Abstract
The physical state of components in the unfrozen solute phase of frozen solutions was determined by using differential scanning calorimetry (DSC) for mixtures of lactose, sucrose, and trehalose with albumin, gelatine, or cornstarch. An equal weight ratio (1:1) of lactose-sucrose, lactose-trehalose and sucrose-trehalose mixtures with polymer systems (sugar mixture-albumen (1:1:1), gelatine (1:1:1), and cornstarch (1:1:1), as well as cornstarch-gelatine (1:1:1:1) was used. Mixed sugar mixture (lactose/sucrose)-polymer systems were further studied for maximum freeze-concentration in complex systems. A comparative thermal study between sugar-polymer and mixed sugar-polymer systems was conducted. The sugar-polymer and mixed sugar-polymer systems showed similarities and differences in thermal behaviour. The similarities included maximum freeze-concentration of a frozen system at an annealing temperature (Tm´-1) °C, constant initial concentration independent onset of glass transition Tg´, and onset of ice melting temperature Tm´, and increased in temperature difference between Tg´ and Tm´ or increase in broadness of transition with the addition of polymeric compound.
INTRODUCTION
The stability of food and biological systems is affected by the solute-solvent interactions. The freezing of food and the biological systems changes solute-solvent interactions because of separation of water (solvent) into crystallized ice and solutes in an amorphous unfrozen solute phase.[Citation1,Citation2] The amorphous phase exists in a thermodynamically non-equilibrium (metastable) state, and it is temperature dependent.[Citation3,Citation4,Citation5,Citation6,Citation7,Citation8] The quality and stability of frozen foods depend on the physical state of constituent solids present in the unfrozen phase.[Citation9]
The properties of an unfrozen phase affect ice formation in a frozen system. Ice formation in a frozen system depends on the heat and mass transfer properties and composition of the unfrozen phase.[Citation10,Citation11] It has been reported that ice formation in the frozen systems is greatly influenced by system composition and its mass transferring properties.[Citation5,Citation6,Citation8,Citation10,Citation11]
Muhr and Blanshard[Citation12] found that food hydrocolloids (polymers) in the unfrozen phase influenced frozen systems by affecting ice crystals size and morphology. Harper and Shoemaker[Citation13] and Budiaman and Fennema[Citation14,Citation15] reported that sugars in a solute phase affect ice formation more than polymeric components. The rate of crystallization as affected by food hydrocolloids depends on the type of food hydrocolloid used.[Citation14,Citation15] Buyong and Fennema[Citation14,Citation15,Citation16] reported that hydrocolloids retard the growth of ice crystals to greater extent in combination with sugars than alone. The use of polymers as stabilizers usually modified the kinetic properties of an unfrozen phase rather than thermal properties, such as glass transition.[Citation7,Citation10] Recently, Singh and Roos[Citation17,Citation18,Citation19] reported that the viscosity of the unfrozen phase is likely to control ice formation in disaccharides-polymers food systems.
In addition to less ice formation, rapidly cooled vitrified systems consist of high amounts of unfrozen water in the unfrozen solute phase.[Citation5,Citation8,Citation9,Citation11] Rewarming of a rapidly vitrified system showed ice crystal growth before melting.[Citation6] Ice crystal growth before melting was designated as devitrification when studied on DSC.[Citation6,Citation8] The presence of a devitrification peak on thermographs of frozen systems was considered as a stability related process in frozen foods.[Citation14,Citation15,Citation20–27]
An exotherm or devitrification peak before melting of ice on DSC graphs was found to be due to partial freeze-concentration and the associated high amount of unfrozen water in the unfrozen phase.[Citation9] The high amount of unfrozen water in a rapidly cooled system dilutes the glassy matrix and increases the mobility above its low Tg.[Citation6,Citation9,Citation10] The increase in mobility in a frozen system above this Tg favours the ice formation before melting.[Citation6–10]
Roos and Karel[Citation9] suggested that sufficient annealing of a system at a temperature slightly below the temperature of onset of ice melting (Tm´) eliminates devitrification, as time is allowed for ice formation at conditions allowing maximum freezing. Annealed systems may have maximum amount of solute concentration in an unfrozen phase and have concentration independent and constant glass transition temperature (Tg´) and onset temperature of melting (Tm´).[Citation9,Citation11]
The state diagrams suggested by Franks and others[Citation28] have been used by various authors to describe the maximum concentration of solutes in the unfrozen phase of a maximally freeze-concentrated system.[Citation5,Citation6] Roos[Citation11] and Sahagian and Goff[Citation10] reported that in the maximally freeze-concentrated state a frozen system often contains about 80% concentration of solute (Cg´) in an unfrozen phase.
Quality changes in frozen food systems are often a result of physico-chemical changes occurring at temperatures above Tg´.[Citation29–35] It has been reported that a decrease in viscosity above Tg´ and further above Tm´ was to increases rates of diffusion controlled chemical reactions in frozen foods.[Citation29–35] Above Tg´ and Tm´ an increase in free volume or unfrozen water results in increased molecular mobility, which enhance diffusion controlled chemical reactions.[Citation32,Citation35] It has been reported that the rates of diffusion controlled chemical reactions inversely relate to the viscosity of the unfrozen phase.[Citation29–35]
Large efforts have been made to determine the thermal behaviour of pure carbohydrates. This study is the continuation of previous studies on a model system composed of a single sugar and a polymer.[Citation17–19] Frozen foods are, however, a complex mixture of food ingredients. Ice cream, as an example, is a mixture of several sugars (lactose, sucrose) and various polymeric compounds like proteins and polysaccharides. This study was carried out with the aims 1) to determine thermal behaviour of a model system with two sugars; and 2) to study how the thermal behaviour and maximum ice formation are affected by the presence of polymers in this system. The results from the present study can be further used to point out the differences between reported single sugar-polymer systems and present mixed sugar-polymer systems.
