Abstract
A true equilibrium process usually takes very long to achieve and is very difficult, therefore, a pseudo-equilibrium process is often employed. Several methods exist for predicting pseudo-equilibrium conditions and their accuracies vary. Dynamic equilibrium and pseudo-equilibrium moisture loss (ML) and solids gain (SG) during osmotic dehydration of apple cylinders at different temperature (40, 50, and 60°C) and concentrations (30, 40, 50, and 60°Brix) were evaluated in this study. Pseudo-equilibrium achieved depended on product and processing conditions. Higher concentrations increased the pseudo-equilibrium ML and decreased SG. Two kinds of pseudo-equilibration appear to exist, one for the liquid, which is reached in about 24 h, depending on sample size, the other for the solid matrix, which takes much longer to achieve. Solute penetration in to the product is a much slower process during osmotic dehydration equilibration.
INTRODUCTION
Osmotic dehydration is a technique for partial removal of water from plant tissues and other materials by immersion in a hypertonic solution. The driving force for water removal is the chemical potential between the solution and the intracellular fluid. The mass transfer mechanisms during the process include osmosis, diffusion, hydrodynamic mechanism penetration[Citation1] and other specific/active mechanisms effective in the temperature range where the tissue is living.[Citation2] Modeling of the mass transfer phenomenon is a useful tool to optimize the osmotic dehydration processes, aimed at improving product quality and lowering energy costs. Modeling of such simultaneous mass transfer kinetics requires limiting data under equilibrium conditions.
In the past decade, several studies have been carried out to better understand the behavior of plant tissue and mass transfer mechanisms during osmotic dehydration equilibration.[Citation3–15] Only a few studies have reported details on osmotic equilibration kinetics from different points of view.[Citation8 Citation9] This poses a problem since the modeling of the mass transfer processes requires data on the equilibrium status of the product to define the driving force for drying.[Citation1 Citation4 Citation16] Food engineers depend on theoretical or empirical models for the design of equipment and optimization of processes. Reliable data on the equilibrium properties is necessary in such models involving osmotic dehydration.
The osmotic dehydration process is characterized by dynamic, pseudo-equilibrium and equilibrium periods. The dynamic period refers to the transient conditions during which the bulk of the mass exchange process takes place. Equilibrium is the period when both chemical and physical (mechanical) equilibrium are reached with no further changes in sample composition or weight. This true equilibrium is hard to achieve in an osmotic dehydration process. Such equilibrium relationships have not been well established because of system complexity and non-ideal behavior of different phases. Even when no changes either in sample composition or weight occur, the system might not have reached chemical equilibrium; such equilibrium kinetics can take up to 100 days.[Citation6] In some cases, it is also not possible to attain equilibrium due to biological and/or physical instability.[Citation17] Pseudo-equilibrium (practical equilibrium) is the relatively short period of time, about 24 h depending on processing conditions, when sample compositional equilibrium is achieved. In the dynamic period, the mass transfer rates are increased or decreased until pseudo-equilibrium is reached.
