicates the relative dispersion of the experimental points from the prediction of the model. The three-dimensional plots were drawn by keeping one variable constant at the center point and varying the other two variables within the experimental range. Response surface plots were generated with the same software. Optimal conditions for the osmotic dehydration of plum depended on sugar concentration, temperature and immersion time were obtained using the predictive equations of RSM.
RESULTS AND DISCUSSION
Fitting Models
Regression equations describing the effect of osmotic dehydration variables on the shrinkage, weight reduction (WR), water loss (WL) and sugar gain (SG) of plums are given in . Coefficient of determination, R2, is defined as the ratio of the explained variation to the total variation and is a measure of the degree of fit.[Citation38] The response surface models developed in this study for predicting the shrinkage, WR, WL and SG were adequate. The R2 values were 0.994, 995, 0.972, and 0.989, for WL, WR, SG, and shrinkage, respectively. Since the R2 values were higher than 0.80, the regression models explain the process well.[Citation39] These results showed that the model for WL, SG, WR, and shrinkage could explain 99.4, 99.5, 97.2, and 98.9% of the variations, respectively. This means only less than 3% of the variation was due to other factors not included in the model.
Table 2 ANOVA table showing the variables as a linear, quadratic and interaction terms on each response variable and coefficients for the prediction models
The lack of fit (), which measures the fitness of the model, did not result in a significant F-value in case of WL, WR, SG, and shrinkage, indicating that these models are sufficiently accurate for predicting those responses. As a rule, the coefficient of variation (CV) should not be greater than 10%. In this study, the coefficients of variation were less than 10% for all the responses (), a relatively lower value of the coefficient of variation indicates better precision and reliability of the experiments carried out.
Water loss (WL) was mainly affected linearly by immersion time, temperature and sucrose concentration and the effect of these factors were highly significant (p < 0.001). The quadratic effect of temperature was only significant at 5% level. The results also showed that the interaction between immersion time and sucrose concentration were also significant for WL at 1% level. These results were also true for weight reduction (WR), meaning that WR was also effected by all three factors. As for sugar gain (SG), sucrose concentration, temperature and immersion time were found significant. The quadratic effect of immersion time and temperature on SG was significant at 0.001 level whereas sucrose concentration was significant at 5% level. The interaction of temperature and time and sucrose concentration and time were significant at 0.001% and 0.01% level, respectively. Shrinkage was mainly affected linearly by temperature and sucrose concentration whereas immersion time was not significant. The quadratic effects of sucrose concentration were significant at 0.001 level wile emersion time and temperature was significant at 0.05 levels. It showed only a significant interaction effect between temperature with time and temperature with sucrose concentration at p < 0.001 with a negative effect.
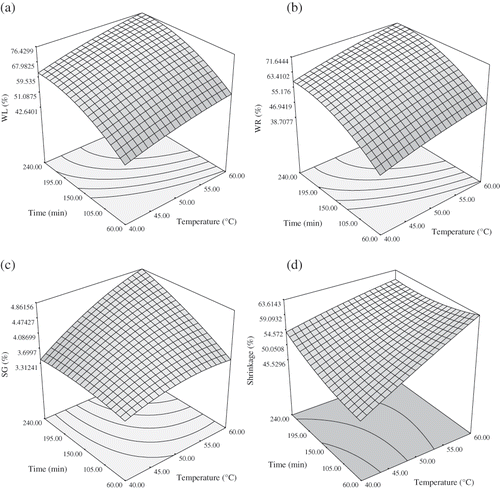
To visualize the combined effects of the two factors on the response, the response surface was generated for each of the fitted models in function of two variables, while keeping other variable at the central values. show the effect of sucrose concentration and temperature on WL, WR, SG, and shrinkage, when immersion time is kept constant. The WL, WR and SG increases with sucrose concentration over the entire osmotic dehydration process. Generally the larger the osmotic solution concentration the larger the water loss from the food is obtained.[Citation14] The rate of water loss and weight reduction gradually slowed down with increased concentration and then decreased slightly at high temperature. This result was similar to those reported by Cao et al.[Citation12] and Ponting, Walters, and Wang.[Citation40] Cao et al.[Citation12] found that above 60% sugar concentration, additional increase in sugar concentration did not promote further water loss in kiwifruit. The increase in solid gain with increasing sugar concentration is higher at high temperatures (50–60°C). These result agreed with Eren and Kaymak-Ertekin[Citation31] and Cao et al.[Citation12] Shrinkage of samples reduced with increasing the sucrose concentration (). Sugar movement into intracellular space might be the reason of shrinkage reduction.
