Abstract
Kokum (Garcinia indica Choisy), a tropical fruit, is a potential source of anthocyanin, which has a great potential as a natural colorant. The major acid present in it is hydroxy citric acid, which is used as an antiobesity ingredient in pharmaceutical industries. The fruit also contains garcinol, a polyisoprenylated benzophenone derivative, which is an antioxidant and has a chelating activity. It is used in making health beverages or squash and jellies like products. The review highlights the bioactive constituents present in kokum fruit and also discusses the extraction, purification and concentration of anthocyanins from kokum and its applications in foods.
INTRODUCTION
Color is an important constituent of any food as every food is associated and identified with certain color. Color is the first characteristic the consumer perceives of a food, which confers expectations of quality and flavor.Citation[1] Synthetic colors are very commonly used in medicines, foods, clothes, and in other products. Consumer awareness of the toxicological effects associated with synthetic colors is increasing and hence the use of natural colorants is increasing. The demand for natural pigment is high, however, the existing colorants and their methods of extraction are not able to meet this demand.Citation[2] The size of the color market on a global scale was estimated to be about US $940 million, out of which for natural colors it is estimated to be US $250 million (∼27% market share). This demand is met by betalains, anthocyanin, algal pigments, carotenoids, etc.[Citation3,Citation4] The use of natural pigments as food colorants permitted by the regulatory authorities is very limited. Any new source that has a potential to be used as food colorant requires the approval of the Food and Drug Administration (FDA).
Anthocyanins are present in nature as flavonoids. These are water-soluble and vacuolar pigments present in the epidermal cells and flowers of plants.Citation[5] The term anthocyanin is derived from the two Greek words “anthos” and “kyanos,” which mean flower and blue, respectively. Anthocyanins comprise of diverse groups of intensely colored pigments. These are responsible for appealing spectacular orange, red, purple, and blue colors of many fruits, vegetables, flowers, leaves, roots and other plant organs.[Citation6–Citation9] The solubility of anthocyanins in water makes them as the best candidates for the incorporation into aqueous food systems.[Citation5,Citation10] The various sources of anthocyanins along with the pigment concentration are provided in , which indicates that the kokum contain highest concentration (2400 mg/100g of fresh fruit) of anthocyanins as compared to other sources.[Citation11]
Table 1 Anthocyanin content (mg/100 g FW) in selected common edible plants
Garcinia indica Choisy is an underexploited fruit species, which is a rich source of anthocyanin. It is popularly known in India as Kokum, brindon or bhirand, or anslil, murgal, punarpulli. It belongs to the family of Guttiferae. Garcinia species are also distributed throughout the tropical Asian and African countries and have a tremendous potential as a colorant, spice with medicinal value. It is found in India in tropical humid evergreen rain forests of Western Ghats of south India as well as in northeast states of India. More than 200 listed species of Garcinia are available worldwide and out of these 30 species are reported to be available in India.Citation[35]
In India, the present level of production of kokum is estimated to 10,200 metric tones with productivity of 8.50 tons/ha. However, there is a continuous increase in its demand as it is evident by the inland market trends and export scenario. The estimated demand of kokum fruits for the year 2004–2005 was approximately 774.2 metric tons and India exported 31.32 metric tons of kokum fruit in the year 1997–1998.Citation[35]