Assuming that the tissue surface is in equilibrium with the contact solution, pseudo-equilibrium kinetics can be determined by considering equilibration between the osmotic solution and liquid in the fruit tissue. Del Valle et al.[Citation3] set the equilibrium condition as equality of concentration of salt in the brine and in the total water inside the muscle. Favetto et al.[Citation18] studied beef slices in a sodium chloride glycerol solution and found that the equilibrium condition between beef and the external solution was given by the equality of sodium chloride and glycerol concentration in the water of the solution and in the muscle tissue water. Lenart and Flink[Citation16] reported that 4–20 hours were required to achieve equilibrium, defined as equal soluble solid concentration and water activity in the product and osmotic solution, in potato cubes (10 mm) after osmosis in 10–70% sucrose and NaCl solutions. Biswal and Le Maguer[Citation4] and Biswal and Bozorgmehr,[Citation19] by considering that fruit solute activities matched those of the osmotic solution at equilibrium, found good agreement between experimental and predicted densities of carrots and potatoes after osmosis in NaCl and ethanol solutions for 16–36 h. Azuara et al.[Citation17] proposed a model to estimate mass transfer coefficients and equilibrium water loss and solids gain with linear regression. Rahman[Citation20] characterized equilibrium kinetics by defining equilibrium constants and dynamic periods. Rastogi et al.[Citation21] plotted the rate of change of moisture content versus average moisture content, and used the slope to define the osmotic dehydration rate and intercept to infer equilibrium moisture and solids contents. Nzonsi and Ramaswamy[Citation22] reported a detailed osmotic dehydration kinetics for blueberries. Barat et al.[Citation5] studied the mechanism of equilibrium kinetics during osmosis. They identified two periods in equilibration. First, the compositional equilibrium was achieved in a relatively short time, depending on conditions. Then a bulk flux of osmotic solution into the fruit tissue occurred due to relaxation of previously shrunken cellular structure. In such a state, differences in both activity and pressure disappeared, and the solid cellular matrix became fully relaxed. Waliszewski et al.[Citation9] studied equilibrium concentration and water and sucrose diffusivity in osmotic dehydration of pineapple slabs. Sablani and Rahman[Citation7] studied the effect of syrup concentration, temperature and sample geometry on equilibrium distribution coefficients during osmotic dehydration of mango. Nieto et al.[Citation15] investigated the structural changes of apple tissue during glucose and sucrose osmotic dehydration equilibration.
In spite of the above studies, the evaluation of equilibrium kinetics in the OD process is still limited and the available data are scattered and mostly disconnected. It is also difficult to establish general rules about equilibrium kinetics; even then only a pseudo-equilibrium state can realistically be achieved. Long time equilibrium kinetics signified the practical end of the osmotic process and set limits to the range of potential data applications. The challenge therefore, is to try to come up with a way to exploit the equilibrium kinetics data for osmotic dehydration process. The objectives of this work were: (1) to study osmotic dehydration dynamic period moisture loss, solids gain relation with equilibrium moisture loss and solids gain with Azuara's model; (2) to evaluate the influence of different process parameters such as temperature and concentration of sucrose syrup on pseudo-equilibrium ML, SG and the ratio of ML/SG change (pseudo-equilibrium dehydration efficiency, PEDE); (3) to study sample size influence on pseudo-equilibrium ML and SG variance; and (4) to investigate sample internal moisture loss and solid gain variance trends during osmotic dehydration pseudo-equilibration.
MATERIALS AND METHODS
Sample Preparation
A batch of Idared apples of uniform size and ripeness were obtained from Macdonald Campus farm and commercial sucrose (sugar) was obtained from a local supermarket. The fruits were refrigerated at 2–5°C and at 95% relative humidity until used for experiments. Cylinders of three different sizes were cut for the osmotic dehydration study: large, 2.0 cm in diameter, medium, 1.0 cm in diameter, and small, 0.5 cm in diameter, with 2.0 cm height for all the samples. These were cut along the vertical direction clearly excluding the central seed cavity. Long cylinders of different diameters were first cut out from the apples using a cork borer and then the cylinder heights were adjusted to 2 cm using a special handmade cutting device with two wide knifes, spaced 2 cm apart.
Experimental Procedure
Apple cylinders were placed inside a wire mesh steel cage (60 cm in diameter 60 cm in height) and immersed into a sucrose-water solution in a stainless steel pot placed inside a water bath to maintain the required experimental temperature. If necessary during the experiments, a small amount of distilled water was added to the sucrose solution to compensate for the water loss caused by evaporation. The osmotic medium was agitated continuously with a stirrer to maintain uniform temperatures throughout the experiment and enhance the equilibration process. Temperatures were monitored and maintained within ±0.1°C. Test conditions included 30, 40, 50, and 60°Brix, and 40, 50, and 60°C. The fruit-syrup mass ratio (R) was kept very high (1:30), since ideally infinite fruit-syrup mass ration is necessary to avoid the effect of syrup dilution effect during the process. The duration of osmotic drying varied from 0.5–48 h depending on process conditions.