Figure 1 Response surface for WL (a), WR (b), SG (c), and shrinkage (d) during osmotic dehydration of plum as function of sucrose concentration and temperature (at constant immersion time, 150).
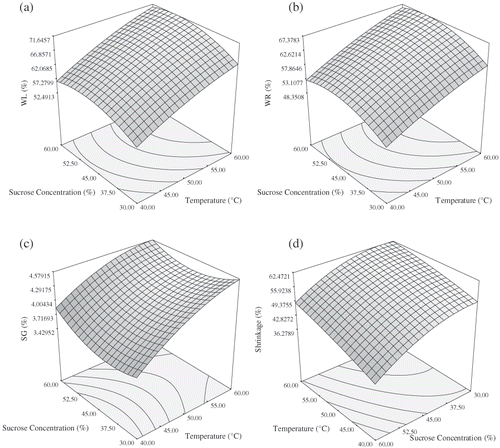
The interaction effect of sucrose concentration and temperature on WL shows that water loss is favored by increasing concentrations sucrose and increasing temperature () which was similar to the study by Cao et al.[Citation12], Uddin, Ainsworth, and Ibanoglu[Citation29], Rodrigues and Fernandes,[Citation41] and Eren and Kaymak-Ertekin.[Citation31] WR, SG and shrinkage also increase with increase in temperature (), which were consistent with literature values.[Citation12,Citation14] Contreras and Smyral[Citation43] proposed that the reason for this phenomenon is that higher temperatures seem to promote faster water loss through swelling and plasticizing of cell membranes as well as the better water transfer characteristics on the product surface due to lower viscosity of the osmotic medium. Weight reduction reached nearly the equilibrium conditions at 50% sucrose concentration at higher temperatures. These result had agreement with other studies.[Citation29,Citation31,Citation42]
show that the immersion time and the temperature of the osmotic solution were the most important effects on WL, WR, and SG. At the beginning of the process, because osmotic dehydration was not completed and due to the high osmotic driving force between the concentrated solution and the fresh sample, the rate of water removal and solid gain was relatively high. The WL, WR, and SG increases sharply with immersion time but at higher temperature, time didn't have a significant effect on shrinkage. Similar results have been reported by a vast number of researchers.[Citation29,Citation31,Citation41,Citation44 Citation,45]
Optimization
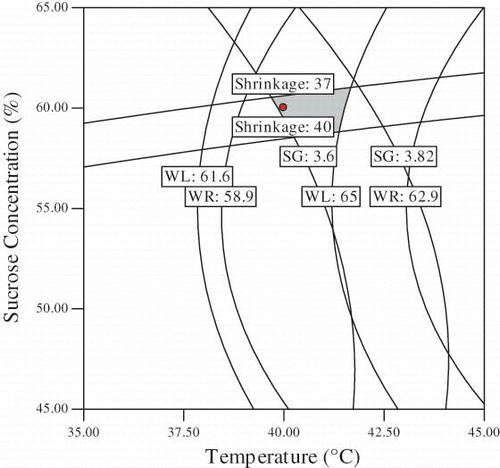
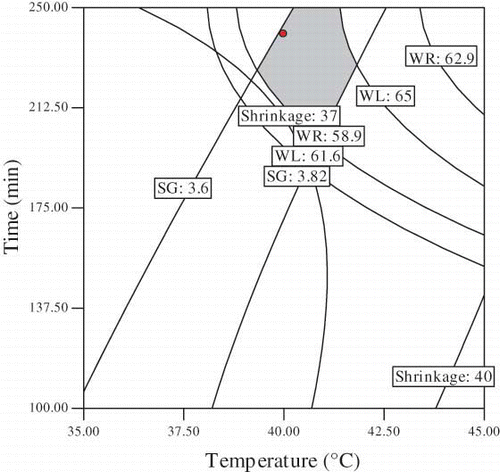
Numerical and graphical optimization was carried out for the process parameters for osmotic dehydration of plum. Optimum condition for osmotic dehydration was determined to obtain maximum water lost, and weight reduction with minimum solid gain and shrinkage. In this study, sucrose concentration, temperature and immersion time were selected in the range of 30–60 g/100 g sample, 40-60°C and 60–240 min, respectively. The highest value of WL: 63.49% and WR: 59.87% with lowest, SG: 3.62% and shrinkage: 37.88% were provided by using sucrose concentration of 60 (g/100 g sample) and temperature of 40°C during 240 min of immersion time.
