Abstract
Flavonoids of sweet potato vines (FSPV) were examined for their antioxidant effects in vitro. The scavenging capacities of FSPV on 1,1-diphenyl 2-picrylhydrazyl (DPPH), superoxide anion radical, and hydroxyl radical were measured, and their antioxidant activity in a linoleic acid model system was studied using thiobarbituric acid (TBA) method. The results showed that FSPV had a strong scavenging effect on DPPH. Their scavenging activity on superoxide radicals was stronger than that of rutin, and the scavenging activity on hydroxyl radical was higher than that of rutin and vitamin C. Moreover, FSPV had strong antioxidant activities in the linoleic acid model system.
INTRODUCTION
Sweet potato is the world's sixth largest food crop and is important for the growing populations in Asian and African countries. More and more people began to pay attention to sweet potato vines, including leaves, stalks, and stems, although their value had been completely neglected in 1960s. Internationally, most materials used to extract flavonoids were herbal medicine or food resources, which were resource-limited and expensive, resulting in high operating costs. Sweet potato vines, the castoff of farm production in most countries containing flavonoids.[Citation1,Citation2] Flavonoids of sweet potato vines have been found to have effects on antilipidemics[Citation3] and hypoglycemic,[Citation4,Citation5] and can effectively cure fatty liver and increase the antioxidant activity of the organisms in vivo and thus prevent oxidative damage of the lipid.[Citation1]
Reactive oxygen species (ROS), including free radicals such as superoxide anion radical (), hydroxyl radical (
), singlet oxygen (1O2), and hydrogen peroxide (H2O2), are active oxygen species that often generate biological reactions by exogenous factors. These active oxygen species play important roles in oxidative decomposition of invaders such as bacteria. The overproduced harmful radicals, on the other hand, not only cause aging, cancer, and many other ill-effects to the human body, but also have to be eliminated in biological systems by some enzymes, such as superoxide dismutase, catalase, and peroxidase,[Citation6] which possess antioxidant abilities. In recent years, interest in the study of antioxidants has been grown. The objective of this study is to examine in vitro antioxidant effects of FSPV by measuring the scavenging capacities of FSPV on 1,1-diphenyl 2-picrylhydrazyl, superoxide radical anion, and hydroxyl radical, and investigating the antioxidant capability of FSPV in a linoleic acid model system by using the thiobarbituric acid (TBA) method. The results of these studies can provide a theoretical basis for further development of the FSPV resources.
MATERIALS AND METHODS
Materials
Fresh sweet potato vines (Xushu18) were obtained from Jiangxi Good Proliferous Place (Jiangxi, China). All reagents such as rutin, linoleic acid, butylated hydroxytoluene (BHT), thiobarbituric acid (TBA), trichloroacetic acid (TCA), n-butanol, ethanol (95%, 100%), and vitamin C (ascorbic acid) were analytical grade and purchased from Shanghai Chemical Reagent Co. (Shanghai, China), except for DPPH, which was obtained from Sigma Chemical Co (St. Louis, Missouri, USA). A reagent kit of superoxide radical anion and hydroxyl radical was obtained from Nanjing Department of Biologic Engineering Research (Nanjing, China).
Preparation of Freeze Dried Extracts of Flavonoids of Sweet Potato Vines (FSPV)
Fifty grams of sweet potato vines (60-mesh particle size) were suspended in 500 ml ultrapure water and hydrodistilled for 4 h to extract polysaccharides. The extract was filtered and the residue was further extracted with the same volume of 70% ethanol under reflux in a water bath at 80°C for 2 h, and the same extraction process was repeated twice. Then the ethanol extracts were filtered and the filtrates were combined, evaporated, and dried. The dried materials were re-dissolved in 100 ml of distilled water. The materials were then adsorbed by the macroporous resin and subsequently desorbed with 95% ethanol. The eluant (95% ethanol) was reduced in volume in a vacuum drier (45°C), freeze dried, and stored at 4°C. Then flavonoid aglycones in the freeze-dried extracts were identified by HPLC (Varian Inc., Palo Alto, California, USA). Seven types of aglycones were identified in the freeze-dried extracts. They are genistein, leteolin, coumarin, quercetin, formononetin, apigenin, and kaempferol. The freeze-dried extracts were named as flavonoids of sweet potato vines (FSPV). 10 batches of fresh sweet potato vines were tested for extracting FSPV.