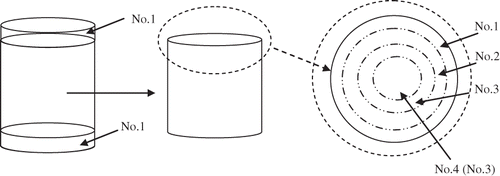
After selected treatment times, samples were removed and sample weight, moisture and solids content were determined. In addition, the large size sample (2-cm diameter) was cut across at the two ends to remove two slices (0.2-cm thick), and categorized as No. 1. The remaining portion cylinder was cut lengthwise using large cork borers to separate several cylindrical layers (0.2-cm thick) each numbered from one to four, based on distance from the center. The schematic explanation is shown in . These individual sections were evaluated for moisture and solids content.
Analytical Methods
Sample soluble solids content and solution concentration were measured in °Brix with a portable refractometer (ATAGO, Japan) at ambient temperature. Moisture content of fresh and osmotically treated apple cylinders was determined by oven method. Initially experiments were conducted to determine the time required to reach equilibration. The moisture content and total solids were measured gravimetrically on apple cylinders after different contact times. The cylinders were quickly rinsed and gently blotted with a wet paper towel to remove surface water before weighing. For measuring solids content, samples were air dried at 105°C for 24 h. All experiments were performed at least in triplicate and average values were reported.
Model Development
The most common mass transport terminologies used in osmotic dehydration are moisture loss (water loss), solid gain and weight reduction. The calculations are based on the general balance of concentration driven mass transfer between the liquid and solid phases, and are all on an initial mass basis. Moisture loss (ML), the net water loss is:
The corresponding solids gain (SG), the net solids (soluble) transport into the, is obtained from:
The weight reduction (WR), the net mass loss of the sample, is:
The solids gain (SG) was correlated with ML and WR (23):
In this study, only dynamic equilibrium and pseudo-equilibrium (practical equilibrium) period kinetics were evaluated.
Dynamic Period
Most theoretical models on moisture loss and solid gain kinetics are based on Fick's law of diffusion in an unsteady state one-dimension transfer. However, there are some limitations, such as when simultaneous mass transfer is reduced to a single mass transfer, resulting in diffusivities assumed as internal transfers and biological properties of the cell membrane not considered. Raoult-Wack[Citation24] reported that the two-parameter Azuara-kinetic model[Citation17] avoided some of the difficulties of Fick's diffusion model and found good accuracy to predict the mass transport dynamics of osmotic dehydration. Therefore, the Azuara-model was used to estimate the dynamic period ML∞ and SG∞,
Substituting EquationEq. (7) into (6) and (5), and rearranging terms, the following is obtained:
This equation associates the moisture loss (ML) with time (t), by means of two constants, s and ML∞. In order to predict the fraction of water lost by the food at time t in EquationEq. (8), it is necessary to know the values for s and ML∞. These values can be estimated using a non-linear regression program, or by linear regression, using experimental data and the linearized form of EquationEq. (9):
Similar EquationEqs. (10) and Equation(12) can be written for the weight reduction and solid gain of the products:
Pseudo-Equilibrium Period
Previous studies have revealed that similar compositions for product and osmotic solution were achieved after relatively long treatment times (about 20 h). Pseudo-equilibration dehydration efficiency (PEDE), the ratio of pseudo-equilibrium ML and SG, is
Moisture content (MC), the net water content on wet mass basis is:
The corresponding solids content (SC), the net soluble solids transport into the sample (again on an initial mass basis), can be obtained from:
RESULTS AND DISCUSSION
Dynamic Period Moisture Loss and Solid Gain in Relation with Equilibrium
Moisture Loss and Solid Gain
In order to test the applicability of EquationEqs. (8–Equation9) and (Equation12–Equation13), different time period experimental data were used and compared in . Different time periods were selected to test the minimum treatment time required for accurately evaluating the equilibrium data. Azuara's model is a dynamic model, and hence the predicted ML∞ and SG∞ at 3, 12, and 24 h differed considerably. The relative ML∞% difference was 114% for 40°C–30°Brix and 48% for 60°C–60°Brix while the relative SG∞% difference was 381% for 40°C–30°Brix and for 25% for 60°C–60°Brix. This means that simple extrapolation of this model to infinite time using 3 h data cannot give true equilibrium moisture content results. Waliszewski et al.[Citation9] reported similar results. The equilibrium moisture loss and solids gain of apple cylinders in the dynamic period increased with increasing contact time, temperature and concentration (), and samples appear to converge to pseudo-equilibrium condition after 24 h (). The ML∞% was 64.67, 64.98, and 65.40% for 24, 36, and 48 h dehydration time, respectively. Thus, 24 h can be considered as the pseudo-equilibrium time for all experiments in this study. The values of t/ML%, t/SG% and t/WR% as a function of time (t) at a selected condition are plotted in to demonstrate the linearity of the Azuara model. demonstrates the acceptability of the model for mass transport studies in dynamic period.