MATERIALS AND METHODS
Materials
α-lactose monohydrate, sucrose (Cane sugar, SigmaUltra), trehalose dihydrate, gelatin (Type B: from Bovine Skin), egg albumin (Chicken Egg, Grade II), and corn starch were products of Sigma-Aldrich Chemie Gmbh, 89552 Steinheim, Germany. These and distilled water were used in the present study.
Preparation of Solutions
Lactose, sucrose, trehalose, albumin, gelatin, and cornstarch, were weighed with water and solutions with 10%, 20%, and 30% total solids (w/w) were made to analyze their frozen state behaviour using DSC. Systems were also made using various components in an equal weight ratio (lactose/ sucrose/ albumin (1:1:1), lactose/ trehalose/ albumin (1:1:1), sucrose/ trehalose/ albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ trehalose/ gelatin (1:1:1), sucrose/ trehalose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), lactose/ trehalose/ cornstarch (1:1:1), sucrose/ trehalose/ cornstarch (1:1:1), and lactose/ sucrose/ gelatin/ cornstarch (1:1:1:1)).
The sugar solutions with albumin were prepared at room temperature using magnetic stirrer to obtain clear solutions. A water bath at 60°C was used to obtain clear solutions of sugar-gelatine mixtures. Heated solutions were cooled to room temperature and weighed. Any weight loss resulting from evaporation of water during heating was added to the solution on an analytical balance.
For differential scanning calorimetry (DSC) samples with cornstarch were gelatinized with sugar mixtures in DSC pans before determining their thermal behaviour. The procedure used for gelatinizing (before cooling in DSC cell) included first heating to 85°C and isothermal holding at this temperature for 5 min to complete gelatinization of starch. The solutions containing cornstarch used for freeze-drying were gelatinized in a water bath above 85°C for 5 min before freeze-drying. All solutions were cooled at room temperature and water loss because of evaporation was added on an analytical balance.
Preparation of Anhydrous Solid Materials
Anhydrous, amorphous materials were obtained by freeze-drying (Amsco, Finn Aqua, Lyovac GT2) 20% model system solutions on petri dishes. Aliquots of model solutions were filled into petri dishes up to an approximate height of 0.5–1 cm. Solutions in petri dishes were immediately pre-frozen at −20°C for 12 h and subsequently transferred to a −80°C freezer for 24 h. From −80°C freezer, samples were rapidly transferred to a freeze-drier (within a time interval of less than 1 min) and vacuum was applied. The solutions on petri dishes were freeze-dried for 72 h at a pressure < 0.1 mbar corresponding to ice temperature of −45°C.[Citation17–19] The freeze-dried samples were stored for 5 days at room temperature in vacuum desiccators over P2O5 for further dehydration. After P2O5 treatment, freeze-dried materials were crushed to a fine powder with a mortar and pestle. 5–25 mg samples of the powder were filled in DSC pans and placed over P2O5 at room temperature for 3 days for further the removal of residual moisture.
Differential Scanning Calorimetry (DSC)
DSC (DSC, Mettler Toledo 821e with liquid N2 cooling) was used to observe phase and state transitions. The instrument was calibrated for heat flow and temperature as reported by Singh and Roos.[Citation17–19] Frozen-state behaviour was determined using 10–35 mg samples weighed in 40 μl aluminum pans (Mettler Toledo 27331, Switzerland). Each sample was weighed accurately using a Mettler Toledo AG245 balance. Filled pans were hermetically sealed. An empty aluminum pan was used as a reference in all measurements. Thermograms were analysed for the onset temperature of glass transition, Tg´, and onset temperature of ice melting, Tm´ using Stare, version 6.0 (Mettler Toledo, Switzerland) thermal analysis software.
Annealing
Annealing below Tm´ was used to obtain a maximum freeze-concentration in sugar solutions.[Citation9] In the present study, annealing was done using Singh and Roos[Citation17] method. The glass transition (Tg´) of the maximally freeze concentrated phase was an endothermal change indicating a change in heat capacity.[Citation6,Citation9,Citation11,Citation17–19] A Tg´ curve followed by an endotherm was considered as an onset temperature of ice melting and denoted by Tm´.[Citation9,Citation11,Citation17,–Citation19]
Analysis of Dried Samples
To determine Tg, 5–25 mg samples of powdered freeze-dried materials were stored in open DSC pans for 144 h at room temperature in evacuated desiccators over P2O5 and different saturated salt solutions: LiCl, CH3COOK, MgCl2, K2CO3, Mg(NO3)2, NaNO2, and NaCl (Sigma-Aldrich Chemie Gmbh, 89552 Steinheim, Germany) with respective relative water vapor pressures (RVP) of 11.4, 23.1, 33.2, 44.1, 54.5, 65.6 and 76.1%, giving an aw of 0.01 × percentage of RVP at equilibrium (Labuza and others 1985). Water activity of the saturated salt solutions was confirmed with an Aqua Lab CX-2 (Decagon Devices, Inc. Pullman, Washington, USA) water activity meter. After equilibration, the pans were hermitically sealed. Triplicate samples of each material were analysed.