Scavenging Activity of FSPV on DPPH Radicals
The capability of the freeze-dried extracts to scavenge DPPH radicals was determined by the method of Yan et al.[Citation7] The freeze-dried extracts were dissolved in 95% ethanol to make FSPV solutions. Ten different concentrations of FSPV solutions: 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 mg/L were accurately prepared. A 2 × 10-4 mol/L solution of DPPH in ethanol was prepared, and 2 mL of this DPPH solution were added to 2 mL of FSPV solution (total volume of 4 mL). After gentle mixing, the mixture was kept in a water bath at 25ºC for 30 min. The absorbance was measured at 517 nm, and then the inhibition (k) of FSPV on DPPH can be calculated using the following equation:
Scavenging Activity of FSPV on Super Oxide Radical ( )
)
In order to mimic the xanthine and purine oxidase reaction system in the human body to obtain superoxide radical anion, both electronic transmit substance and Gress reagents[Citation8] were added. Absorbance of the fuchsia mixture was measure at 550 nm and then the inhibition rates were calculated. According to the specification on reagent kit, twelve concentrations of FSPV solutions: 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, and 40 mg/L were accurately prepared and the same concentrations of vitamin C and rutin were used as control experiments. The scavenging activity was calculated by the following equation:
Scavenging activity of FSPV on hydroxyl radicals ( )
)
According to the Fenton principle, the amount of hydroxyl radical should be produced in proportion to the amount of H2O2.[Citation9]When the electron acceptor and Gress reagent were provided, the mixture turned purple and the color intensity was proportional to the amount of 12. For this purpose, concentrations of FSPV solutions were accurately prepared: 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, and 40 mg/L and vitamin C and rutin were used as control experiments. The detailed procedure was according to Burtis and Bucar.[Citation9]The color intensity was measured at 550 nm. The Scavenging activity was calculated using EquationEq. (2).
Antioxidant Activity of FSPV Against Oxidation of Linoleate
The antioxidant activity of FSPV was determined using a linoleic acid model system.[Citation10] Different concentrations of FSPV solutions (0.20, 0.10, and 0.05%) and 0.10% BHT solution were prepared using 95% (w/v) ethanol. Each FSPV solution (4.0 mL), BHT solution (4.0 mL), and ultrapure water (4.0 mL) was mixed with linoleic acid (2.50%, v/v) in 95% ethanol (4.1 mL), 0.05 M phosphate buffer (pH = 7.5, 8.0 mL) and utralpure water (3.9 mL) and the mixture was kept in a screw-cap container in the dark at 40 ± 1ºC for 24 h incubation. 1.0 mL of this solution was mixed with 1.0 mL of 20% trichloroacetic acid (TCA) and 2.0 ml thiobarbituric acid solution (TBA, 0.30% thiobarbituric acid in 0.50% trichloroacetic acid, v/v). This mixture was then placed in a boiling water bath at 100ºC for 15 min. After the mixture was cooled down, 4.0 mL of n-butanol was added and the mixture was shaken vigorously. After being centrifuged at 3000 g force for 15 min, the organic layer was taken, and the absorbance of supernatant at 532 nm was measured.