and presents the effect of sucrose concentration, emersion temperature and time on WL, WR, SG and shrinkage. Superposition of these contour plots was carried out to obtain and , which were utilized to determine the best combination of osmotic dehydration of plum. A small shaded area, namely overlay area of the four responses is assigned as the optimum area of osmotic dehydration of plum which represents a higher amount of WL and WR and low SG and shrinkage. The optimum ranges obtained are sucrose concentration: 60–61.19 (g/100 g sample) and temperature: 39.31–41.6°C when the time is 240 min (). The optimum combination of temperature and time of emersion when the sucrose concentration was kept at 60 (g/100 g sample) was derived averaging those values: temperature = 40.91–41.73°C and time = 200–287.6 min.
CONCLUSION
The results showed that in most cases, an increase of sucrose concentration, temperature and immersion time increased WL, SG, WR, and shrinkage, except the increasing of immersion time for osmotic treatment has no effect on shrinkage. It was found that immersion time and temperature were the most significant factors affecting the WL and WR during osmotic dehydration. Effect of temperature and time were more pronounced for SG than the concentration of sucrose solution. Predictive equations of WL, SG, WR and shrinkage using a response surface methodology can be used to find optimum conditions for the physical properties of sweet plum products. The process conditions were optimized by numerical and graphical optimization methods and the optimum product qualities in terms of WL (63.49%), WR (59.87%), SG (3.62%), and shrinkage (37.88%) were obtained at sucrose concentration of 60 (g/100 g sample), temperature of 40°C and 240 min of immersion time.
REFERENCES
- Chakraverty , A. , Mujumdar , A.S. , Raghavan , G.S.V. and Ramaswamy , H.S. 2003 . “ Handbook of postharvest technology: cereals, fruits, vegetables, tea, and spices ” . 1 – 55 . New York : Marcel Dekker Inc .
- Sacilik , K. , Elicin , A.K. and Unal , G. 2006 . Drying kinetics of Uryani plum in a convective hot-air dryer . Journal of Food Engineering , 76 : 362 – 368 .
- Doymaz , I. 2006 . Effect of dipping treatment on air drying of plums . Journal of Food Engineering , 64 : 465 – 470 .
- Goyal , R.K. , Kingsly , A.R.P. , Manikantan , M.R. and Ilyas , S.M. 2007 . Mathematical modelling of thin layer drying kinetics of plum in a tunnel dryer . Journal of Food Engineering , 79 : 176 – 180 .
- Sabarez , H.T. and Price , W.E. 1999 . A diffusion model for prune dehydration . Journal of Food Engineering , 42 : 167 – 172 .
- FAO. Statistical database, 2004 http://www.fao.org/ (Accessed: 10 December 2009 ).