The samples were scanned first over the glass transition temperature range at 5°C min−1, followed by cooling at 10ºC min−1 to below glass transition, and a second heating scan at 5°C/min was run to well above the glass transition. Anhydrous samples were scanned using pans with punctured lids to allow evaporation of any residual water during the measurements. Samples with different water contents were scanned in hermetically sealed pans. Transition temperatures (Tg onset) were determined using STARe thermal analysis software, version 6.0 (Mettler Toledo, Switzerland).
The Gordon and Taylor,[Citation37] equation was fitted to the experimental Tg data as reported by Singh and Roos.[Citation17] The Gordon and Taylor equation was applied in the present study for predicting water plasticization of lactose/ sucrose, lactose/ sucrose/ protein, lactose/ sucrose/ cornstarch and lactose/ sucrose/ protein/ cornstarch mixtures, considering that all solid components contributing to the observed glass transition were miscible and formed a single phase.[Citation33,Citation35]
Statistical Analysis
A 1-way ANOVA followed by multiple range (LSD) test were performed using SPSS 11.0 (SPSS Inc., Chicago, III., U.S.A.) software to compare the Tg, Tg´, and Tm´ values for different compositions. The level of confidence required for significance was selected as P ≤ 0.05.
RESULTS AND DISCUSSION
Effect of Water and Composition on Glass Transition Behavior
The foundation of glassy dynamic concept of food stability is based on the stability properties of the glassy state. The physical state of the sugars at low water contents has been an important factor affecting the stability of sugar containing foods.[Citation36] The Tg of anhydrous, amorphous lactose/sucrose (1:1) was found at 80°C (onset of transition), being slightly higher than the Tg previously reported.[Citation38] This confirms that punctured pans allowed water to evaporate during measurements, and a more accurate anhydrous higher Tg.
It was found that the Tg of lactose/sucrose mixtures was higher than previous values determined for sucrose Tg[Citation17] but lower than the Tg of individual lactose.[Citation19] It has been reported that the Tg and Tc (temperature of crystallization) of lactose/sucrose mixtures increased with lactose content and decreased with higher sucrose levels.[Citation38] Arvanitoyannis and Blanshard[Citation38] assumed the intermolecular interaction between sugars (sucrose and lactose) results in plasticization by the lower Tg component in the lactose-sucrose mixture.
It has been reported that the Tg of sugar systems increased with miscible polymeric substances.[Citation17–19,Citation33,Citation38–40] Similarly, significant (P ≤ 0.05) increases in Tg values were observed in the present work for lactose/ sucrose/ albumin, lactose/ sucrose/ gelatin, lactose/ sucrose/ cornstarch, and lactose/ sucrose/ cornstarch/ gelatin mixtures (, ) in comparison to lactose-sucrose mixture. It was assumed that the higher molecular weight proteins and polysaccharides increased the average molecular weight of the mixed systems. This confirmed earlier observations.[Citation17–19,Citation36]
Table 1 Anhydrous glass transition temperature (Tg) as well as glass transition temperature (Tg´), and onset of ice melting temperature (Tm´) of maximally freeze-concentrated solutions of lactose/sucrose and lactose/sucrose protein and lactose/sucrose cornstarch mixtures (Cg´) for various sugar/proteins and polysaccharide mixtures. A 15 min annealing time was used for all solutions
Figure 1 Thermograms of 20%, lactose/ sucrose, lactose/ sucrose/ albumin (1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), and lactose/ sucrose/ gelatin/c ornstarch (1:1:1:1) solutions isothermally annealed at (Tm´−1)°C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1.
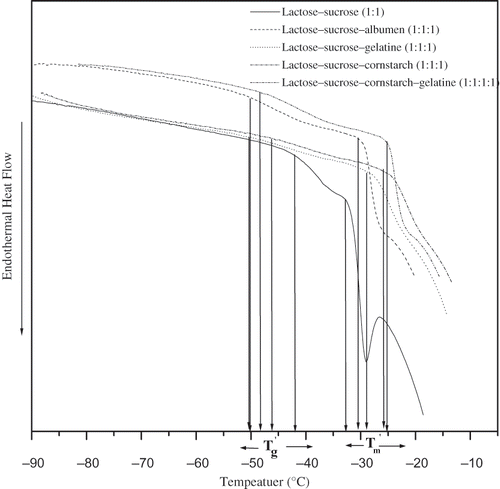
A single glass transition was found in reported sugar-polymer model systems[Citation17–19] and for the sugar mixture (lactose/sucrose)-polymer model systems. Antipova and Semenova[Citation39] stated that sugars (like sucrose) increase the interaction and miscibility of biopolymers in mixed systems. Singh and Roos[Citation17–19] reported that miscibility of components in a sugar-polymer system resulted in a single glass transition as observed from DSC thermographs. Low level of phase separation could also be below the DSC determination limits.
It is well known that increased water content of a system results in increased plasticisation of the amorphous solids and reduced Tg. In the present study, the Tg of all materials decreased with increasing storage RVP (aw 0.231–0.761). The increase in RVP increased water contents of stored samples and sorbed water plasticized the amorphous materials and lowered their Tg.[Citation36,Citation37] The phenomenon of water plasticization was similar for sugar-polymer systems reported by Singh and Roos[Citation17–19] and mixed sugar-polymer systems.