Kokum (Garcinia indica Choisy) tree is an ornamental tree, with a dense canopy of green and red-tinged tender emerging leaves and well known as Indian butter tree. It does not require rainfall and generally not affected by pest attack or any diseases. Kokum tree is a slow growing slender tree with drooping branches growing to a height of 10–18 meters. It belongs to Garcinia genus and is either dioecious or polygamous. The flowers of kokum tree are small and unisexual; the male and female flowers are found on the same tree. The calyx is of four free sepals and corolla of four free petals.Citation[36] There are two main stages of growing of kokum crop. The first stage is establishment stage and the second one is the production stage. The kokum crop requires six to seven years to bear fruits. The tree flowers from November to February and yields ripened fruits from April to May in every year. A simple and efficient method has been developed for rapid regenerations of plantlets via adventitious bud differentiation on mature seeds of kokum tree.Citation[38] The growth of tree is slow and propagation is usually done by seeds and softwood grafting. Malik and coworkersCitation[37] has developed an efficient and reproducible method for rapid in vitro multiplication, conservation, and ex-vitro establishment of this species using apomictic seeds. The life of the tree is around 70 years and gives maximum bearing of fruits between 20–50 years.Citation[38]
The fruits are harvested manually in spring and sun dried for preservation. The normal shelf life of the fresh fruit is about a week. Hence, these are cut into halves and sun dried. It takes around 6–8 days for complete drying. The ripe Kokum fruit is dark purple or red in color with a yellow tinge. It contains 3 to 8 large seeds embedded in a regular pattern like orange segments in the white pulpy material as shown in . The fruit shape varies, round, oblong, oval, fruits with pointed tips and it weighs around 21–85 g.[Citation35,Citation39]
The fruit has a pleasant flavor and sour taste. It is traditionally used as an acidulant in many Indian dishes. Kokum was found to be effective in the treatment of dysentery, tumors, and heart complaints. Kokum juice is also used as natural remedy for stomach and liver disorders.[Citation35,Citation38,Citation39,Citation40] The major organic acid component that imparts the savory taste to the fruit is hydroxyl citric acid (HCA), which is an important ingredient in many fat reducing supplements and it is claimed to increase fat burning.Citation[41]
The seed of Garcinia indica contains oil, which remains solid at room temperature. It finds its applications in foods, pharmaceuticals as well as in cosmetics.Citation[38] The chemical and spectral investigations revealed the kokum contains Garcinol, a fat-soluble yellow pigment and two water soluble anthocyanin pigments as cyanidin-3-glucoside and cyanidin-3-sambubioside. The constituents of kokum are as shown in .Citation[42]
Table 2 Chemical constituents of kokum fruit
The present review discusses the availability of bioactive constituents in kokum, types of anthocyanins, methods for their determination, methods for the extraction, concentration, and stabilization of anthocyanin from kokum. The other important components present in kokum such as hydroxyl citric acid and benzophenone derivative have been provided. A brief account of potential application of anthocyanins from kokum in food and allied industries has also been presented.
MAIN CONSTITUENTS OF THE KOKUM FRUIT
Anthocyanin
Kokum is rich in red color natural pigments, which constitute approximately 2.4% of total fruit biomass. These pigments are water soluble and help in scavenging free radicals. Cyanidin-3-glucoside and cyanidin-3-sambubioside are the major pigment present in kokum and these occur in the ratio of 4:1. These two anthocyanins were identified by thin layer chromatography using acetic acid: HCl: water in the ratio of 15:3:82.Citation[42] The sugars present along with these cyanidin are glucose and xylose, respectively.[Citation38,Citation43] The general structure of cyandin present in kokum is as shown in .
Figure 2 General structure of Cyanidin in kokum. (Source: Wrolstad et al.Citation[44]).
![Figure 2 General structure of Cyanidin in kokum. (Source: Wrolstad et al.Citation[44]).](/cms/asset/4fb55883-8cf0-4bb8-9ba1-63936c82db03/ljfp_a_362843_o_f0002g.gif)
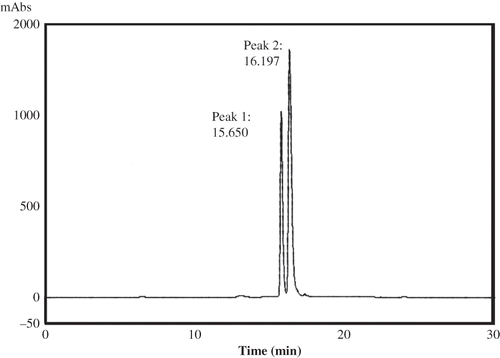
In order to identify the types of anthocyanins present in kokum, Nayak et al.[Citation11] adopted the HPLC procedure as described by Wrolstad et al.Citation[44] The color was extracted in 1% acidified solution, which was subjected to ultrafiltration using 10 kDa membrane. The permeate collected after ultrafiltration was subjected to HPLC and the chromatogram obtained for kokum extract is shown in , which indicated that the two major peaks were obtained, which corresponded to two types of anthocyanins, i.e., cyanidin-3-glucoside and cyanidin-3-sambubioside.[Citation11] Nayak et al.[Citation11] completely characterized the kokum anthocyanins using modern analytical techniques such as high performance liquid chromatography (HPLC), electron spray ionization mass spectroscopy (ESI-MS), and nuclear magnetic resonance (NMR).