RESULTS AND DISCUSSION
Scavenging Activity of FSPV on DPPH Radicals
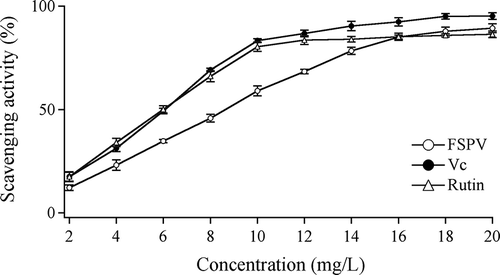
DPPH method was a convenient way to select radical scavengers and evaluate antioxidant activity. The principle of the method is based on the assumption that DPPH is a stable free radical with three benzol rings and an N atom with a pair of isolated electrons. The mixture is purple when dispersed in ethanol with a strong absorbance at 517 nm. When radical scavengers were added, the monopole was matched, the color turned to yellow from purple and thus the absorbance at 517 nm became weak, and there was a close linear correlation between DPPH radical-scavenging activity and the reduction in absorbance. FSPV solutions, the same as vitamin C and rutin, showed a dose-dependent inhibitory activity on DPPH, as shown in . The inhibition reached a plateau when the concentration became high enough (). The scavenging activity was observed for the FSPV solution with an IC50 of 8.06 mg/L, followed by vitamin C (6.07 mg/L) and rutin (5.99 mg/L). The DPPH radical-scavenging activity of FSPV is less effective than vitamin C and rutin, whose DPPH scavenging activity had no significant differences from those of extracts of plant flavonoids reported, such as flavonoids of celery.[Citation7] It was reported that the DPPH scavenging activity of plant extracts might be affected by the presence and position of the phenolic hydroxyl group.[Citation11]
Scavenging Activity on Super oxide Free Radicals ( )
)
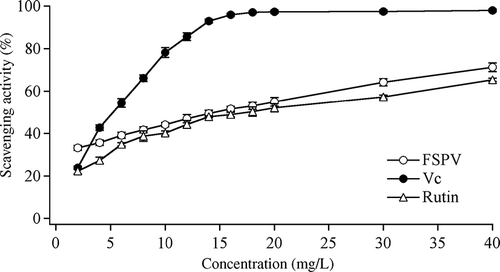
showed that as the concentration of extracts increased, the inhibition was increased. The antioxidant activity on of FSPV, vitamin C, and rutin were expressed by the parameter of IC50. The IC50 value of rutin was 17.48 mg/L, followed by FSPV (14.56 mg/L) and vitamin C (5.29 mg/L). The experimental results were analyzed using ANOVA. P value (<0.01) indicates that different concentrations of FSPV solutions had significant effects on the scavenging activity. In order to test this hypothesis, the scavenging activities of FSPV solutions with different concentrations were analyzed by SSR method (). showed that the scavenging activities between 4 and 6 mg/L of FSPV solutions were significantly different. The most remarkable differences in the scavenging activity of FSPV were between 20 and 30 mg/L, and 30 and 40 mg/L. Other close concentrations of FSPV solutions were not remarkably different.
Table 1 SSR table of FSPV scavenging activities on  and
and 
Scavenging Activity on Hydroxyl Radicals ( )
)
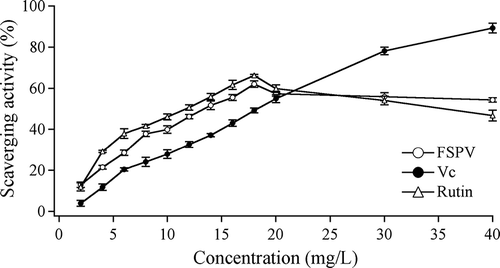
The scavenging activity of FSPV, vitamin C and rutin on has shown in . The scavenging activity on
increased with the concentrations of extracts increased. The IC50 value was 13.38 mg/L for FSPV, 18.27 mg/L for vitamin C, and 13.73 mg/L for rutin, indicating that FSPV has a higher scavenging activity on hydroxyl free radicals than both rutin and vitamin C. The scavenging activity of vitamin C showed concentration dependent over 20 mg/L. However, this was not the case for FPSV and rutin solutions. It was noticed that the scavenging activity of FPSV and rutin solutions on
decreased as their concentrations increased when the concentrations of FPSV and rutin Solution were over 18 mg/L It was known that super oxide or oxide could be produced if flavonoids, iorn hydronium, and oxygen were concomitant. This was called the stimulative oxygen effect, and would be the reason why the scavenging activity of FSPV and rutin on
decreased in the FeSO4-H2O2 system when their concentrations reached a certain value.
The scavenging activity of FSPV on was analyzed using ANOVA. P value (<0.01) indicated that the scavenging activity of FSPV on
was remarkably different among the concentrations tested. The scavenging activities of FSPV solutions with different concentrations were analyzed by SSR method (). The scavenging activities were significantly different for all the close concentrations from 2 to 20 mg/L except two solutions of 8 and 10 mg/L. When concentration was beyond 20 mg/L, the scavenging activities were decreased because of the stimulatory effect of oxygen. Similar results were reported by Chen and Yen.[Citation12] So the optimal concentration of FSPV on
was 18 mg/L.