Table 1 Relation of the experimental data and Azuara's model predicted equilibrium value of osmotic dehydration during dynamic period
Figure 2 Moisture loss, solids gain and weight reduction of apple cylinders as a function of time at 50°C 50°Brix.
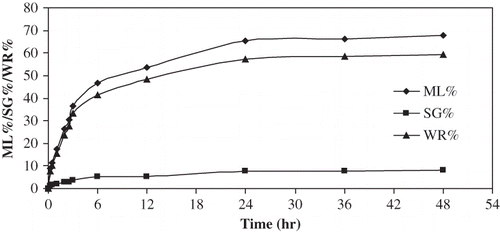
Figure 3 Plot of t/ML / t/SG / t/WR vs t for osmotic dehydration of apple cylinders at 50°C 50°Brix.
If experimental data did not include equilibrium time, the parameters obtained by linear regression would be impossible to fit.[Citation9] In all cases, the model fitted experimental moisture loss, weight reduction and solids gain quite well with the 24 h experimental equilibration data (). Thus, the Azuara model permits the evaluation of mass change at equilibrium with a linear regression equation, provided that experimental data were calculated in long-time trials.
Table 2 Relation of Azuara's model predicted equilibrium moisture loss, weight reduction and solid gain with 24-h period experiment data
Effect of Temperature and Concentration on Pseudo-Equilibration
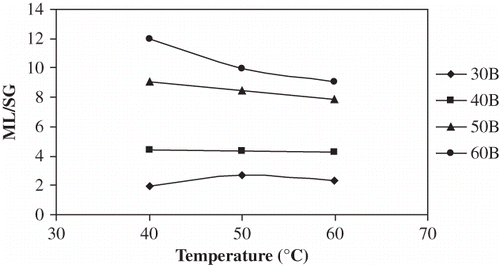
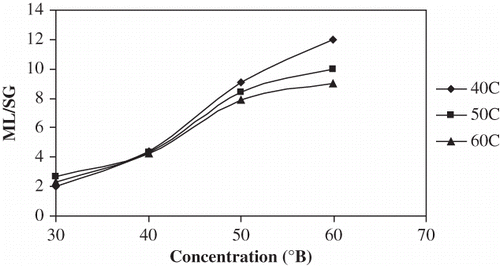
As expected, higher concentrations of osmotic solutions gave a higher ML∞ ( and ). ML∞ was not a strong function of temperature except for the 40°C–30°Brix combination in which case the chemical potential was relatively very low, and thus the ML rate was slow and the ML∞ was probably not reached within the time period. Statistical comparison of the various temperature-concentration treatments is presented in . Concentration effects were significant to ML∞ (P < 0.05); as concentration was increased from 30 to 60°Brix, ML∞ increased by 199% at 40°C, by 100% at 50°C and by 107% at 60°C. Increasing temperature from 40 to 50°C, ML∞ associated with 30°Brix increased by almost 50%, but between 50 and 60°C for 30°Brix and between 40 and 60°C for all other concentrations, the temperature effect on ML∞ was not significant (P > 0.05). The combined increase in ML∞ from 30°Brix 40°C to 60°Brix 60°C was 207%. Parjoko et al.[Citation25] also reported that at constant temperature, the equilibrium constants for both water and solids increased with increase in syrup concentration. Waliszewski et al.[Citation9] found the concentration effects on ML∞ to have a very positive effect and temperature to have a less obvious effect.