- Price , W.E. , Sabarez , H.T. , Storey , R. and Back , P.J. 2000 . Role of the waxy skin layer in moisture loss during dehydration of prunes . Journal of Agricultural and Food Chemistry , 48 : 4193 – 4198 .
- Mazza , G. and LeMaguer , M. 1980 . Dehydration of onion: some theoretical and practical considerations . Journal of Food Technology , 15 ( 2 ) : 81 – 194 .
- Lewicki , P.P. , Witrowa-Rajchert , D. , Pomaranska-Lazuka , W. and Nowak , D. 1998 . Rehydration properties of dried onion . International Journal of Food Properties , 1 ( 3 ) : 275 – 290 .
- Pan , Y.K. , Zhao , L.J. , Zhang , Y. , Chen , G. and Mujumdar , A.S. 2003 . Osmotic Dehydration Pretreatment in Drying of Fruits and Vegetables . Drying Technology , 21 : 1101 – 1114 .
- Dixon , G.M. and Jen , J.J. 1977 . Changes of sugar and acid in osmotic dried apple slices . Journal of Food Science , 42 : 1126 – 1131 .
- Cao , H. , Zhang , M. , Mujumdar , A.S. , Du , W. and Sun , J. 2006 . Omptimization of Osmotic Dehydration of Kiwifruit . Drying Technology , 24 : 89 – 94 .
- Torreggiani , D. 1993 . Osmotic dehydration in fruit and vegetables . Process and Food International , 26 ( 3 ) : 59 – 68 .
- Panagiotou , N.M. , Karathanos , V.T. and Maroulis , Z.B. 1999 . Effect of osmotic agent on osmotic dehydration of fruits . Drying Technology , 17 ( 1&2 ) : 175 – 189 .
- Madamba , P.S. and Lopez , R. I. 2002 . Optimization of osmotic dehydration of the osmotic dehydration dehydration of mango (Mangifera indica L.) . Drying Technology , 20 ( 6 ) : 1227 – 1242 .
- Saurel , R. , Raoult-Wack , A.L. , Rios , G. and Guilbert , S. 1994 . Mass transfer phenomena during osmotic dehydration of apple I. Fresh plant tissue . International Journal of Food Science and Technology , 29 : 531 – 542 .
- Krokida , M.K. , Maroulis , Z.B. and Saravacos , G.D. 2001 . The effect of the method of drying on the colour of dehydrated products . International Journal of Food Science and Technology , 36 : 53 – 59 .
- Lerici , C.R. , Pinnavaia , G. , Dalla Rosa , M. and Bartolucci , L. 1985 . Osmotic dehydration of fruits: influence of osmotic agents on drying behavior and product quality . Journal of Food Science , 50 : 1217 – 1220 .
- Rastogi , N.K. and Raghavarao , K.S.M.S. 1997 . Water and solute diffusion coefficients of carrot as a function of temperature and concentration . Journal of Food Engineering , 34 : 429 – 440 .
- Lenart , A. 1996 . Osmo-concective drying of fruits and vegetable tissues undergoing osmotic processing . Drying Technology , 14 : 2 – 10 .
- Myers , R. and Montgomery , D.C. 2002 . Response surface methodology , New York : Wiley .
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory evaluation techniques, 2nd ed.; CRC Press: Boca Raton, FL, 1991. pp. 357–404.
- Resurreccion , A.V.A. 1998 . “ Quantitative of quality attributes as perceived by the consumer ” . In Consumer sensory testing for product development , 203 – 228 . Gaithersburg , MD : Aspen Publishers, Inc .
- Ibanoglu , S. and Ainsworth , P. 2004 . Application of response surface methodology for studying the viscosity changes during canning of tarhana, a cereal-based food . Journal of Food Engineering , 64 : 273 – 275 .
- Quanhong , L. and Caili , F. 2005 . Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein . Food Chemistry , 92 : 701 – 706 .
