Abstract
The equilibrium moisture contents of litchi (Litchi Chinensis Sonn.) were experimentally determined using the dynamic method at temperatures of 30, 40, and 50°C over a range of relative humidity values of 12 to 95%. Five models were tested to fit the experimental isotherm data of litchi. The GAB model fitted the best to experimental isotherm data. The agreement between experimental and predicted values of this model is excellent (RMSE 1.8 to 3.4%). The isosteric heats of sorption water were also determined from the equilibrium data using the Clausius-Clapeyron equation and it was found to be a function of equilibrium moisture content.
INTRODUCTION
Litchi (Litchi Chinensis Sonn.) is a tropical fruit and it is grown mainly in northern Thailand and northern Vietnam. This fruit is consumed as fresh and dried product. It is a seasonal fruit and drying of the fresh fruit during harvesting season ensures the year round taste of litchi. Furthermore, dried fruits as an alternative to fresh fruits are becoming popular because of the taste and flavor of the dried fruits. Once dried, this product is on sale for consumption and storage. For proper handling of drying process and storage of litchi, it is essential to know its sorption isotherm.
Moisture sorption isotherms describe the relationship between the equilibrium moisture content and relative humidity at a constant temperature.[Citation1] The moisture sorption isotherm is an extremely useful tool for food scientists and technologists because it is required for predicting shelf life stability, predicting moisture changes during storage, selecting and designing packaging systems and modeling of drying and optimization of drying equipment.
Based on the authors' knowledge, there is no information available on sorption isotherms of litchi for its use in drying, packaging and storage. The objective of this study was to conduct experimental measurement of isotherms for litchi at various temperatures and relative humilities, to fit the moisture isotherms data to sorption isotherm models and to calculate the net isosteric heat of sorption from the experimental data and develop an equation for the net isosteric heat of sorption as a function of moisture content.
MATHEMATICAL MODELS OF SORPTION ISOTHERMS
Chen and Morey[Citation2–3] evaluated four models: the modified Henderson model, modified Chung- Pfost model, the modified Halsey model and the modified Oswin model for fitting the sorption isotherms data from 18 grains and seed crops. Sun and Woods[Citation4] reviewed more than 200 EMC/ERH purely empirical equations developed for cereal grains and food materials. But no single equation accurately describes the EMC/ERH relationships for various crops over a broad range of the relative humidity and temperature and also there is no universal equation for sorption isotherms of agricultural and food materials.
Numerous mathematical models for isotherms of food and food materials are available. Some of these are based on theory of sorption mechanism and others are either semi-theoretical or purely empirical. Van den Berg and Bruin[Citation5] have classified 77 such equations, and Chirife and Iglesias[Citation6] reviewed 23 such isotherm equations. None of these equations described adequately the sorption isotherms over the whole range of relative humidity and for all types of food material tested. Lomauro et al.[Citation7] evaluated two two-parameter equations and one three-parameter equation for 163 food materials including fruits, vegetables, spices and starchy foods. They found that the three-parameter Guggenheim, Anderson and de Boer (GAB) equation[Citation8] described the sorption isotherms for most food better than two parameters equations. Kuye and Ariri[Citation9] tested the applicapability of nine sorption models to 30 isotherm data of some Nigerian foods and they also found that the GAB model fits well most of the data.
Recently the GAB model has been proposed by food engineers as the universal model to fit the sorption data for all foods. Lomauro et al.[Citation7] reported that moisture sorption of foods can be described by more than one sorption model and the GAB gives the best fit for more than 50% of the fruits, meats and vegetables analyzed. But Chen and Jayas[Citation10] repeated that GAB model could not be used to describe the sorption isotherms of starchy grains. Furthermore, it is difficult to extend the model by adding a temperature term.
Mir and Nath[Citation11] fitted five isotherm models (BET, GAB, Henderson, Oswin and Smith) to the sorption isotherm data of three types of mango bars (plain mango bar, mango-coconut bar and mango-soya protein concentrate bar). The BET and GAB models were found to predict well the moisture content only for the plain mango bar whereas the Oswin model was applicable to all the three types of the mango bars. Hossain et al.[Citation12] developed six two-parameter and one-three parameter models to fit the observed data of pineapple and the modified BET model was found to be the best model for pineapple.
Lahsasni et al.[Citation13] determined the equilibrium moisture contents of prickly pear fruit using the gravimetric static method at three temperatures of 30, 40, and 50 °C over a range of relative humidity values from 5 to 90%. The sorption curves of prickly pear fruit decreased with increase in temperature at constant relative humidity. The GAB, modified Halsey, modified Chung-Pfost, modified Oswin and modified Henderson models were tested to fit the experimental data. The GAB model was found to be the most suitable for describing the sorption curves.