DSC analysis of sugar mixtures showed that glass transitions were associated with endothermic relaxation peaks, which were also reported for sugars (lactose, sucrose, and trehalose).[Citation17–19] Such relaxations were not observed in the second scans of lactose/ sucrose/ protein and lactose/ sucrose/ cornstarch mixtures (). Singh and Roos[Citation17–19] found the differences in enthalpy relaxation behavior of sugar-polymer glasses from the sugar glasses. Sahagian and Goff[Citation11] reported that the difference in molecular packing in various compound may appear as difference for relaxation peak.
Figure 2 DSC thermograms (Second scan) of freeze-dried anhydrous, lactose/ sucrose (1:1), lactose/ sucrose/ albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ ucrose/ cornstarch (1:1:1), and lactose/ sucrose/ gelatin/ cornstarch (1:1:1:1). The arrow indicates the onset of Tg.
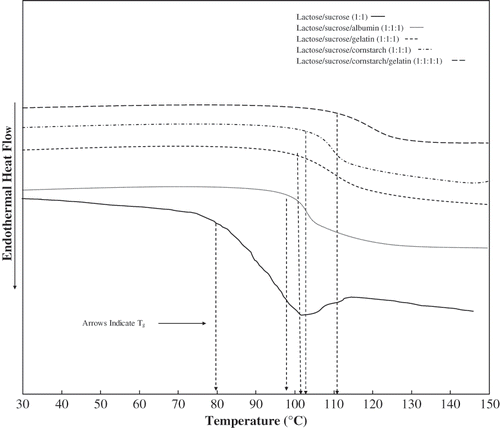
It can be assumed that a polymer-sugar system requires a longer relaxation time because of additional free volume in a polymer system. The experimental Tg values for lactose/ sucrose, lactose/ sucrose/ albumin, lactose/ sucrose/ gelatin, lactose/ sucrose/ cornstarch, and lactose/ sucrose/ cornstarch/ gelatin mixtures were successfully modeled by the Gordon-Taylor equation at various water contents. The constant, k, as for of Tg as a function of water content for lactose/ sucrose, lactose/ sucrose/ albumin, lactose/ sucrose/ gelatin, lactose/ sucrose/ cornstarch, and lactose/ sucrose/ cornstarch/ gelatin mixtures with respective k values were of 5.4, 6.8, 5.8, 6.4, and 6.4. In general, the constant k for sugar-polymer systems reported by Singh and Roos[Citation17–19] or lactose-sucrose-polymer systems, were in the range of 4.5–9.2. Water plasticization of lactose/ sucrose and lactose/ sucrose protein and cornstarch mixtures with their respective experimental data are shown in .
Figure 3 State diagrams for lactose/sucrose, lactose/sucrose/albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), and lactose/ sucrose/ cornstarch/ gelatin (1:1:1:1). Tg curve was predicted by the Gordon and Taylor equation (Gordon and Taylor, 1952).[Citation37] Below Tg all solutions were in the glassy state. Maximally freeze concentrated solutions showed constant Tg´ (onset of glass transition temperature) and Tm´ (onset of ice melting temperature) values. All solution showed maximum concentration of solute in the unfrozen phase at Cg´.
![Figure 3 State diagrams for lactose/sucrose, lactose/sucrose/albumin (1:1:1), lactose/ sucrose/ gelatin (1:1:1), lactose/ sucrose/ cornstarch (1:1:1), and lactose/ sucrose/ cornstarch/ gelatin (1:1:1:1). Tg curve was predicted by the Gordon and Taylor equation (Gordon and Taylor, 1952).[Citation37] Below Tg all solutions were in the glassy state. Maximally freeze concentrated solutions showed constant Tg´ (onset of glass transition temperature) and Tm´ (onset of ice melting temperature) values. All solution showed maximum concentration of solute in the unfrozen phase at Cg´.](/cms/asset/8a13ece6-95aa-4f90-b8df-8d67a8c08ba1/ljfp_a_326085_o_f0003g.gif)
Thermal Behaviour of Frozen Systems
Annealing at a temperature slightly below the Tm´ allows maximum freeze-concentration in frozen systems.[Citation9] Annealed systems exhibit thermographs with initial concentration independent and constant Tg´ and Tm´ values with no devitrification peak.[Citation9,Citation10,Citation11,Citation17–19] This was true also for sugar-polymer systems i.e. annealing temperature of (Tm´–1) °C () led to maximally freeze-concentrated mixed sugar-polymer systems. The initial concentration independent of Tg´ and Tm´ values with no devitrification peak on thermographs of mixed sugar-polymer systems (, ) were the indications of maximum freeze-concentration of solutes in the unfrozen phase. Singh and Roos[Citation17–19] reported similar thermal behaviour for other sugar-polymer systems. The maximum concentration of solutes in the unfrozen phase enhances quality retention and stability of frozen systems.[Citation17–19]
Table 2 The glass transition temperature (Tg´), and onset of ice melting temperature (Tm´) of maximally freeze-concentrated solutions of sugar mixtures/proteins/cornstarch mixtures. A 15 min annealing time was used for all solutions
In the present study, Tg´ and Tm´ values were determined for mixed sugar-polymer mixtures (). The Tg´ of lactose-trehalose was significantly higher than that of lactose-sucrose and sucrose-trehalose. The lactose-sucrose mixture had significantly lower Tg´ value (P ≤ 0.05) than any other sugar mixtures studied. Previously reported Tg´ values for sugars, lactose, sucrose, and trehalose[Citation17,Citation18,Citation19] and the present sugar mixtures (lactose-sucrose, lactose-trehalose, and sucrose-trehalose) were in the range of −40 − −46°C. The Tg´ value of lactose, trehalose reported by Singh and Roos[Citation18–19] and lactose-trehalose were in the same range, whereas Tg´ values of sucrose-trehalose and lactose-sucrose were higher than that of sucrose reported by Singh and Roos.[Citation17] This suggested that components with high Tg´ increased the Tg´ in mixtures with sugars of low Tg´. Tm´ values for sugars[Citation17–19] and sugar mixtures were approximately in the same range of −30 – −35°C.
