Abstract
Wavelength-dispersive x-ray fluorescence (WDXRF) was applied to determine bromate (Br) as an indication of pre-baking bromate addition in bread. The proposed methodology needed a minimum sample preparation procedure because it was carried out directly on solid samples. The calibration of Br in bread obtained showed low detection limit and high sensitivity to distinguish precisely Br concentrations greater than natural Br. The excellent performance of the present methodology would be useful to identify pre-baking bromation in bread, which can be used to help set up a programme to control bromation in bread. Application of this methodology to bakery control caused an important reduction of bromate use in province of Córdoba, Argentina.
INTRODUCTION
Due to different baking qualities of wheat flours, the new technologies and the different properties of baked goods elaborated all over the world, the use of additives to control their rheology and texture have become a common practice in the baking industry. The main groups of additives used successfully in food industry to modify the rheological and textural properties of emulsions, suspensions, and foams are enzymes, emulsifiers, oxidizers, and hydrocolloids.[Citation1,Citation2]
Strong oxidizers, as potassium bromate, are commonly used in bread production. They produce an increase in the volume of bread and make the kneading of dough easier. In many countries, the use of potassium bromate in bread production is forbidden because of its toxicity. The most common acute signs of bromate ingestion are severe gastrointestinal irritation (vomiting, pain and diarrhoea)[Citation3] and central nervous system depression (lethargy, hypotension, hypotonicity, and loss of reflexes).[Citation4] These effects are reversible, but bromate exposure for several days can induce permanent renal injury and hearing loss.[Citation3,Citation5] Bromate may exert its toxicity through the production of reactive oxygen species, which results in oxidative stress.[Citation6] Toxicological studies in rats have shown that bromate is a potent animal carcinogen.[Citation7,Citation8]
Seventy three percent of the total production of flour in Argentina is used in the manufacture of bread products. Small and medium bakeries consume 70% of this quantity and industrial establishments consume 30%.[Citation9] In small and medium bakeries, a special breadmaking process, which makes a good product with minimum infrastructure, is currently employed. Until 1998, this method was based on the use of potassium bromate as oxidizer. From 1998, MERCOSUR Legislation forbade potassium bromate in bakery production, so the replacement of this salt by other oxidizers was necessary.[Citation10] However, other salts or enzymes successfully applied in other countries cannot be directly adopted in Argentina because the breadmaking process currently employed in our country is an original process. This process requires baking times longer than eight hours and low quantity of yeast, which are not common in other procedures of manufacture. Consequently, a high proportion of commercial bakeries in Argentina still use potassium bromate to produce apparently good bread. To change this situation, bakery inspections are necessary to stop the use of bromate using a reproducible and simple analytical procedure.
Reduction of bromate to bromide occurs because of the reductive properties of bread dough. Reduction begins when ingredients are mixed and continues during baking. When the reduction process is finished, the amount of bromate residue in the final product is very low. It is the principal reason for which the conventional analytical techniques for bromate determination are only applied in flour, additives, and dough.[Citation11] Other methods are capable of measuring bromate residues as low as 10 ppb in bread. [Citation12–15] These methods are laborious, time consuming, require extensive analytical clean-up, and in some cases, derivation prior to chromatographic determination. However, the amount of bromide could be elevated in bread products when potassium bromate is used.[Citation15] Therefore, Br determination in bread products would be useful to identify which products would be most likely to have bromate.
In the present work, the wavelength-dispersive x-ray fluorescence (WDXRF) was applied to Br determination as an indication of pre-baking bromation of bread. There are previous studies showing the good performance of XRF in measuring Br in bread[Citation16]. However, the present procedure has the advantage that the measurements can be carried out directly on the solid samples pressed into pellets. It avoids the sample digestion-dissolution step, using corrosive and toxic reagents. As a consequence, this procedure is less time consuming in preparation and manipulation.
MATERIAL AND METHODS
Preparation of Bread with Known Amounts of Potassium Bromate
Bread was prepared using the following recipe: flour (500 g), water (300 g), yeast (15 g) and salt (0.9 g). Commercial bread flour was obtained from the local market (Carlos Boero Romano S.A.I.C., Argentina). Four bread samples were prepared with different amounts of potassium bromate. Ingredients were mixed in a Philips HR 1495 mixer (Philips, Argentina) for 2 min and rested for 20 min in a cabinet at 30ºC. The dough was then fermented for 10 h. Finally, the dough was cooked at 180ºC for 17 min.
Bromate Measurements
Potassium bromate determinations were performed by applying the AACC 48-42 method (11). Ammonium molybdate, zinc sulphate, and potassium iodate were purchased from Merck (Darmstadt, Germany). All the other chemicals and reagents used in this method were purchased from Sigma Chemical Co. (St. Louis, USA).