Kaymak-Ertekin and Gedik[Citation14] determined the moisture isotherms of grapes, apricots, apples and potatoes at 30, 45, and 60°C using the standard static-gravimetric method. Six two-parameter and five three-parameter sorption models were tested to fit the experimental data. The Halsey equation gave the best fit to the experimental data for all the materials tested over the range of temperatures and water activities investigated. The GAB model gave also the closest fit to the sorption data for potatoes and grapes.
MATERIALS AND METHODS
Determination of Equilibrium Moisture Content of Litchi
Equilibrium moisture contents of litchi were determined experimentally in the Department of Physics, Silpakorn University, Nakhon Pathom, Thailand using the dynamic method. To determine the equilibrium moisture contents, three sets of equipment were constructed and each set consists of a hot air chamber containing six sample boxes in three trays. The sample box was essentially an airtight plastic box containing saturated salt solution to maintain constant relative humidity inside the sample box. Each sample box is divided by plastic wall into two sections for holding two samples separately and simultaneously. The sample boxes were half filled with salt solution. The samples were placed inside the perforated sample containers of 3 cm diameter and 2 cm height and the sample boxes were placed on the perforated plastic supports just above the salt solution. Two small electric fans were fitted to circulate the air inside the sample box to accelerate moisture transfer between the samples and air inside the sample box. The sample boxes were placed inside the hot air chamber. The hot air chamber was equipped with a 3 kW electrical heater and an electronic temperature controller to maintain the temperature and the relative humidity was maintained by saturated salt solution.
In conducting the experiments, 50 g of the flesh of litchi was placed inside the sample containers located inside the sample boxes and the sample boxes were placed inside the hot air chamber. The samples were weighed regularly until they reached equilibrium. The final moisture contents of the product were determined by the standard oven method (temperature of 103°C for 24 hours). The selected temperatures for sorption isotherm determination were 30, 40, and 50°C and the water activity was 0.12 to 0.95.
The models selected to fit the sorption isotherms of litchi are shown in . These models were selected based on their effectiveness for describing isotherms of several food and plant materials and simplicity of computation. The temperature effect in the GAB model is included as:
Table 1 Selected isotherm models for fitting experimental data
The parameters of the models were determined using Marquardt-Levenberg optimization methods. Reduced mean relative error (RMRE), value of the root mean square error (RMSE) and randomness of residuals (ei) were computed.
Determination of the Isosteric Heat of Sorption
The net isosteric heat of sorption can be used to determine the energy requirements of drying and it provides useful information on the state of water in food products. The moisture content level of a material at which the net isosteric heat of sorption reaches the value of the latent heat of sorption is often considered as an indication of the amount bound water existing in the food.[Citation15–16] Several researchers have determined isosteric heats of sorption of food materials such fruits, vegetables, fish, meat and spices.[Citation12,Citation16,Citation17–20] Heat of sorption phenomena can be explained by Clausius-Clayperon equation[Citation16,Citation20] as follows:
Integrating EquationEq. (2), assuming that the isosteric heat of sorption (Qst ) is independent of temperature, gives the following equation:
The value of Qst is calculated from the slope of the EquationEq. (3).
RESULTS AND DISCUSSIONS
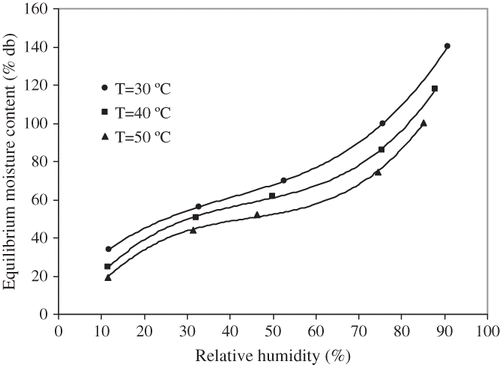
Sorption isotherms (points) of litchi at three temperature levels of 30, 40, and 50 °C, in the range of 12–95% relative humidity values are shown in . The isotherm curves have sigmoid shape and similar patterns. shows that the EMC values decreased with the increase in temperature at all levels of relative humidity. The kinetic energy associated with water molecules present in litchi increased with increasing temperature. This in turn, resulted in decreasing attractive forces, and consequently, escapes of water molecules. This led to a decrease in EMC values with increase in temperature at a given relative humidity. Several researches[Citation12,Citation20,Citation22–25] have reported similar trends for food materials.