The mixed sugar mixture-polymer systems showed similarity in thermal behaviour to that of sugar-polymer systems.[Citation17–19] The mixed sugar-polymer systems showed lower Tg´ and higher Tm´ values than sugar mixtures (, , ). It was found that lactose-trehalose-cornstarch had the highest Tg´ value and lactose-sucrose-gelatine had the lowest Tg´ of mixed sugar -polymer system studied (). It was observed that albumen had similar Tg´ values to lactose-sucrose, lactose-trehalose, and sucrose-trehalose sugar mixtures. A common Tg´ value for mixed sugar-albumen systems was presumed due to the similar compatibility of albumen with all mixed sugar systems studied (, ). The Tm´ values for mixed sugar-polymer systems were in the range of −26 – −31°C (, ). These observations were different from the assumption of Goff and other[Citation7] who suggested that polymers might not affect the glass transition behaviour of frozen systems. However, later Hagiwara and Hartel[Citation2] and Miller-Livney[Citation40–41] showed the possibility of polymers to influence the glass temperature of frozen sugar systems.
Figure 4 a) Thermograms of 20% solutions, lactose/sucrose (1:1), lactose/ trehalose (1:1), and sucrose/ trehalose isothermally annealed at (Tm´−1) °C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1. b) Thermograms of 20% solutions, lactose/ sucrose/ albumin (1:1:1), lactose/ trehalose/ albumin (1:1:1), and sucrose/ trehalose/ albumin (1:1:1) isothermally annealed at (Tm´−1) °C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1. c) Thermograms of 20% solutions, lactose/ sucrose/ gelatin (1:1:1), lactose/ trehalose/ gelatin (1:1:1), and sucrose/trehalose/gelatin (1:1:1) isothermally annealed at (Tm´−1) °C for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1. d) Thermograms of 20%, lactose/sucrose/cornstarch (1:1:1), lactose/ trehalose /cornstarch (1:1:1), and sucrose/ trehalose/ cornstarch (1:1:1) isothermally annealed at Tm´−1 for 15 minutes before determination of the transitions during rewarming from –100 to 20°C at 5°C min−1.
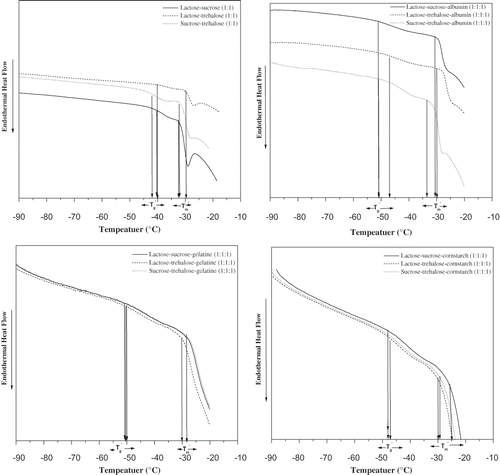
A low Tg´ with a high Tm´ indicates differences in the ice formation and that crystallization ceases at higher unfrozen water content. The Tg´ value of a frozen system gives a measured amount of ice formed in the system. Maximum ice formation or ice crystallization rate in a frozen system is affected by the viscosity of the freeze-concentrated phase.[Citation2,Citation3] The rate of ice crystallization includes two distinct physical processes, the rate at which molecules diffuse into the crystal lattice, and the diffusion of heat and water molecules in the neighbourhood of the growing interface.[Citation2] The high molecular weight food polymers may reduce the ice crystallization rate by increasing its viscosity.[Citation1] At a lower temperature, ice formation becomes limited by diffusion of water from the unfrozen phase to the ice phase.[Citation2,Citation3,Citation5,Citation6,Citation7] Roos and KarelCitation[9] predicted a minimum viscosity of 107 for ice formation in carbohydrate solutions. Singh and Roos[Citation17–19] recently reported a critical viscosity for the ice formation in carbohydrate-polymer systems.
The viscosity of unfrozen solute phase depends on the composition of frozen food matrix. Harper and Shoemaker[Citation13] reported variations in the amount of ice formation in a frozen system with the type of sugar (sweeteners). Budiaman and Fennema[Citation14] found similar observations for hydrocolloids or stabilizers used in frozen dairy products, i.e., the ice formation in a frozen system was dependent on the type of stabilizer used. Like sugars stabilizers or hydrocolloids increase the viscosity of the unfrozen phase and reduce the recrystallization of ice in frozen dairy systems.[Citation15]
Stabilizers were less effective than sweeteners in retarding ice formation in frozen dairy systems.[Citation15,Citation16] Buyong and Fennema[Citation16] reported that stabilizers retarded ice crystal growth to a greater extent in conjunction with sweetener. However, sweeteners and stabilizers were mutually important composition determinants for ice crystallization.[Citation22,Citation41] Sweeteners affect ice crystallization by freezing point depression, increasing the microviscosity and influencing glass transition temperature of a frozen system.[Citation2,Citation22,Citation41] Where as stabilizers affect ice crystallization by increasing the viscosity of unfrozen phase and reducing the mobility in a frozen system.[Citation41]
Roos and Karel[Citation9] concluded that annealing of a frozen system separated maximum amount of water into an ice phase and maximum amount of solute in the unfrozen phase. Annealed systems have an unfrozen phase with solid like properties and constant values for the onset of Tg´ and Tm´.[Citation8,Citation10] It seems that an annealing temperature of (Tm´−1) allowed maximum amount of ice formation.