Br Measurements
Br determinations were done by WDXRF in a BRUKER SRS3400 spectrometer, which has an end-window x-ray tube with Rh-anode. The spectrometer was operated at 60 kV and 67 mA in vacuum atmosphere in order to produce the optimum excitation of Br-Kα fluorescence line in the sample. The measurement time, including background, was of only 30 seconds. Interference effects in the energy region of Br-Kα fluorescence line were not present in bread samples, so the spectral analysis was simple. Besides, the size grain effect, which is common in pressed pellets methodology, did not show major influence on the intensity of Br-Kα fluorescence line. This means that the Br determinations in bread by XRF were very reproducible.
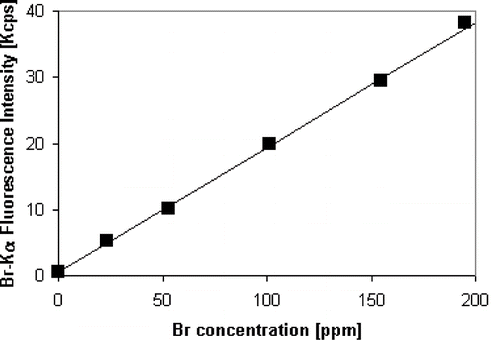
To calibrate the spectrometer, the standard addition method was applied. Five standard materials with known amounts of Br were prepared using a standard aqueous solution of Br at 1000 ppm (Tritisol, MERCK, Darmstadt, Germany). First, a known volume of the Br solution was added to 4 g of cellulose (PrepAid cellulose binder, SPEX, Metuchen, USA). After that, the wet sample was dried at 60ºC for 24 h. Then, the sample was mixed for 15 min with 1 g of wax (Hoechst wax C micropowder, MERCK, Darmstadt, Germany) using a 5100 SPEX Mixer/Mill. Finally, the sample was deposited into a 40 mm aluminium cup and pressed at 10 Ton in a pellet die with tungsten carbide pellets. As a result, a solid and stable 40 mm sample disc was obtained. The volumes of Br solution added for the five calibration standards were 0.1, 0.2, 0.5, 0.75, and 1 ml; which generated the standard samples of 25, 50, 100, 150, and 200 ppm, respectively (ppm: parts per million by weight). The calibration curve obtained with the five calibration samples had low dispersion and high sensitivity as shown in . In , the vertical axis represents the Br Kα fluorescence intensity corrected by matrix effects by fundamental parameters. The detection limit of the calibration was 1 ppm, which is adequate to measure the levels of Br in bread. A standard material with 40 ppm of Br was prepared in the same way than the standards materials described previously. The sample disc was quantified by XRF using the calibration curve of . The absolute error of the result was 0.5 ppm showing the good accuracy of the proposed methodology.
The sample preparation of bread for Br determination by XRF proposed in this work was found very simple. Firstly, the bread was dried at 60ºC for 24 h. Then, the sample was pulverized in a disc mill with zirconia grinding container for 2 min. Then, the sample was mixed for 5 min with 1 g of wax using a 5100 SPEX Mixer/Mill. Finally, the sample was pressed to form a 40 mm sample disc as was described by calibration standards. To study the precision of our methodology we measured by duplicate samples of bread. Breads from ten bakeries randomly selected were divided into two groups. Each group was analysed by XRF using the procedure described above. With the results from the same bread, the relative error of the determination was calculated. Taking average of these errors we obtained a mean relative error of 10%, which was used to calculate the absolute errors of the Br determinations by XRF presented in this work.
In each sample analyzed in this work, Br concentration was measured in dried bread, because the water content showed a high variability. This procedure allowed a direct comparison of the results and simplified the analytical procedure because it avoided the water content determination.
RESULTS AND DISCUSSIONS
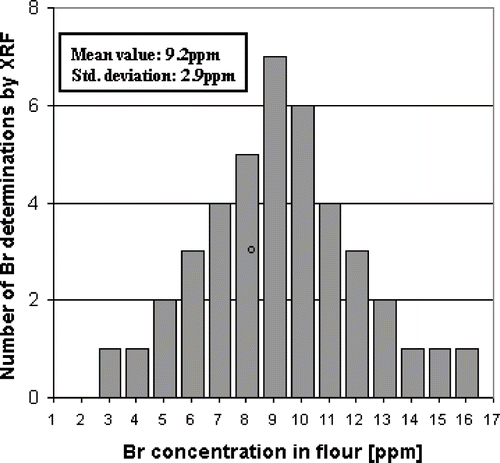
In order to detect the Br contribution of Potassium Bromate in dried bread samples, the typical values of natural Br in breads were required. Bread contained a relatively small but significant amount of natural Br when contributions from bromation and contaminants are excluded. Since flour is the main contribution of Br in bread,[Citation17] it is a good approximation to consider the flour contribution equal to the natural Br in dried bread. In order to know the typical values of natural Br in flour, 41 samples of commercial flour produced in Argentina were measured using the proposed methodology. The obtained results in revealed a mean value of 9.2 ppm for Br in flour with a standard deviation of 2.9 ppm. To calculate added Br in dried breads, 9.2 ppm were subtracted from the total Br concentration, which accounts for the natural Br in bread. For the detection of bromation in dried bread samples, the screening value of 16 ppm were considered, which represents possible bread without potassium bromate produced with the flour of the highest natural Br concentration.