Figure 1 Measured sorption isotherms (points) for litchi at the temperature levels of 30, 40, and 50°C.
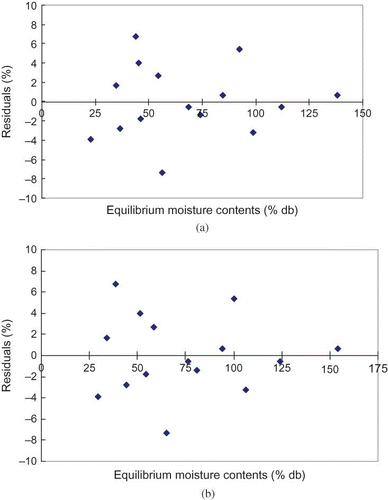
Computed parameters and their coefficients of determination for sorption isotherms of litchi are shown in . The characteristic parameters of the GAB model are shown in . Among the isotherm models tested, GAB model fitted the best to the experimental isotherm data of litchi for the temperature levels of 30, 40, and 50°C. The GAB model was the best among the five fitted models with the lowest values of standard error of estimate (RMSE) and mean relative error (RMRE), and the highest value of coefficient of determination. The modified Oswin model was next to the GAB model in terms of standard error of estimate and mean relative error, and the highest value of coefficient of determination. The predicted and observed equilibrium moisture contents are shown in for the GAB model and the modified Oswin model. The agreement between the observed and predicted values is very good. shows the residuals of the predicted equilibrium moisture contents for the GAB model and the modified Oswin model and the residuals for both the models are random in pattern. Hence, the GAB model and the modified Oswin model are acceptable.
Table 2 The coefficients of the selected models, reduced mean relative error (RMRE), standard error of estimate (RMSE) and the coefficient of determination (R2) for litchi
Figure 2 (a) Predicted and measured sorption isotherms for litchi at the temperature levels of 30, 40, and 50°C (GAB model); and (b) predicted and measured sorption isotherms for litchi at the temperature levels of 30°C, 40°C and 50°C (Modified Oswin model).
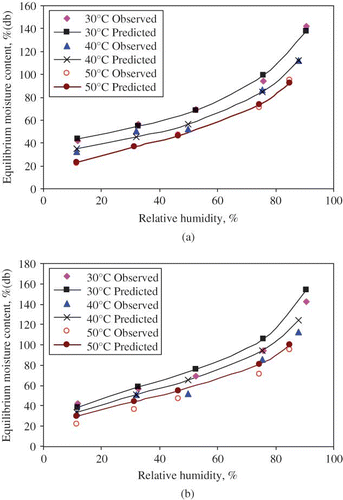
Table 3 Characteristic parameters of the GAB model
Figure 3 (a) Residuals of the predicted equilibrium moisture contents (GAB model); and (b) residuals of the predicted equilibrium moisture contents (Modified Oswin model).
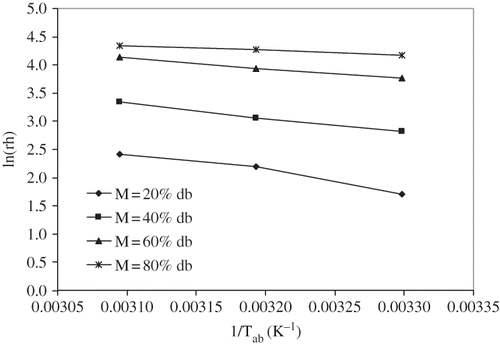
The plot of ln(rh) as a function of 1/Tab at constant moisture content is shown in . The slopes of the lines at constant moisture contents were determined by regression analysis to find the net isosteric heat of sorption of litchi. The heat of sorption for litchi at different moisture contents is presented in . The net isosteric heat of sorption was found to decrease exponentially with increase in moisture content. This trend is similar to those reported by other researchers for agricultural, food, and medicinal and aromatic plants.[Citation12–13,Citation17,Citation26–32] The net isosteric heat of sorption was found to be a function of equilibrium moisture content and the following equation is developed:
Figure 5 Net isosteric heat of sorption (Qst ) of litchi for different for different values of equilibrium moisture contents.