Broadness in Transition by Polymers in Frozen Sugar Systems
In frozen sugar-polymer and mixed sugar-polymer systems Tg´ was lower and Tm´ was higher and appeared as broadness of transition (). A high molecular weight polymeric substance develops high viscosity around ice crystals and retards the deformation of unfrozen phase at a high temperature, above Tg´.[Citation7] An increased Tm´ increased the temperature difference between Tg´ and Tm´and appeared as broadness of transition.[Citation17–19] In mixed sugar-albumen it was observed that increased broadness of transition was similar to sugar-polymer[Citation17–19] (). In general, it was observed that gelatine increased the broadness of transition to the grater extent in sucrose-polymer and lactose-polymer systems[Citation17,Citation19] and in the lactose-sucrose-polymer systems () than albumen and cornstarch. But cornstarch showed dominance on transition broadness in trehalose-polymer systems.[Citation18] The variation in the broadness of transition temperature in sugar-polymer reported by Singh and Roos[Citation17–19] and mixed sugar-polymer systems () may due to the compatibility of polymer with sugars or sugar mixtures.
Thermal Behaviour of Lactose-Sucrose-Polymer Systems
Lactose is commonly present in combination with sucrose in frozen dairy products and investigated in mixed form with sucrose in the present study. Analysis of thermal-graphs for lactose-sucrose-polymer systems revealed that lactose-sucrose-albumin and lactose-sucrose-gelatin had non-significantly different Tg´ values (). The Tg´ values of lactose-sucrose-albumin and lactose-sucrose-gelatin were lower and significantly different from Tg´s of lactose-sucrose-cornstarch and lactose-sucrose-cornstarch-gelatin mixtures (P ≤ 0.05). In general, the lactose-sucrose-cornstarch-gelatin mixture had significantly higher and lactose-sucrose-albumin mixture significantly lower values of Tg´ and Tm´ than lactose-sucrose-cornstarch and lactose-sucrose-gelatine (, ) systems.
State diagrams were used to estimate maximum concentration of solutes in the unfrozen phase. On the state diagram, the intersection of Tg´ on Tg curve and the corresponding value on the concentration axis (x-axis) was considered as maximum concentration of solute, Cg´ in unfrozen phase. We found that maximally freeze-concentrated lactose/sucrose had Cg´ of 82%, while lactose-sucrose-polymer systems (lactose-sucrose-albumin, lactose-sucrose-gelatin, and lactose-sucrose-cornstarch) had Cg´ of 80% (, ), except lactose-sucrose-gelatine system. It was noted that like lactose-polymer and sucrose-polymer systems the lactose-sucrose-gelatine system had the lowest Cg´ of 77% from other lactose-sucrose-polymer systems. It was presumed that gelatine increased the critical viscosity for a mixed sugar system at lower temperature. The higher critical viscosity for a system at a lower temperature retarded the separation of unfrozen water that decreased concentration of solute in the unfrozen phase.
It was found that Cg´ values for lactose-sucrose-polymer systems were higher than lactose-polymers and sucrose-polymer systems.[Citation17,Citation19] Antipova and Semenova[Citation39] reported that sugars in mixtures were more compatible with food biopolymers. Ibrahim and others[Citation42] stated that sugars in mixture increased the compatibility of sugars toward polymers and affected the viscosity of the unfrozen phase and hence unfrozen water components in a frozen system. Wang and others[Citation43] described that due to structural differences, food polymers vary in their water hydration capacity affecting unfrozen water in frozen systems. Flores and Goff[Citation25] reported that structural and molecular differences in food polymers used were responsible for the variations in viscosity values of unfrozen phase, and hence, cause variations in unfrozen water contents in the unfrozen phase. In this work, it was assumed that higher values of Cg´ for lactose-sucrose-polymer might be due to critical viscosity of the unfrozen phase achieved at a high temperature. The low viscosity of unfrozen phase at higher temperature favored the separation of maximum amount of unfrozen water into ice phase and hence increased the concentration of solute in unfrozen phase. There are evidences in literature that less concentrated unfrozen phase or unfrozen phase with high amounts of unfrozen water resulted in quality and stability degradation in frozen systems.[Citation9,Citation10,Citation11,Citation31,Citation35]
CONCLUSIONS
The thermal study of mixed sugar-polymer systems showed similarities and differences respect to sugar-polymer systems earlier reported by Singh and Roos.17–19 Annealing temperature (Tm´−1) °C for mixed sugar-polymer and sugar-polymer was successfully used for maximum freeze-concentration in frozen systems. Maximally freeze-concentrated mixed sugar-polymer and sugar-polymer systems showed constant and initial concentration independent onset of glass transition, Tg´ and onset ice melting temperature, Tm´. The presence of polymers in unfrozen phase of the maximally freeze-concentrated sugar systems sustained high viscosity above Tg´ and shifted the Tm´ to higher temperature. Melting of ice at high Tm´ eliminated devitrification peak from the DSC thermographs and increased the temperature difference between Tg´ and Tm´ and hence broadness of thermal curves increased. The broadness of thermal curves illustrated the high viscosity of unfrozen phase above Tg´, which provided resistance to ice phase to melt. The difference in DSC traces appeared as dissimilarities between mixed sugar-polymers and sugar-polymer systems.[Citation17–19] The lactose-sucrose-polymer systems, lactose-sucrose (1:1), lactose-sucrose-albumin (1:1:1), lactose-sucrose-gelatine (1:1:1), lactose-sucrose-cornstarch (1:1:1), and lactose-sucrose-cornstarch-gelatine (1:1:1:1) had a Tg´ value of −43, −51, −51, −48, and −46°C, respectively. At Tg´, a sugar mixture had Cg´ of 82%, where as lactose-sucrose-polymer systems had a solute concentration Cg´ of 80%. Decreased Cg´ value for lactose-sucrose-polymer systems revealed that polymeric compound in an unfrozen phase affects the critical viscosity of a frozen system at lower temperature and hence affects the ice formation. The observed Tg´ and Cg´ value for lactose-sucrose-polymer were higher than sucrose-polymer systems[Citation17]. This indicated that in a mixture (lactose-sucrose), a high Tg´ sugar (lactose) increased the Tg´ of a sugar having low Tg´ value (sucrose). The physical state studies of model-frozen systems help to increase quality and stability of frozen and freeze-dried foods by manipulating the composition of unfrozen phase.