- Afoakwa , E.O. and Yenyi , S.E. 2006 . Application of response surface methodology for studying the influence of soaking, blanching and sodium hexametaphosphate salt concentration on some biochemical and physical characteristics of cowpeas (Vigna unguiculata) during canning . Journal of Food Engineering , 77 : 713 – 724 .
- Sin , H.N. , Yusof , S. , Sheikh Abdul Hamid , N. and Rahman , R.A. 2006 . Optimization of enzymatic clarification of sapodilla juice using response surface methodology . Journal of Food Engineering , 73 : 313 – 319 .
- Ravindra , M.R. and Chattopadhyay , P.K. 2000 . Optimization of osmotic preconcentration and fluidized bed drying dehydrated quickcooking potato cubes . Journal of Food Engineering , 44 : 5 – 11 .
- Uddin , M.B. , Ainsworth , P. and Ibanoglu , S. 2004 . Evaluation of mass exchange during osmotic dehydration of carrots using response surface methodology . Journal of Food Engineering , 65 : 473 – 477 .
- El-Aouar , A.A. , Azoubel , P.M. , Barbosa Jr , J.L. and Xidieh Murr , F.E. 2006 . Influence of the osmotic agent on the osmotic dehydration of papaya (Carica papaya L.) . Journal of Food Engineering , 75 : 267 – 274 .
- Eren , I. and Kaymak-Ertekin , F. 2007 . Optimization of osmotic dehydration of potato using response surface methodology . Journal of Food Engineering , 79 : 344 – 352 .
- AOAC . 1990 . Official methods of analysis , Washington , , DC : Association of Official Analytical Chemists .
- Le Marguer, M. Osmotic dehydration: review and future directions. In Proceedings of the symposium in food preservation process, vol. 1 CERFCI: Brussel, 1988; 283–309.
- Mavroudis , N.E. , Gekas , V. and Sjoholm , I. 1998 . Osmotic dehydration of apples—effects of agitation and raw material characteristics . Journal of Food Engineering , 35 : 191 – 209 .
- Mohsenin , N.N. 1970 . Physical properties of plant and animal materials , 79 – 128 . New York : Gordon and Breach Science Publishers .
- Ogut , H. 1998 . Some physical properties of White lupin . Journal of Agricultural Engineering Research , 56 : 273 – 277 .
- Montgomery , D. C. 2001 . “ Design and analysis of experiments ” . In , 5th , 455 – 492 . New York : Wiley .
- Haber , A. and Runyon , R. 1977 . General statistics , 3rd , Reading , MA : Addison-Wesley .
- Joglekar , A.M. and May , A.T. 1987 . Product excellence through design of experiments. C . ereal Foods World , 32 : 857 – 868 .
- Ponting , J.D. , Walters , G.G. and Wang , X.B. 1966 . Osmotic dehydration of fruits . Food Technology , 20 : 125 – 128 .
- Rodrigues , S. and Fernandes , F.A.N. 2006 . Dehydration of melons in a ternary system followed by air-drying . Journal of Food Engineering , 80 : 869 – 872 .
- Corzo , O. and Gomez , E. R. 2004 . Optimization of osmotic dehydration of cantaloupe using desired function methodology . Journal of food engineering , 64 : 213 – 219 .
- Contreras , J.E. and Smyral , T.G. 1981 . An evaluation of osmotic concentration of apple rings using corn syrup solids solutions . Canadian Institute of Food Science and Technology Journal , 14 : 310 – 314 .
- Chenlo , F. , Moreira , R. , Fernandez-Herrero , C. and Vazquez , G. 2006 . Experimental results and modeling of the osmotic dehydration kinetics of chestnut with glucose solutions . Journal of Food Engineering , 74 : 324 – 334 .
- Mendoza , R. and Schmalko , M.E. 2002 . Diffusion coefficients of water and sucrose in osmotic dehydration of papaya . International Journal of Food Properties , 5 : 537 – 546 .