Figure 4 Plot of equilibrium ML% vs temperature for osmotic dehydration of apple cylinders at different concentration.
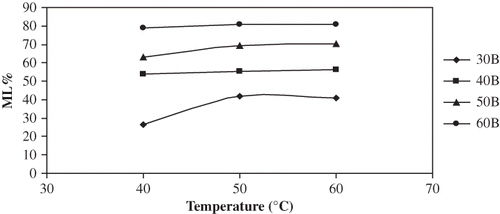
Figure 5 Plot of equilibrium ML% vs concentration for osmotic dehydration of apple cylinders at different temperatures.
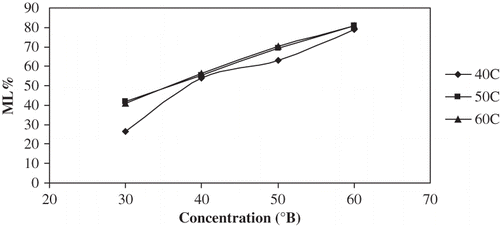
Table 3 Mean values and 95% confidence limits for moisture loss (%) during osmotic treatment of apple cylinders in sugar solution at different concentrations and different temperatures
and show plots of SG∞ versus temperature and concentration for different concentrations and temperatures. SG∞ increased with an increase in temperature while it decreased with an increase in solution concentration. Statistical comparison of the various temperature-concentration treatments is presented in . All temperature effects were significant (P < 0.05), while only concentration effects from 30 to 50°Brix were significant (P < 0.05). Contrary to the observed ML∞ increase, SG∞ reduced by 50–55% when solution concentration was raised from 30 to 60°Brix. Waliszewski et al.[Citation9] reported that sucrose concentration had a positive effect on solids equilibrium while the temperature effect was less evident, confirming the results of this study.
Figure 6 Plot of equilibrium SG% vs temperature for osmotic dehydration of apple cylinders at different concentrations.
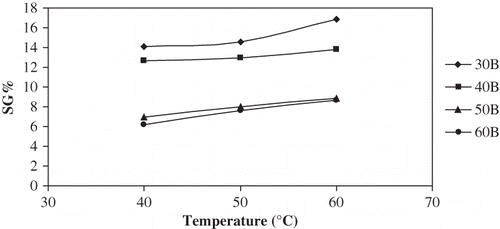
Figure 7 Plot of equilibrium SG% vs concentration for osmotic dehydration of apple cylinders at different temperatures.
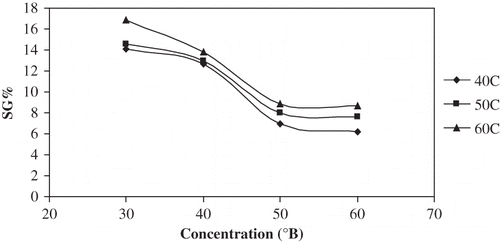
Table 4 Mean values and 95% confidence limits for solids gain (%) during osmotic treatment of apple cylinders in sugar solution at different concentrations and different temperatures
The ratio of ML∞/SG∞ increased with concentration but decreased or remained constant with temperature ( and ). The highest ratio of ML∞/SG∞ was observed with the highest concentration (60°Brix) and at the lowest temperature (40°C). Processing at lower temperatures in higher concentration medium therefore favored better water removal over solute uptake; however, this prolonged the equilibrium time.