Antioxidant Activity of FSPV on Linoleate
Plant tissues contain a range of components that may be broadly classed as antioxidants. The relative activity of each component may differ depending upon the type of system (lipidic or aqueous) used.[Citation3–5] In this study, the antioxidant activities of water and alcohol extracts were evaluated in a linoleic acid model system.
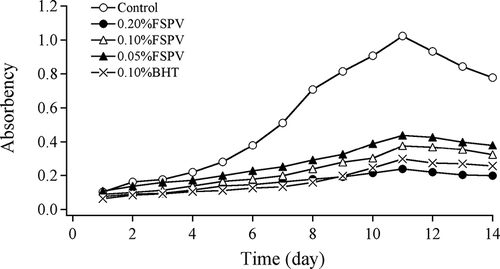
In TBA method, the antioxidant activity increased with the decrease of absorbance. showed FSPV and BHT both had antioxidant capacity against oxidation of linoleate, with FSPV showing a dose-dependent inhibition. The oxidation of linoleate for all solutions reached their maximum on the 11th day. The absorbance of 0.20% FSPV solution was lower than those of 0.10% and 0.05% FSPV solutions at the same period of time, which indicated that 0.20% FSPV solution showed greatest effect on the antioxidant activity and its antioxidant activity was also stronger than 0.10% BHT solution. After the 11th day, the absorbance declined slightly because Maleic dialdehyde was decreased.[Citation18]
A few studies on antioxidant activity of the plants' extracts also showed the same results.[Citation19–20] Antioxidant activity and its strength in each plant depend on the existence of various compounds in that plant. Antioxidative and radical scavenging activities of flavonoids are well studied.[Citation21] Some of phenolic compounds (anthocyanidin, catechines, flavones, flavonols, and isoflavones), tannins (ellagic acid, gallic acid), phenyl isopropanoids (caffeic acid, coumaric acids, and ferulic acid), lignans, catchol, and many others are antioxidants.[Citation22–23] Further investigation is necessary to separate the component of extracted sample and then evaluate the antioxidants activity of each component.
CONCLUSION
FSPV showed a high scavenging activity on DPPH radicals with the IC50 of 8.60 mg/L, but this scavenging effect is weaker than those of vitamin C and rutin. The scavenging activity of FSPV on superoxide radical was stronger than that of rutin but weaker than that of vitamin C, and the scavenging activity of FSPV on hydroxyl radical was higher than both of rutin and vitamin C. Moreover, FSPV had strong antioxidant activity on linoleic acid, and the antioxidant activity of 0.20% FSPV solution was stronger than that of 0.10% BHT solution. These findings demonstrated that FSPV possess free radical and hydroxyl radical scavenging activity as well as antioxidant activity in vitro.
ACKNOWLEDGMENTS
This work was supported by the Open Foundation of the State Key Laboratory of Food Science, Nanchang University, China Ministry of Education PCSIRT Program (IRT0540), and University of Minnesota Center for Biorefining.
REFERENCE
- Luo , L.P. , Gao , Y.Y. , Wang , Y.X. , Xia , D.H. and Hong , X.E. 2005 . Studies on Antioxidant Effect in vivo of Flavonoids and polysaccharides from Sweet Potato Vines . Food Science (Chinese) , 26 ( 8 ) : 408 – 410 .
- Li , W.F. , Tian , C.L. , Huang , M.E. and Shan , D.Y. 2005 . Preliminary Determination of Flavonoids in Sweet Potato leaves and stems . Chinese Agricultural Science Bulletin , 21 ( 4 ) : 119 – 121 .
- Gao , Y.Y. , Luo , L.P. , Wang , Y.X. , Xia , D.H. and Hong , X.E. 2005 . Antilipidemic Effect of Flavonoids from Sweet Potato Vines . Food Science (Chinese) , 26 ( 1 ) : 211 – 215 .
- Gao , Y.Y. , Luo , L. P. , Wang , Y. X. , Xia , D. H. and Hong , X. E. 2005 . Hypoglycemic Effect of Flavonoids from Sweet Potato Vines . Food Science (Chinese) , 26 ( 3 ) : 218 – 220 .