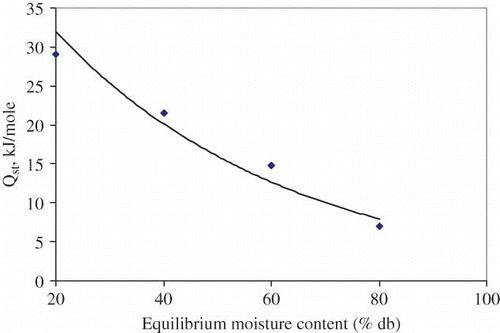
This relation shows that the net isosteric heat of sorption of litchi decreased exponentially with the increase of equilibrium moisture content.
CONCLUSIONS
The equilibrium moisture content isotherms of litchi were determined experimentally at the temperature levels of 30, 40, and 50°C and a relative humidity in the range of 12 to 95% using the dynamic method. The effects of temperature were found to be significant and the equilibrium moisture content increased with decreasing temperature at constant water activity. Five sorption isotherm models were used to fit the experimental data of litchi. Among the sorption models tested, the GAB model fitted the best to the experimental isotherms of litchi at 30, 40, and 50°C and the modified Oswin model was next to the GAB model. The best equation is suggested for its use in drying, storing and packaging. The heat of sorption of litchi is found to decreases with the increase of moisture content and it is an exponential function of moisture content. This equation is suggested for use in the computation of heat of sorption of litchi.
NOMENCLATURE
| aw | = |
water activity, decimal |
| b0,b1, b2, b3, b10, b20 | = |
parameters of isotherm equation |
| EMC | = |
equilibrium moisture content, % db |
| ERH | = |
equilibrium relative humidity, % |
| h1,h2 | = |
parameters of isotherm equation |
| k | = |
constant of the solution of the Clausius-Clayperon equation |
| M | = |
moisture content, % db |
| Me | = |
equilibrium moisture content, % db |
| Qst | = |
isosteric heat of sorption, kJ/mole |
| rh | = |
relative humidity, % |
| R | = |
coefficient of determination |
| R0 | = |
universal gas constant, 8.315 kJ/kg-mol-K |
| T | = |
temperature, °C |
| Tab | = |
temperature, K |
ACKNOWLEDGMENTS
This research is a part of the research project SFB 564 (“Research for Sustainable Land Use and Rural Development in Mountainous Regions of Southeast Asia”), funded by Deutsche Forchungsgemeinschaft (DFG), Germany, and co-funded by the National Research Council of Thailand and the Ministry of Science, Technology and Environment, Vietnam. We gratefully acknowledge the financial support for this research. We would also like to thank the Silpakorn University Research and Development Institute for supporting the experimental work.
REFERENCES
- Bala , B.K. 1997 . Drying and Storage of Cereal Grains , New Delhi, , India : Oxford and IBH Publishing Co. Pvt. Ltd .
- Chen , C. and Morey , R.V. 1989a . Comparison of four EMC/ERH equations . Transactions of the American Society of Agricultural Engineers , 32 : 983 – 990 .
- Chen , C. and Morey , R.V. 1989b . Equilibrium relative humidity (ERH) relationships for yellow-dent corn . Transactions of the American Society of Agricultural Engineers , 32 : 999 – 1006 .
- Sun , D.W. and Woods , J.L. 1994 . The selection of sorption isotherm equations for wheat based on the fitting of available data . Journal of Stored Products Research , 30 ( 1 ) : 27 – 43 .
- Van den Berg , C. and Bruin , S. 1981 . “ Water activity and its estimation in food systems: Theoretical aspects ” . In Water activity: influence of food quality , Edited by: Rockland , L.B. and Stewart , G.E. 45 – 58 . New York : Academic Press .
- Chirfe , J. and Iglesias , H.A. 1978 . Equations for fitting water sorption isotherms of foods. A review . Journal of Food Technology , 13 : 159 – 174 .
- Lomauro , C.J. , Bakshi , A.S. and Labuza , T.P. 1985 . Evaluation of food moisture sorption isotherm equations. Part 1. Fruit, vegetable and meat products . Lebensmittel-Wssenschaff , 18 : 111 – 117 .
- Van den Berg , C. 1984 . “ Description of water activity of foods for engineering purpose by means of the GAB model of sorption ” . In Engineering and Food: Engineering Sciences in the Food Industry , Edited by: McKenna , B.M. Vol. 1 , 311 – 321 . London : Elsevier Applied Science Publishers .
- Kuye , A. and Ariri , I. 2005 . Modeling the eqilibrium moisture sorption data for some Nigerian foods . International Journal of Food Properties , 8 : 1 – 13 .
- Chen , C. and Jayas , D. S. 1998 . Evaluation of the GAB equation for the isotherms of agricultural products . Transactions of the American Society of Agricultural Engineers , 41 : 1755 – 1760 .