Determination of Anthocyanin
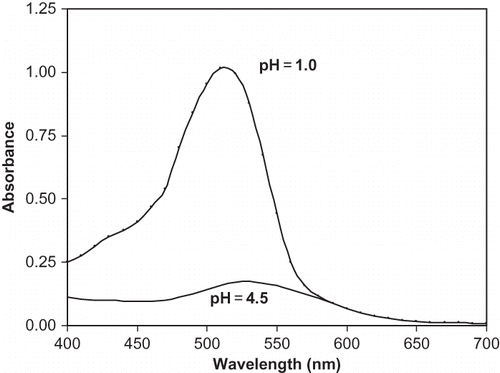
The concentration of anthocyanin in kokum was measured using pH differential method. A 200 μl of colour extract was added to 2.8 ml of respective buffers such as potassium chloride buffer (pH 1.0) or sodium acetate buffer (pH 4.5). The colour of anthocyanin changes from a saturated bright red color at pH 1.0 to colorless at pH 4.5.[Citation44–Citation46] The spectral characteristics of anthocyanin at pH 1.0 and 4.5 in the visible range (400–700 nm) are as shown in . The anthocyanin content for the kokum extract is calculated using the following equationCitation[44]:
where, A is the total absorbance [(A510- A700)at pH 1.0 - (A510- A700) at pH 4.5]; Mw is the molecular weight of anthocyanin (445.2 g mol −1); DF is the dilution factor; ϵ is the extinction coefficient (29,600 L cm−1 mol−1); and L is the path length (1 cm).Citation[45] The kokum colorant (red) is highly stable in the pH range from 1.0–3.0. It turns colorless at pH 4.5. Beyond pH value of 4.5, it changes to blue color.
Extraction of Anthocyanin Colorant
Major constituents in the anthocyanin color extracts are water and sugars, which contributed to the growth of the microorganisms. Shelf life of these extracts can be increased by removal of water. Hence, it is desirable to concentrate these extracts to improve shelf life, stability, and to reduce storage/transportation costs. The conventional method for downstream processing of anthocyanin from Kokum involved extracting anthocyanin from plant material, which involved disintegration of the raw material, extraction with a suitable solvent and then concentrating the extract under optimized conditions. Water, alcohol, and methanol were employed for extraction. Solvents like alcohol and methanol are not preferred in food application due to their harmful effects on health.Citation[10] These health concerns have led to numerous research efforts for the development of new extraction and concentration procedure for the natural color. Citation[35]
An efficient extraction procedure should maximize anthocyanin recovery with minimal amount of adjuncts and minimal degradation or alteration of its native state. Krishnamurthy and coworkersCitation[39] have concentrated anthocyanin from the dried kokum powder using 500 ppm sulphur dioxide water using six percolators (15 cm dia × 58 cm height) connected in series and the extract, thus obtained, was concentrated in a evaporator. Each percolator was packed with 5.0 kg kokum powder. The ratio of material to water and feed flow rate were kept at 1:11 and 20 ml/min, respectively. The anthocyanin extract was concentrated to 60°Brix and the yield of anthocyanin was reported to be 86.8%. It was suggested that the concentration of color can be increased by removal of free sugars present in the extract by yeast fermentation (Saccharomyces cerevisae) prior to concentration step.Citation[38 Citation,39]
Stabilized anthocyanin extract for kokum was developed by Bhaskaran and Mehta,Citation[43] where in the kokum extract was stabilized by addition of antioxidants to promote color retention. The fruit was pressed through a pulper and filtered through a filter cloth. The clear fruit extract, thus obtained was then subsequently subjected to adsorption employing ion-exchange resins (such as Amberlite IRA 120 or equivalent like Tulsion T42 MP, Dowex 50WX8) to remove sugars and pectins. The column was eluted using methanol or ethanol with 8% hydrochloric acid. The eluted methanol extract was concentrated using vacuum drier at 40°C until the pigments were in the form of semi-solid. Citation[43] The semi solid paste was maintained in stabilized condition by addition of suitable antioxidant such as tochopherol, Occuim sanctum extract, or Azadiracta indica extract.
Concentration of natural color extract by conventional methods such as evaporation and distillation resulted in loss of hue and chroma, which led to low quality product of anthocyanin, besides being energy intensive. Hence, membrane processes such as microfiltration, ultrafiltration and reverse osmosis were being employed for clarification, and concentration of natural color extracts. The limitations of these membrane processes are requirement of high pressure, maximum attainable concentration, reduction of flux due to concentration polarization and membrane fouling.