Effect of Sample Size on Pseudo-Equilibrium Moisture Loss and Solid Gain
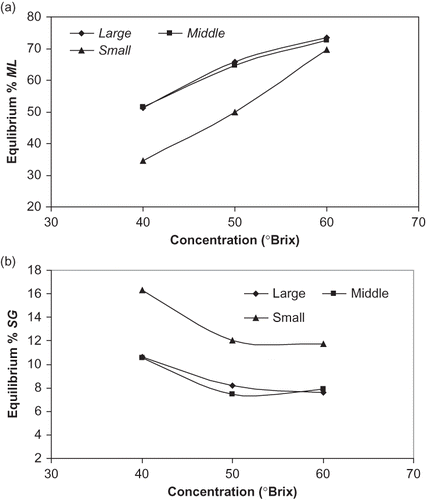
Pseudo-equilibrium moisture loss and solids gain for samples of different size under selected conditions are shown in . While ML∞ increased with increasing solution concentration for three different sizes of sample, SG∞ decreased. The ML∞ for large and middle size samples was in the same trend and level, while the lower ML∞ for small size sample was due to the same level ML∞ coming earlier and reducing afterwards (). The SG∞ for large and middle size sample was the same; however, it was different compared with the SG∞ for the small size sample. From these observations, it can be concluded that sample size may not affect equilibrium moisture loss but may affect equilibrium solids gain. Possible explanations for these phenomena include: both the moisture transfer and solids transfer inside the samples occurred in a progressive way, the moisture transfer was faster than solids transfer even in pseudo-equilibration period, and the equilibrium solids gain was not reached totally. The water flowing out from the surface restricts the sugar penetration into the cellular material.[Citation26 Citation27]
Moisture and Solid Migration During Pseudo-Equilibration
In order to study sample internal mass transfer variation, the osmotic treated sample was divided into different sections and its moisture content (MC) and solids content (SC) measured. The accuracy of the measured MC and SC using the five-time repeat sectioning operation was 95% compared with the control without sectioning. The MC and SC variations with sample positions and osmotic processing time are shown in . These tests were carried out only with the large size sample (2-cm diameter).
Figure 11 Plot of %MC (a) and %SC (b) vs sample section number for osmotic dehydration of apple cylinders at 50°C 50°Brix for large-size sample at different time.
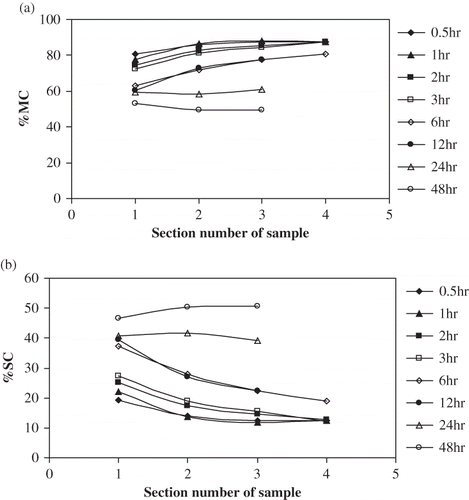
For a short time period (< 6 h), the MC of different sections increased from outside to inside of the sample, e.g. at the first time period (0.5–3 h), the center section moisture content was the same as the fresh sample moisture content; even after 12 h of osmotic dehydration treatment, the center section moisture content was still higher than that of external solution moisture content (). After a treatment time of 12 hours, the osmotic treated sample could only be separated to three different sections:,a surface layer (No.1), a middle layer (No.2), and a central layer (No.3). The MC varied less with position only after 24 and 48 h treatment time, indicating approaching equilibration. Although there was a continued drop in the moisture content of the samples observed.
Similarly, for a short period of time (< 6 h), the SC of different sections decreased from outside to inside of the sample, e.g. at 6 h treatment time, the center section solids content was the same as that in the fresh sample; even after 12 h osmotic dehydration treatment, the center section solute content was still lower than that of external solution solids content (). After 24 h osmotic dehydration treatment, SC for all the three sections was in same level. The SC picture was quite similar to that of MC (but increasing rather than decreasing).
Many aspects of cell structure affect the osmotic dehydration of samples, such as alteration of cell wall, splitting of the middle lamella, lysis of the membranes, tissue shrinkage, etc. These tissue changes could strongly influence the transport properties of the sample during the process.[Citation15] Only after 24 h, both ML and SG varied very slowly with time. From this point, it is suggested that the pseudo-equilibrium kinetics be used in osmotic dehydration studies should be extended to 24 h. Beyond this point, it appeared that there could be further slow changes in MC and SG but without following the conventional gradients.