ACKNOWLEDGMENTS
This study was carried out with financial support from Higher Education Authority of Ireland PRTLI Cycle 3.
REFERENCES
- Némethy , G. 1968 . Low Temperature Biology of Foodstuffs , Edited by: Hawthorn , J. and Rolfe , E.J. 1 – 22 . London : Pergamon Press .
- Hallett , J. 1968 . Low Temperature Biology of Foodstuffs , Edited by: Hawthorn , J. and Rolfe , E.J. 23 – 52 . London : Pergamon Press .
- Luyet , B.J. 1968 . Low Temperature Biology of Foodstuffs , Edited by: Hawthorn , J. and Rolfe , E.J. 53 – 78 . London : Pergamon Press .
- Love , R.M. 1968 . Low Temperature Biology of Foodstuffs , Edited by: Hawthorn , J. and Rolfe , E.J. 105 – 124 . London : Pergamon Press .
- Levine , H. and Slade , L. 1988 . Principle of “cryostabilization” technology from structure/property relationships of carbohydrate/water systems-A review . Cryo-lett. , 9 : 21 – 63 .
- Roos , Y.H. and Karel , M. 1991 . Amorphous state and delayed ice formation in sucrose solutions . Int. J. Food Sci. and Technol. , 26 : 553 – 566 .
- Goff , H.D. , Caldwell , K.B. and Stanley , D. W. 1993 . The influence of polysaccharides on the glass transition in frozen sucrose solutions and ice cream . J. Dairy Sci. , 76 : 1268 – 1277 .
- Sahagian , M.E. and Goff , H.D. 1995 . Thermal mechanical and molecular relaxation properties of stabilized sucrose solution at sub-zero temperatures . Food Res. Int. , 28 ( 1 ) : 1 – 8 .
- Roos , Y.H. and Karel , M. 1991 . Nonequilibrium ice formation in carbohydrate solutions . Cryo-lett. , 12 : 367 – 376 .
- Roos , Y.H. 1995 . Phase Transitions in Foods , 360 San Diego CA : Academic Press .
- Goff , H.D. and Sahagian , M.E. 1996 . “ Freezing of dairy products ” . In Freezing effects on Food Quality , Edited by: Jeremiah , L.E. 299 – 335 . New York : Marcel Dekker Inc .
- Muhr , A.H. and Blanshard , J.M.V. 1986 . Effects of polysaccharide stabilizers on the rate of growth of ice . J. Food Technol. , 21 : 683 – 710 .
- Harper , E.K. and Shoemaker , C.F. 1983 . Effects of locust bean gum and selected sweetening agents on ice recrystallization rates . J. Food Sci. , 48 : 1801 – 1803 .
- Budiaman , E.R. and Fennema , O. 1987 . Linear rate of water crystallization as influenced by temperature of hydrocolloid suspensions . J. Dairy Sci. , 70 : 534 – 546 .
- Budiaman , E.R. and Fennema , O. 1987 . Linear rate of water crystallization as influenced by temperature of hydrocolloid suspensions . J. Dairy Sci. , 70 : 547 – 554 .
- Buyong , N. and Fennema , O. 1988 . Amount and size of ice crystals in frozen samples as influenced by hydrocolloids . J. Dairy Sci. , 71 : 2630 – 2639 .
- Singh , K.J. and Roos , Y.H. 2005 . Frozen State Transitions of Sucrose-Protein-Cornstarch Mixtures . J. Food Sci. , 70 ( 3 ) : 198 – 204 .
- Singh , K.J. and Roos , Y.H. 2006 . State transitions and freeze concentration in trehalose–protein–cornstarch mixtures . Lebensm.-Wiss.u.-Technol. , 39 ( 8 ) : 930 – 938 .
- Singh , K.J. and Roos , Y.H. 2007 . Frozen state transitions in freeze-concentrated lactose-protein-cornstarch systems . Int. J. Food Prop. , 10 ( 3 ) : 577 – 587 .