The measurements of Br in the test bread samples are shown in the . All the values showed the amounts of added Br to the test bread samples, which means that Br loss is negligible during baking process. This result corroborates previous reports on bromation of bread products, which means that it is possible to use the Br determination in bread to help identify those products which have potassium bromate in their recipes. After that, the XRF predictions were compared to the bromate determinations following the AACC 48-42 method. Bread samples collected in 25 bakeries of Córdoba city were analysed with these methodologies. The results showed reduction bromate to bromine in high proportion during the baking process (). For samples with bromate residues, the bromate concentrations were not linearly proportional to the bromine concentrations. It means that the reduction reaction of bromate was incomplete probably due to differences in the baking conditions applied in the bakeries. Bromate residues were only detected in samples with Br concentrations greater than the screening value of 16 ppm. However, there were samples with Br concentrations greater than 16 ppm without bromate detection. Assuming that samples were not contaminated, this means that bromate reduction occurred in the baking process for these samples. Nevertheless, it is important to know that little amounts of bromate mean a risk to consumer health. Therefore, the proposed methodology could identify bromation in bread even when the bromate residues were quite low, this is an advantage over quantitative methods regarding to determination of bromate, which cannot detect bromation in all possible situations. [Citation11–15]
Table 1 Br determinations by XRF in dried test breads
Table 2 Br determinations in dried breads by XRF and AACC 48-42 method
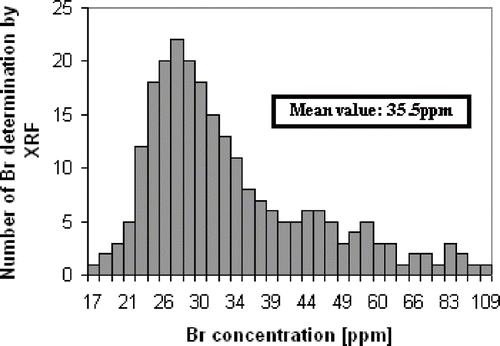
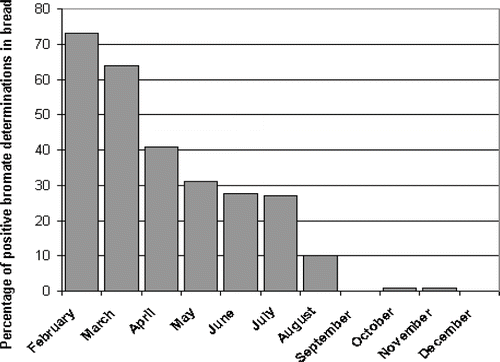
Using the proposed methodology, 1100 analysis of Br in bread were done. Bread loaves were sampled in several bakeries spread over the province of Córdoba. This province is located in the central region of Argentina covering a total area of 165321 square kilometres. shows the distribution of Br concentrations in dried breads above the screening value of 16 ppm. The mean value was 35.5 ppm, which represents an amount of added potassium bromate of 55.3 mg per kg of flour. This value is included in the typical range of the potassium bromate quantities indicated in the old bread recipe employed in our country until 1998. It means that a high proportion of establishments had not updated their bakery process after potassium bromate was forbidden. shows the monthly variation of the number of bread samples with Br concentrations above the screening value of 16 ppm. At the beginning, 73% of analysis was positive, but in a few months the positive results were reduced to approximately 2%. We think that it was the consequence of a publicity campaign conducted in Córdoba province to persuade bakers to use modern bread recipes without potassium bromate. The success of this publicity campaign depended mainly on the efficiency reached in the bakery inspections with the present methodology.
The main advantage of the proposed methodology is that it allows studying the bromation of bread avoiding the digestion of the sample. [Citation11–15] This advantage is shared with other methodologies as NAA or PIXE,[Citation17,Citation18] however the instrumental of XRF is considerable less costly than these alternatives.