Relatively, newer athermal membrane process such as direct osmosis employs a semi-permeable dense hydrophilic membrane, which separates two aqueous solutions (feed and osmotic agent solution) having different osmotic pressures. The difference in osmotic pressure acts as the driving force. The transfer of water occurs from the low to high concentrated solution until the osmotic pressures of both the solutions become equal. Direct osmosis is a non-pressure driven membrane process, which is capable of concentrating liquid foods at ambient conditions without product deterioration.Citation[47] Using direct osmosis, kokum extract containing anthocyanin was concentrated from 49.63 to 3510 mg/L, which resulted in more than 50 times reduction in the feed volume.Citation[34] The flow rates of both feed (kokum extract) as well as osmotic agent (26% sodium chloride) solutions were maintained at 125 ml/min. This nonthermal procedure for the concentration of kokum extract offers several other advantages such as low energy consumption, higher retention of thermo liable components and achievement of higher concentration.
Extraction can be also carried out using different types of nonthermal treatments like gamma irradiation,Citation[4] and pulsed electric field extractionCitation[48] can be employed to enhance the yield of the anthocyanin.
Hydroxy Citric Acid (HCA)
Hydroxy citric acid (HCA) is mainly found in the fruit. It is also known as garcinia acid and has a large number of commercial applications. It is shown that HCA can inhibit ATP-citrate lyase (EC 4.1.3.8 an important enzyme in Kreb's Cycle) which is needed for conversion of carbohydrates into fat.Citation[41] HCA have been extensively studied for its unique regulatory effect on fatty acid synthesis, lipogenesis, appetite, and weight loss.Citation[41 Citation,49] HCA consumption was reported to enhance fat mobilization and fat burning.Citation[50] The HCA is used as an ingredient in anti-obesity formulations. The derivatives of HCA have been incorporated into a wide range of pharmaceutical preparations for claimed purpose of enhancing weight loss, cardio protection, and endurance in exercise. HCA comprises of citric acid with a hydroxyl group at the second carbon.Citation[51] HCA can be determined by HPLC or by titration method. The major organic acid in leaves and rinds are found to be HCA, which is in the range of 4.1–4.6 and 10.3–12.7%, respectively. Minor quantities of hydroxycitric acid lactone and citric acid are also present along with the HCA.Citation[52]
A process for large-scale isolation of HCA was demonstrated by Ibrahim and co-workersCitation[53] from the fresh/dried rinds of Garcinia indica, Garcinia combogia and Garcinia atorvirdis. The dry rinds of the fruit were sliced and soaked in boiling water for about 20 h. The aqueous sodium hydroxide was added to the extract at around 80°C. Methanol was added to the extract until two layers are formed. The lower layer contained the sodium salt of HCA, which was separated and neutralized by hydrochloric acid. Further, acetone was added to filtrate to obtain pure crystals of HCA.
Athermal process of obtaining concentrated HCA using osmotic membrane distillation (OMD) was employed by Anandaramakrishnan and coworkers.Citation[54] Garcinia extract were extracted from Garcinia fruit rinds with deionized water. The extract was subjected to concentrate using OMD in a co-current flat membrane module using micro porous hydrophobic polypropylene membrane. HCA was concentrated from 6.0–62°Brix, without any formation of HCA lactone in this process. This process involves no phase change and can be operated at ambient temperature and pressure and hence no thermal damage of the desired product takes place.
Benzophenone Derivatives
Kokum fruit contains 1.5% of polyisoprenylated benzophenone derivative called Garcinol (C38H50O6, Melting point 122°C), which is a yellow color fat soluble pigment. It is crystallized out from the hexane extract of the fruit rinds of Garcinia indica. It was found to be the potent inhibitor of the enzyme histone acetyl transferase. Garcinol, is a potential anti-cancer agent. It has been reported to be associated with has antibiotic, antioxidative, chelating, free radical scavenging, anti-ulcer, and anti-glycation activity.[Citation55–Citation57]
Garcinol showed three times greater DPPH (1,1 diphenyl-2- picrylhydrazyl) free scavenging activity than DL-α-tocopherol by weight in aqueous ethanol solution. Hence, it was regarded as a potential antioxidant and a glycation inhibitor.Citation[57] Garcinol can be used as a natural to impart yellow color to butter and ghee. The general structure of garcinol is shown in .
Figure 5 General structure of garcinol (Source: Yamaguchi et al.Citation[57]).