CONCLUSIONS
Osmotic dehydration process is characterized by three periods: dynamic, pseudo-equilibrium and equilibrium periods. Dynamic and pseudo-equilibrium (practical equilibrium) period data are necessary for estimating the time of the osmotic process, and ultimate mass transport of the solutes and water. Higher concentrations increased pseudo-equilibrium ML∞ and decreased SG∞. Temperature effect on the result of water loss at equilibrium was less evident. Equilibrium moisture loss and solid gain could be estimated with Azuara-model combined with relatively long time experiment data. The equilibrium estimated here is the initial equilibrium stage without structural relaxation. ML∞, SG∞, PEDE and other related information could be used as reference to design osmotic dehydration processes and equipment for apple products. Two kinds of equilibration might exist: one is soluble solute equilibration, which is reached around 24 h; the other is solid matrix equilibration, which takes a long time to reach. Solute penetration is not uniform throughout the sample, but limited to only a relatively small depth and it was achieved by around 24 h of osmosis. Solute penetration did not strictly follow the diffusion law.
NOMENCLATURE
| K | = |
Function of time and rate of moisture loss or solids gain (dimensionless) | |||||||||||||||||||||||
| MS | = |
Fraction of water that can diffuse out, but remains in the food at time t (dimensionless) | |||||||||||||||||||||||
| Md | = |
After certain osmotic dehydration treatment, the solid mass of the sample | |||||||||||||||||||||||
| Mo Mt | = |
Sample mass at initial time and time t, respectively (kg) | |||||||||||||||||||||||
| ML∞ SG∞ | = |
Sample equilibrium moisture loss and solid gain | |||||||||||||||||||||||
| N | = |
Number of experimental data points | |||||||||||||||||||||||
| s | = |
s1 s2Constants related to the rate of moisture loss, weight reduction and soluble solids gain, respectively (dimensionless) | |||||||||||||||||||||||
| sost | = |
Sample solids fraction at initial time and time t, respectively (kg/kg wet base) | |||||||||||||||||||||||
| xoxt | = |
Sample moisture fraction at initial time and time t, respectively (kg/kg wet base) | |||||||||||||||||||||||
| VeVc | = |
Value got by experimental result and model predicted result, respectively
| |||||||||||||||||||||||
ACKNOWLEDGMENT
This research was partially supported by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
REFERENCES
- Fito , P. 1994 . Modeling of vacuum osmotic dehydration of food . Journal of Food Engineering , 22 : 313 – 328 .
- Yamaki , S. and Ino , M. 1992 . Alteration of cellular compartmentation and membrane permeability to sugars in immature and mature apple fruit . J. Amer. Soc. Hort. Sci. , 117 : 951 – 954 .
- Del Valle , F.R. and Nickerson , J.T.R. 1967 . Studies on salting and drying fish . Journal of Food Science , 32 : 173 – 179 .
- Biswal , R.N. and Le Maguer , M. 1989 . Mass transfer in plant materials in contact with aqueous solutions of ethanol and sodium chloride: Equilibrium data . Journal of Food Processing Engineering , 11 : 159 – 176 .
- Barat , J.M.E. , Chiralt , A. and Fito , P. 1998 . Equilibrium in Cellular Food Osmotic Solution Systems as Related to Structure . Journal of Food Science , 63 ( 5 ) : 836 – 840 .
- Barat , J.M.E. , Chiralt , A. and Fito , P. 1999 . Equilibrium in apple tissue in osmotic dehydration: microstructural changes . Drying Technology , 17 ( 7,8 ) : 125 – 127 .
- Sablani , S.S. and Rahman , M.S. 2003 . Effect of syrup concentration, temperature and sample geometry on equilibrium distribution coefficients during osmotic dehydration of mango . Food Research International , 36 : 65 – 71 .
- Rahman , M.S. , Sablani , S.S. and Al-Ibrahim , M.A. 2001 . Osmotic dehydration of potato: equilibrium kinetics . Drying Technology , 19 ( 6 ) : 1163 – 1176 .