CONCLUSIONS
The proposed methodology implies a minimum sample preparation procedure because it is carried out directly on solid samples avoiding the sample digestion-dissolution step with corrosive and toxic reagents. Besides, it has a short analysis time and does not require qualified technicians for measurements and calibrations. The obtained calibration of Br in bread has a low detection limit and high sensitivity, which is good enough to distinguish precisely Br concentrations greater than Br natural. These properties imply a good reproducibility and efficiency, which are important advantages over other alternative techniques. The excellent performance of the present methodology would be useful to identify pre-baking bromation in bread, which can be used to help set up a programme to bromation control in bread. The present methodology has been successfully applied in 1100 bakery inspections carried out in the province of Córdoba, Argentina; hence, the use of bromate diminished dramatically.
ACKNOWLEDGEMENTS
The authors would like to thank the Laboratorio de Idiomas (FCA-UNC) for providing useful suggestions to improve the English in this paper, and the Agencia Córdoba Ciencia SE for financial support.
REFERENCES
- Kaur , K. , Singh , N. and Singh , H. 2002 . Studies on the effect of skim milk powder, sprouted wheat flour, and pH on rheological and baking properties of flour . International Journal of Food Properties , 5 : 13 – 24 .
- Sidhu , J.P.S. 2002 . Bawa A.S. Dough Characteristics and baking studies of wheat flour fortified with xanthan gum . International Journal of Food Properties , 5 : 1 – 11 .
- Matsumoto , I. , Morizono , T. and Paparella , M.M. 1980 . Hearing loss following potassium bromate: two case reports . Otolaryngology-Head and Neck Surgery , 88 : 625 – 629 .
- Parker , W.A. and Barr , J.R. 1951 . Potassium bromate poisoning . British Medical Journal , 1 : 1363 – 1363 .
- Kuwahara , T. , Ikehara , Y. , Kanatsu , K. , Doi , T. , Nagai , H. , Nakayashiki , H. , Tamura , T. and Kawai , C. 1984 . Two Cases of potassium bromate poisoning requiring long-term hemodialysis therapy for irreversible tubular damage . Nephron , 37 : 278 – 280 .
- Giri , U. , Iqbal , M. and Athar , M. 1999 . Potassium bromate (KBrO3) induces renal proliferative response and damage by elaborating oxidative stress . Cancer Letter , 135 : 181 – 188 .
- Kurokawa , Y. , Hayashi , A. , Maekawa , T. , Takahashi , T. and Kokubo , T. 1983 . Odashima, S. Carcenogenicity of potassium bromate administered orally to F344 rats . Journal of the National Cancer Institute , 71 : 965 – 972 .
- United State Environmental Protection Agency. Toxicological review of bromate. CAS No 15541-45-4. In support of summary information on the integrated risk information system (IRIS). 2001. http://www.epa.gov/iris
- Pantanelli , A. 2001 . Cadenas alimentarias: harina de trigo . Alimentos Argentinos , 22 : 25 – 30 .
- Código Alimentario Argentino. Anexo Mercosur - GMC - RES 073/93. 1998. Cap IX, p. 54.
- 11. American Association of Cereal Chemists, 2000. AACC Method 48-42. Approved Methods of the American Association of Cereal Chemists, 10th ed. St. Paul, MN, USA: AACC International. Method 48–42. pp. 1–2.
- Himata , K. , Kuwahara , T. , Ando , S. and Maruoka , H. 1994 . Determination of bromate in bread by capillary gas chromatography with a mass detector (GC/MS) . Foods additives and contaminants , 11 : 559 – 569 .
- Dennis , M. J. , Burrel , A. , Mathieson , K. , Willetts , P. and Massey , R. C. 1994 . The determination of the flour improver potassium bromate in bread by gas chromatographic and ICP-MS methods . Foods additives and contaminants , 11 : 633 – 639 .
- Himata , K. , Noda , M. , Ando , S. and Yamada , Y. 1997 . Measurement of bromate in bread by high performance liquid chromatography with post-column flow reactor detection . Food Additives and Contaminants , 14 : 809 – 818 .
- Heitkemper , D.T. , Kaine , L.A. , Jackson , D.S. and Wolnik , K.A. 1994 . Practical applications of element-specific detection by inductively coupled plasma mass spectrometry and inductively coupled plasma mass spectrometry to ion chromatography of foods . Journal of Chromatography A , 671 : 101 – 108 .
- Adelfang , P. , López , C.P. , Bosso , S. , Gandía , S. and Benzi , C. 1998 . Avances en Análisis por Técnicas de Rayos X . 10 : 66 – 71 .
- Cunningham , W.C. and Warner , C.R. 2000 . Br concentration as an indication of pre-baking bromation of bread products . Food Additives and Contaminants , 17 : 143 – 148 .
- Romo-Kröger , C. M. 2002 . An overview of x-ray spectrometry at research centers in Chile 2002 . X-ray spectrometry , 31 : 128 – 131 .