![Figure 5 General structure of garcinol (Source: Yamaguchi et al.Citation[57]).](/cms/asset/331be178-8d4c-488e-bad2-33b2c8175c87/ljfp_a_362843_o_f0005g.gif)
POTENTIAL APPLICATIONS OF KOKUM IN FOOD AND ALLIED INDUSTRIES
The color from kokum can be used in many food and food formulations. Selected examples are discussed in the following section.
Kokum Beverages
Kokum extract is having approximately 4% sugars, which can be fermented to produce a high quality red wine. The extract from kokum can be converted to many health beverages and squash like products with the addition of sugar. Red syrup extracted from the ripe fruit with the addition of sugar to kokum rinds can be stored in the house hold for making cool health drinks in summer.Citation[40] Due to high sugar content, the syrup will have a expected shelf life of 6–8 months. Another popular beverage made from kokum is known as ‘solkhadi’, which can be prepared by addition of jaggrey and coconut milk to kokum extract. It can be served as a digestive drink with meals.
Dehydrated Kokum
Kokum powder is prepared by drying kokum pieces in a drier followed by grinding. The powder is sieved and stored in airtight containers. This product finds its use in many Indian cuisines as an acidulant for many dishes such as fish and coconut curries as well as in many other food preparations.
Kokum Butter
Kokum seed contains 23–26% edible oil, which can be extracted by subjecting kokum seed to boiling water. The oil collected in the top layer can be separated and referred to as kokum butter. It remains in its solid state at room temperature and it is cream in color. Kokum butter is reported to find its application in many chocolates and confectionary preparations.Citation[58] It can also be used for the production of soaps, candle as well as many pharmaceutical products.Citation[35]
Cosmetic Industry
Apart from the food applications, kokum can have a number of non-food uses. It is reported that the kokum pigments has a potential to absorb UV light. This property may be exploited for the production of sun screens lotions and pastes in cosmetic industry.Citation[35]
Kokum Pigments Based pH Indicators
The color of the kokum pigment changes from red to blue/violet as the pH is increased beyond 5.0. This property was utilized to develop pH indicators.Citation[35] Kokum as a natural food colorants have some limitations as compared to other artificial colors such as increased susceptibility to the exposure to the oxygen, light and high or low pH. These limitations should be evaluated and recognized before using Kokum in food processing.
CONCLUSION
Kokum is a rich source of anthocyanin, which has a great export potential as natural colorant and major source of hydroxy citric acid. Kokum color was found to contain two types of anthocyanins, i.e., cyanidin-3-glucoside and cyanidin-3-sambubioside. Non thermal process such as direct osmosis membrane process could concentrate the kokum anthocyanins from 49.63–3510 mg/L, which resulted in more than 50 times reduction in the feed volume without formation of HCA lactone, when compared to the conventional process. The future R&D work may be focused to explore the untapped potential kokum. Rigorous efforts are needed to establish the large-scale commercial plantations, export oriented marketing, as well as development of suitable downstream processing technologies. A little is known about the various amino acids, vitamins, proteins, enzymes, lecitins, and polyphenols from kokum. The chemical screening of the unexploited kokum fruit may yield novel compounds, which may find their varied applications in food and pharmaceutical applications.
Acknowledgments
Authors would like to thank Dr. V Prakash, Director, CFTRI, Mysore for his encouragement and keen interest in downstream processing. The authors also thank Dr. Nandakumar Kamat, Department of Botany, Goa University, Goa for his help to provide very useful information on kokum. Chetan A Nayak expresses his gratitude and sincere thanks to the Council of Scientific and Industrial Research, New Delhi for providing Senior Research Fellowship.
References
- Chokshi , P.M. and Joshi , V.Y. 2007 . A review on Lycopene –Extraction, purification, stability and applications . International of Journal of Food Properties , 10 : 289 – 298 .
- Chethana , S. , Nayak , C.A and Raghavarao , K.S.M.S. 2007 . Aqueous two phase extraction for purification and concentration of betalains . Journal of Food Engineering , 81 : 679 – 687 .
- Downham , A. and Collins , P. 2000 . Colouring on foods in last and next millennium . International Journal of Food Science and Technology , 35 : 5 – 22 .
- Nayak , C.A. , Chethana , S. , Rastogi , N.K. and Raghavarao , K.S.M.S. 2006 . Enhanced mass transfer during solid-liquid extraction of gamma irradiated red beetroot . Radiation Physics and Chemistry , 75 : 173 – 178 .