- Waliszewski , K.N. , Delgado , J.I. and García , M.A. 2002 . Equilibrium concentration and water and sucrose diffusivity in osmotic dehydration of pineapple slabs . Drying Technology , 20 ( 2 ) : 527 – 528 .
- Tsamo , C.V.P and Bilame, A.-F.; Tsamo, R.N . 2006 . Air drying behaviour of fresh and osmotically dehydrated onion slices (Allium cepa) and tomato fruits (Lycopersicon esculentum) . International Journal of Food Properties , 9 ( 4 ) : 877 – 888 .
- González-Martínez , C. and ; Cháfer, M.; Xue, K.; Chiralt, A . 2006 . Effect of the osmotic pretreatment on the convective air drying kinetics of pear var. blanquilla . International Journal of Food Properties , 9 ( 3 ) : 541 – 549 .
- Li , H. and Ramaswamy , H.S. 2006a . Osmotic dehydration of apple cylinders: I. conventional batch processing conditions . Drying Technology , 24 ( 5 ) : 619 – 630 .
- Li , H. and Ramaswamy , H.S. 2006b . Osmotic dehydration of apple cylinders: II. continuous medium flow heating conditions . Drying Technology , 24 ( 5 ) : 631 – 642 .
- Li , H. and Ramaswamy , H.S. 2006c . Osmotic dehydration of apple cylinders: III. continuous medium flow microwave heating conditions . Drying Technology , 24 ( 5 ) : 643 – 651 .
- Nieto , A.B. , Salvatori , D.M. , Castro , M.A. and Alzamora , S.M. 2004 . Structural changes in apple tissue during glucose and sucrose osmotic dehydration: shrinkage, porosity, density and microscopic features . Journal of Food Engineering , 61 : 269 – 278 .
- Lenart , A. and Flink , J.M. 1984 . Osmotic concentration of potato: I. Criteria for end point of the osmotic process . Journal of Food Technology , 19 : 45 – 63 .
- Azuara , E. , Cortes , R. , Garcia , H.S. and Beristain , C.I. 1992 . Kinetic model for osmotic dehydration and its relationship with Fick's second law . International Journal of Food Science and Technology , 27 : 409 – 418 .
- Favetto , G. , Chirife , J. and Bartholomai , G.B. 1981 . A study of water activity lowering in meat during immersion cooking in sodium chloride-glycerol solutions. I Equilibrium considerations and diffusional analysis of solute uptake . Journal. Food. Technology , 16 : 609 – 619 .
- Biswal , R.N. and Bozorgmehr , K. 1991 . Equilibrium data for osmotic concentration of potato in NaCl-water solution . Journal of Food Process Engineering , 14 : 237 – 245 .
- Rahman , M.S. 1992 . Osmotic dehydration kinetics of foods . Indian Food Industry , 15 : 20 – 24 .
- Rastogi , N.K. and Raghavarao , K.S.M.S. 1995 . Kinetics of osmotic dehydration of coconut . Journal of Food Processing Engineering , 18 : 187 – 197 .
- Nsonzi , F. and Ramaswamy , H.S. 1998 . Osmotic dehydration kinetics of blueberries . Drying Technology , 16 ( 3–5 ) : 725 – 741 .
- Rahman , M.S. and Lamb , J. 1990 . Osmotic dehydration of pineapple . Journal of Food Science and Technology , 27 : 150 – 152 .
- Raoult-Walk , A.L. 1994 . Recent advances in the osmotic dehydration of foods, Trends in Food Science and Technology , 5 : 255 – 260 .
- Parjoko , Rahman , M.S. , Buckle , K.A. and Perera , C.O. 1996 . Osmotic dehydration kinetics of pineapple wedges using palm sugar . Lebensmittel Wissenschaft und Technologie , 27 : 564 – 567 .
- Marcotte , M. and Le Maguer , M. 1992 . Mass transfer in cellular tissues. Part II: Computer simulation vs. experimental data . Journal of Food Engineering , 19 : 27 – 48 .
- Marcotte , M. , Toupin , C.J. and LeMaguer , M. 1991 . Mass transfer in cellular tissues. Part I: the mathematical model . Journal of Food Engineering , 13 : 199 – 220 .