- Mir , M. A. and Nath , N. 1995 . Sorption isotherms of fortified mango bars . Journal of Food Engineering , 25 : 141 – 150 .
- Hossain , M. D. , Bala , B. K. , Hossain , M. A. and Mondol , M. R. A. 2001 . Sorption isotherms and heat of sorption of pineapple . Journal of Food Engineering , 48 : 103 – 107 .
- Lahsasni , N. , Kouhila , M. and Mahrouz , M. 2004 . Adsorption-desorption isotherms and heat of sorption of pickly pear fruit (Opuntia ficus indica) . Energy Conservation and Management , 45 : 249 – 261 .
- Kaymak-Ertekin , F. and Gedik , A. 2004 . Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes . Lebensm.-Wiss.u.-Technol. , 37 : 429 – 438 .
- Duckworth , R.B. 1972 . The properties of water around the surfaces of food colloids . Proceedings of the Institute of Food Science and Technology , 5 : 50 – 62 .
- Wang , N. and Brennan , J.G. 1991 . Moisture sorption isotherm characteristics of potatoes at four temperatures . Journal of Food Engineering , 14 : 269 – 282 .
- Iglesias , H.A. and Chirife , J. 1976 . Prediction of the effect of temperature on water sorption isotherms of food materials . Journal Food Technology , 11 ( 2 ) : 109 – 116 .
- Iglesias , H. A. and Chirife , J. 1978 . Isosteric heats of water vapour on dehydrated foods . Lebensmittel-Wssenschaff.und Tecnolgie , 9 : 116 – 122 .
- Tsami , E. 1991 . Net isosteric heat of sorption in dried fruits' . Journal of Food Engineering , 14 : 327 – 335 .
- McLaughlin , C.P. and Magge , T.R. A. 1998 . The determination of sorption isotherm and the isosteric heats of sorption for potatoes . Journal of Food Engineering , 35 : 267 – 280 .
- Okos , M.R. , Narsimhan , B. , Singh , R.P. and Weimauer , A.C. 1992 . “ Food Dehydration ” . In Handbook of Food Engineering , Edited by: Heldman , D.R. and Lund , D.B. 437 – 562 . New York : Marcel Dekker, Inc .
- Iglesias , H.A. and Chirife , J. Handbook of Food Isotherms: Water Sorption Parameters for Food and Food Components , 11 – 261 . New York : Academic Press .
- Hossain , M.A. and Bala , B.K. 2000a . Moisture sorption isotherms of green chilli . International Journal of Food Properties , 3 ( 1 ) : 93 – 104 .
- Hossain , M.A. and Bala , B.K. 2000b . Moisture isotherms characteristics of red chilli . Drying Technology , 18 ( 1&2 ) : 503 – 515 .
- Shivhare , U.S. , Arora , S. , Ahmed , J. and Raghavan , G.V.S. 2004 . Moisture adsorption isotherms for mushroom . Lebensm-Wiss. u-Technol. , 37 : 133 – 137 .
- Soysal , Y. and Oztekin . 2001 . Sorption isosteric heat for some medicinal and aromatic plants . Journal of Agricultural Engineering Research , 78 : 159 – 166 .
- Corzo , O. and Fuentes , A. 2004 . Moisture sorption isotherms and modeling for pre-cooked flours of pigeon pea (Cajanus ensiformis) and lima bean (Canavalia ensiformis) . Journal of Food Engineering , 65 : 443 – 448 .
- Swami , S.B. , Das , S.K. and Maiti , B. 2005 . Moisture sorption isotherms of black gram nuggets (bori) at varied temperatures . Journal of Food Engineering , 67 : 477 – 482 .
- Sobukola , O.P. , Dairo , O.U. , Afe , T.T. and Coker , O.J. 2007 . Water sorption isotherms and cripsness of fried yam chips in the temperature range from 293K to 313K . International Journal of Food Properties , 10 : 561 – 575 .
- Pagano , A.M. and Mascheroni , R.H. 2003 . Water sorption of Amaranthus cruentus L. seeds modeled by GAB equation . International Journal of Food Properties , 6 : 369 – 391 .
- Vullioud , M. , Marquez , C.A. and De Michelis , A. 2006 . Equilibrium sorption isotherms and isosteric heat of rose hip fruits (Rosa Eglanteria) . International Journal of Food Properties , 9 : 823 – 833 .
- Ikhu-Omoregbe , D.I.O. 2006 . Comparison of the sorption isotherm characteristics of two cassava products . International Journal of Food Properties , 9 : 167 – 177 .