- Gusti , M.M. and Wrolstad , R.E. 1996 . Radish anthocyanin extract as a natural red colorant from maraschino cherries . Journal of Food Science , 61 : 688 – 694 .
- Sharma , R. , Kaur , D. , Oberoi , D.P.S. and Sogi , D.S. 2008 . Thermal degradation kinetics of pigments and visual color in watermelon juice . International Journal of Food Properties , 11 : 439 – 449 .
- Righetto , A.M. and Netto , F.M. 2005 . Effect of encapsulating materials on water sorption, glass transition and stability of juice from immature Acerola . International Journal of Food Properties , 8 : 337 – 346 .
- Jyothi , A.N. , Moorthy , S.N. and Eswariamma , C.S. 2005 . Anthocyanins in sweet potato leaves- varietal screening, growth phase studies and stability in a model phases in a model system . International Journal of Food Properties , 8 : 221 – 232 .
- Rababah , T.M. , Khalil , I.E. , Majdi , A.A. , Khalid , I. , Hidar , A. and Yang , W. 2008 . Total phenolics, antioxidant activities and anthocyanins of different grape seed cultivars grown in Jordan . International Journal of Food Properties , 11 : 472 – 479 .
- Delgado-Vargas , F. , Jiménez , A. R. and Lopez , O. P. 2000 . Natural Pigments: Cartenoids, Anthocyanins and Betalains – Characteristics, Biosynthesis processing and Stability . Critical Reviews of Food Science and Nutrition , 40 ( 3 ) : 173 – 289 .
- Nayak , C.A. , Srinivas , P. and Rastogi , N.K. 2010 . Characterization of anthocyanin from Garcinia indica Choisy . Food Chemistry , 118 : 719 – 724 .
- Kampuse , S. , Kampuss , K. and Pizika , L. 2002 . Stability of anthocyanins and ascorbic acid in raspberry and blackcurrant cultivars during frozen storage . Acta Horticulturae , 2 : 507 – 510 .
- Wu , X. , Gu , L. , Prior , R. L. and McKay , S. 2004 . Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity . Journal of Agricultural and Food Chemistry , 52 : 7846 – 7856 .
- Wang , S.Y. and Lin , H.S. 2000 . Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental Stage . Journal of Agricultural and Food Chemistry , 48 : 140 – 146 .
- Clark , J.R. , Howard , L. and Talcott , S. 2002 . Antioxidant activity of blackberry genotypes . Acta Horticulturae , 2 : 475 – 480 .
- Prior , R.L. , Cao , G. , Martin , A. , Sofic , E. , McEwen , J. , O'Brien , C. , Lischner , N. , Ehlenfeldt , M. , Kalt , W. , Krewer , G. and Mainland , C. M. 1998 . Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species . Journal of Agricultural and Food Chemistry , 46 : 2686 – 2693 .
- Moyer , R.A. , Hummer , K.E. , Finn , C.E. , Frei , B. and Wrolstad , R.E. 2002 . Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes . Journal of Agricultural and Food Chemistry , 50 : 519 – 525 .
- Maatta-Riihinen , K.R. , Kamal-Eldin , A. , Mattila , P.H. , Gonzalez-Paramas , A.M. and Torronen , A.R. 2004 . Distribution and Contents of Phenolic Compounds in Eighteen Scandinavian Berry Species . Journal of Agricultural and Food Chemistry , 52 : 4477 – 4486 .
- Giusti , M.M. and Wrolstad , R.E. 1996 . Characterization of Red Radish Anthocyanins . Journal of Food Science , 61 : 322 – 326 .
- Wu , X. , Beecher , G.R. , Holden , J.M. , Haytowitz , D.B. , Gebhardt , S.E. and Prior , R.L. 2006 . Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption . Journal of Agricultural and Food Chemistry , 54 : 4069 – 4075 .
- Jing , P. and Giusti , M.M. 2005 . Characterization of anthocyanin-rich waste from purple corncobs (Zea mays L.) and its application to color milk . Journal of Agricultural and Food Chemistry , 53 : 8775 – 8781 .
- Hannum , S.M. 2004 . Potential impact of strawberries on human health: A review of the science . Critical Reviews in Food Science and Nutrition , 44 ( 1 ) : 1 – 17 .