- Fennema , O. R. 1973 . Low-Temperature Preservation of Foods and Living Matte , Edited by: Fennema , O.R. , Powrie , W.D. and Marth , E.H. 153 New York : Marcel Dekker .
- Sutton , R.L. , Lips , A. , Piccirillo , G. and Sztehlo , A. 1996 . Kinetics of ice recrystallization in aqueous fructose solutions . J. Food Sci. , 61 ( 4 ) : 741 – 744 .
- Hagiwara , T. and Hartel , R.W. 1996 . Effect of sweetener, Stabilizer, and storage temperature on ice recrystallization in ice cream . J. Dairy Sci. , 79 : 735 – 744 .
- Sutton , R.L. and Wilcox , J. 1998 . Recrystallization in model ice cream solutions as affected by stabilizer concentration . J. Food Sci. , 63 ( 1 ) : 9 – 11 .
- Martin , D.R. , Ablett , S. , Darke , A. , Sutton , R.L. and Sahagian , M. 1999 . Diffusion of aqueous sugar solutions as affected by locust bean gum studied by NMR . J Food Sci. , 64 ( 1 ) : 46 – 49 .
- Flores , A.A. and Goff , H.D. 1999 . Recrystallization in ice cream after constant and cycling temperature storage conditions as affected by stabilizers . J. Dairy Sci. , 82 : 1408 – 1415 .
- Bolliger , S. , Wildmoser , H. , Goff , H.D. and Tharp , B.W. 2000 . Relationships between ice cream mix viscoelasticity and ice crystal growth in ice cream . Int. Dairy J. , 10 : 791 – 797 .
- Regand , A. and Goff , H.D. 2003 . Structure and ice recrystallization in frozen stabilized ice cream model systems . Food Hydrocoll. , 17 : 95 – 102 .
- Franks , F. , Asquith , M.H. , Hammond , C.C. , Skaer , H.B. and Echlin , P. 1977 . Polymeric cryoprotectants in the preservation of biological ultra structure. I. Low temperature states of aqueous solutions of hydrophilic polymers . J. of Microsc. , 110 : 223
- Roos , Y.H. 1995 . Characterization of food polymers using state diagrams . J Food Eng. , 24 : 339 – 360 .
- Meste , Le and M. Huang , V. 1992 . Thermomechanical properties of frozen sucrose solutions . J. Food Sci. , 57 ( 5 ) : 1230 – 1233 .
- Maltini , E. and Anese , M.. 1995 . Evaluation of viscosities of amorphous phase in partially frozen systems by WLF kinetics and glass transition temperatures . Food Res. Int. , 28 ( 4 ) : 367 – 372 .
- Biliaderis , C.G , Swan , R.S. and Arvanitoyannis , I. 1999 . Physicochemical properties of commercial starch hydrolyzates in the frozen state . Food Chem. , 64 : 537 – 546 .
- Herrera , J.R. , Pastoriza , L. and Smapedro , G. 2000 . Inhibition of formaldehyde production in frozen-stored minced blue whiting (Micromesistius poutassou)Muscle by cryostabilizers: An approach from the glassy state theory . J. Agric. Food Chem. , 48 : 5256 – 5262 .
- Herrera , J.R. and Roos , Y.H. 2001 . A kinetic study on formaldehyde production in cryostabilized water-soluble fish muscle extracts . Innov. Food Sci. Emerg. Technol. , 1 : 227 – 235 .
- Le Meste , M. , Champion , D. , Roudaut , G. , Blond , G. and Simatos , D. 2002 . Glass transition and food technology: A Critical Appraisal . J. Food Sci. , 67 ( 7 ) : 2444 – 2458 .
- Haque , K. and Roos , Y.H. 2004 . Water plasticization and crystallization of lactose in spray-dried lactose/protein mixtures . J. Food Sci. , 69 ( 1 ) : 23 – 29 .
- Gordon , M. and Taylor , J.S. 1952 . Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers . J. Appl. Chem. , 2 : 493 – 500 .
- Arvanitoyannis , I and Blanshard , J. M. V. 1994 . Rates of Crystallization of Dried Lactose-sucrose Mixtures . J. Food Sci. , 59 ( 1 ) : 197 – 205 .
- Antipova , A. S. and Semenova , M.G. 1996 . Effect of sucrose on the thermodynamic incompatibility of different biopolymers . Carbohydr. Polym. , 28 ( 4 ) : 359 – 365 .
- Blond , G. and Simatos , D. 1998 . Optimal thermal treatments to obtain reproducible DSC thermograms with sucrose + dextran frozen solutions . Food Hydrocoll. , 12 : 133 – 139 .
- Miller-Livney , T. and Hartel , R.W. 1997 . Ice recrystallization in ice cream: interactions between sweeteners and stabilizers . J. Dairy Sci. , 80 : 447 – 456 .
- Al-Ruqaie , I.M. , Kasapis , S. and Abeysekera , R. 1997 . Structural properties of pectin-gelatin gels. Part II: effect of sucrose/glucose syrup . Carbohydr. Polym. , 34 ( 4 ) : 309 – 321 .
- Wang , S. T. , Barringer , S.A. and Hasen , P.M.T. 1998 . Effects of carboxymethylcellulose and guar gum on ice crystal propagation in a sucrose-lactose solution . Food Hydrocoll. , 12 : 211 – 215 .