- Hu , L.M. 2002 . Study on extracting and analyzing polysaccharides and flavonoids from sweet potato leaves . Master degree thesis of Nanchang University , 6 : 8 – 12 .
- Toyo'oka , T. , Kashiwazaki , T. and Kato , M. 2003 . On-line screening methods for antioxidants scavenging superoxide anion radical and hydrogen peroxide by liquidchromatography with indirect chemiluminescence detection . Talanta , 60 : 467 – 475 .
- Yan , J.G. , Zhang , M.W. , Yang , G.M. and Chi , J.W. 2005 . Extraction conditions of celery favone and its antioxidative activity . Journal of Northwestern Sci-Tech University of Agricultural (nature science edition) , 33 ( 1 ) : 32 – 36 .
- Carrer , I. , Cusmai , P. , Zanozottera , E. , Martinotti , W. and Realini , F. 1995 . Anal . Chim. Acta , 308 : 20
- Burtis , M. and Bucar , F. 2000 . Antioxidant activity of Nigella sativa essential oil . Phytotherapy Research , 14 : 323 – 328 .
- Jung , C.H. , Seog , H.M. , Choi , I.W. and Cho , H.Y. 2005 . Antioxidant activities of cultivated and wild Korean ginseng leaves . Food Chemistry , 92 : 535 – 540 .
- Pyo , Y.H. , Lee , T.C. , Logendra , L. and Rosen , R.T. 2004 . Antioxidant activity and phenolic compounds of Swiss chard (Betavulgaris subspecies) extracts . Food Chemistry , 85 : 19 – 26 .
- Chen , H.-Y. and Yen , G.-C. 2007 . Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves . Food Chemistry , 101 ( 2 ) : 686 – 694 .
- Al-Saikhon , M.S. , Howard , L.R. and Miller , J.C. 1995 . Antioxidant activity and total phenolics in different genotypes of potato (Solonum tuberosum) . Journal of Food Science , 60 : 341 – 343 .
- Cao , G. , Verdon , C.P. , Wu , A.H.B. , Wang , H. and Prior , R.L. 1995 . Automated oxygen radical absorbance capacity assay using COBAS FARA II . Clin. Chem. , 41 : 1735 – 1737 .
- Gazzani , G. , Papetti , A. , Massolini , G. and Daglia , M. 1998 . Antioxidant and prooxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment . J Food Chem. , 6 : 4118 – 4122 .
- Velioglu , Y.S. , Mazza , G. , Gao , L. and Oomah , B.D. 1998 . Antioxidative activity and total phenolics in selected fruits, vegetables, and grain products . J Agric. Food Chem. , 46 : 4113 – 4117 .
- Mohd , Zin, Z. 2007 . Isolation and Identification of Antioxidative Compound from Fruit of Mengkudu (Morinda Citrifolia) . International Journal of Food Properties , 10 : 363 – 373 .
- Yao , X.S. 1995 . Chemistry of nature medication , 198 Beijing : People Healthful Publishing Company .
- Jong , P.L. , Young , C.S. , Jin , W.K. , Chung , H.K. , Jae , S.E. Kang , H.L. 2002 . Free radical scavengers from the heartwood of Juniperus chinensis. Arch . Pharmacal Res. , 25 : 449 – 452 .
- Elmastas , M , Gulcin , I , Beydemir , S , Kufrevioglu , OI and Aboul-Enein , HY . 2006 . A study on the in vitro antioxidant activity of Juniper (Juniperus communis L.) fruit extracts . Anal, Lett. , 39 : 47 – 65 .
- Heinonen , M. , Lehtonen , P.J. and Hopia , A.I. 1998 . Antioxidant activity of berry and fruit wines and liquor . J. Agric. Food Chem. , 46 : 25 – 31 .
- Kakhonen , M.P. , Hopia , A.I. and Vuoela , H.J. 1999 . Antioxidant activity of plant extracts containing phenolic compounds . J. Agric. Food Chem. , 47 : 3954 – 3962 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (morus alba l.) fruits . International Journal of Food Properties , 11 : 44 – 52 .