- Cordenunsi , B.R. , OliveiradoNascimento , J.R. , Genovese , M.I. and Lajolo , F.M. 2002 . Influence of cultivar on quality parameters and chemical composition of strawberry fruits grown in Brazil . Journal of Agricultural and Food Chemistry , 50 : 2581 – 2586 .
- Wang , S.Y. and Lin , H.S. 2000 . Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage . Journal of Agricultural and Food Chemistry , 48 : 40 – 146 .
- Maatta-Riihinen , K.R. , Kamal-Eldin , A. , Mattila , P.H. , Gonzalez-Paramas , A.M. and Torronen , A.R. 2004 . Distribution and Contents of Phenolic Compounds in Eighteen Scandinavian Berry Species . J Agric Food Chem , 52 : 4477 – 4486 .
- Strigl , A.W. , Leitner , E. and Pfannhauser , W. 1995 . Chokeberries as natural food colorant source . Deutsche Lebensmittel-Rundschau , 91 : 177 – 180 .
- McGhie , T.K. , Hall , H.K. , Ainge , G.D. and Mowat , A.D. 2002 . Breeding Rubus Cultivars for high Anthocyanin Content and High Antioxidant Capacity . Acta Horticulturae , 2 : 495 – 500 .
- Moyer , R.A. , Hummer , K.E. , Finn , C.E. , Frei , B. and Wrolstad , R.E. 2002 . Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes . Journal of Agricultural and Food Chemistry , 50 : 519 – 525 .
- Prior , R.L. , Lazarus , S.A. , Cao , G. , Muccitelli , H. and Hammerstone , J.F. 2001 . Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium Spp.) using High-Performance Liquid Chromatography/Mass Spectrometry . Journal of Agricultural and Food Chemistry , 49 : 1270 – 1276 .
- Wang , S.Y. and Stretch , A.W. 2001 . Antioxidant capacity in cranberry is influenced by cultivar and storage temperature . Journal of Agricultural and Food Chemistry , 49 : 969 – 974 .
- Wang , S.Y. , Feng , R. , Bowman , L. , Penhallegon , R. , Ding , M. and Lu , Y . 2005 . Antioxidant activity in lingonberries (Vaccinium vitis-idaea L.) and its inhibitory effect on activator protein-1, nuclear factor-kappaB, and mitogen-activated protein kinases activation . Journal of Agricultural and Food Chemistry , 53 : 3156 – 3166 .
- Deighton , N. , Brennan , R. , Finn , C. and Davies , H.V. 2000 . Antioxidant properties of domesticated and wild Rubus species . Journal of the Science of Food and Agriculture , 80 : 1307 – 1313 .
- Wada , L. and Ou , B. 2002 . Antioxidant activity and phenolic content of Oregon cranberries . Journal of Agricultural and Food Chemistry , 50 : 3495 – 3500 .
- Nayak , C.A. , Rastogi , NK. and Raghavarao , K.S.M.S. 2007 . Direct osmosis process for concentration of anthocyanin from Kokum (Garcinia indica Choisy) a natural food colorant, presented at the 19th Indian Convention of Food Scientists and Technologists at the Indian Institute of Technology (IIT), Kharagpur, India. 31 December–1 February
- Bhat , J.D. , Kamat , N. and Shirodkar , A. 2005 . Compendium and proceedings of 2nd National seminar on KOKUM (Garcinia indica Choisy), Goa University March 4–5, 2005
- Chandran , M.D.S. 1996 . Nature watch: The Kokum Tree . Resonance , 1 : 86 – 89 .
- Malik , S.K. , Chaudhury , R. and Kalia , K.R. 2005 . Rapid in vitro multiplication and conservation of Garcinia indica: A tropical medicinal tree species . Scientia Horticulturae , 106 : 539 – 553 .
- Krishnamurthy , N. 1984 . Chemical and technological studies on coloring matters from natural sources for use in foods, PhD Thesis, Mysore University, Mysore Karnataka, , India
- Krishnamurthy , N. , Lewis , Y.S. and Ravindranath , B. 1982 . Chemical constituents of Kokum fruit rind . Journal of Food Science and Technology , 19 ( 3 ) : 97 – 100 .
- Mishra , A. , Bapat , M.M. , Tilak , J.C. and Devasagayam , T.P.A. 2006 . Antioxiant activity of Garcinia indica (kokam) and its syrup, Current Science . 91 ( 1 ) : 90 – 93 .
- Jena , B.S. , Jayaprakasha , G.K. , Singh , R.P. and Sakariah , K.K. 2002 . Chemistry and biochemistry of Hydroxycitric acid from Garcinia . Journal of Agricultural and Food Chemistry , 50 : 10 – 22 .
- Krishnamurthy , N. and Sampathu , S.R. 1988 . Antioxidant principles of Kokum rind . Journal of Food Science and Technology , 25 ( 1 ) : 44 – 45 .
- Bhaskaran , S. and Mehta , S. 2006 . Stabilized anthocyanin extract from Garcina indica , United States Patent No. US2006/0230983A1 . Filed July 1, 2004; Issued August 28, 2007.
- Wrolstad , R.E. , Acree , T.E. , Decker , E.A. , Reid , D.S. , Schawartz , S.J. , Shoemaker , C.F. , Smith , D. and Sporns , P. 2005 . Hand book of Food Analytical Chemistry-Pigments, colorants, flavor, texture, and bioactive components , 5 – 70 . NJ : Wiley Interscience Publications .
- Ozkanli , O. and Tekin , A.R. 2008 . Rheological behaviors of sumac concentrate, International . Journal of Food Properties , 11 : 213 – 222 .
- Giusti , M.M. and Jing , P. 2008 . “ Analysis of Anthocyanins ” . In In Food Colorants Chemical and Functional Properties , Edited by: Carmen , S. 479 – 506 . New York : CRC Press .
- Wrolstad , R.E. , McDaniel , M.R. , Durst , R.W. , Micheals , N. , Lampi , K.A. and Beaudry , E.G. 1993 . Composition and sensory characterization of red raspberry juice concentration by direct–osmosis or evaporation . Journal of Food Science , 58 ( 3 ) : 633
- Corrales , M. , Toepfl , S. , Butz , P. , Knorr , D. and Tauscher , B. 2008 . Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields . A comparison, Innovative Food Science and Emerging Technologies , 9 : 85 – 91 .
- Majeed, M , V. and Rajendran , R. Pottassium hydroxycitrate for the suppression of appetite and induction of weight loss, 1998, US Patent 5783603 Filed March 13, 1997; Issued July 21, 1998.
- Hastings , C W. and Barnes , DJ . Weight loss composition for burning and reducing synthesis of fats, 1997, US Patent 5626849 Filed June 7, 1999; Issued May 6 1997.
- Yamada , Y. , Hida , H. and Yamada , Y. 2007 . Chemistry, physiological, and microbial production of hydroxycitric acid, Applied Microbiology and Biotechnology . 75 : 977 – 982 .
- Jayaprakasha , G.K. and Sakariah , K.K. 2000 . Determination of (-) Hydroxycitric acid in commercial samples of Garcinia combogia extract by liquid chromatography with ultraviolet detection . Journal of Liquid Chromatography and Related Technologies , 23 ( 6 ) : 915 – 923 .
- Ibnusaud , I. , Thomas , P. and Thomas , B. Convenient method for the large scale isolation of Garcinia acid. 2000, US Patent No. 6,147,228 Filed July 30, 1999; Issued November 14, 2000.
- Anandaramakrishnan , C. , Nagaraj , N. , Jayaprakasha , G.K. , Jena , B.S. , Varadraj , M.C. and Raghavarao , K.S.M.S. 2007 . Athermal process for the concentration of Garcinia Acid , US Patent 2007/0154578A1 . Filed August 24, 2006, Issued October 7, 2008.
- Krishnamurthy , N. and Sampathu , S.R. 1988 312–313002E . Application of Garcinol as a colorant for butter and ghee and method of its estimation . Journal of Food Science and Technology, India , pp. 312–313.
- Yamaguchi , F. , Saito , M. , Ariga , T. , Yoshimura , Y. and Nakazawa , H. 2000 . Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind . Journal of Agricultural and Food Chemistry , 48 ( 6 ) : 2320 – 2325 .
- Yamaguchi , F. , Ariga , T. , Yoshimura , Y. and Nakazawa , H. 2000 . Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind . Journal of Agricultural and Food Chemistry , 48 ( 2 ) : 180 – 185 .
- Jeyarani , T. and Yella Reddy , S. 1999 . Heat-resistant cocoa butter extenders from mahua (Madhuca latifolia) and Kokum (Garcinia indica) fats . Journal of the American Oil Chemists’ Society , 76 ( 12 ) : 1431 – 1436 .