Abstract
Polyphenol oxidase from Rosmarinus officinalis L. (PPO, EC 1:14:18.1) was extracted and partially purified by using (NH4)2SO4 precipitation and dialysis. Stable and highly active PPO extracts were obtained using 0.5% (w/v) PEG and 0.5% (w/v) Triton X-100 in 0.1 M phosphate buffer 7.0. KM values were found to be as 14.3 mM for catechol. Four isoenzymes of Rosemary PPO were detected by PAGE with DL-dopa substrate. The enzyme was strongly inhibited by dithiotreitol, sodium metasulfite, sodium thiosulfate, ascorbic acid, and L-cysteine. Metal ions Ca++, Mg++, and Mn++ were poor inhibitors of rosemary PPO at 10 Mm.
INTRODUCTION
Polyphenol oxidase (PPO) is widely distributed copper proteins from bacteria to mammals in the whole phylogenetic process. In the presence of molecular oxygen, PPO catalyzes the o-hydroxylation of monophenols to o-diphenols (cresolase activity) and further oxidation of o-diphenols to o-quinones (catecholase activity).Citation[1,Citation2] Quinones are electrophilic highly reactive molecules that can polymerize, and leading to the formation of brown or black pigments.Citation[3] These reactions have important implications in food processing and preservation: upon browning, significant post harvest losses of several crops to occur.Citation[3,Citation4,Citation5,Citation6]
Two main problems are found in the optimization of extraction conditions of plant PPO: the difficulty in obtaining full solubilization of membrane-bound PPO, and avoiding phenolic oxidation during and after extraction. Several methods have been described to prevent the reaction of phenols with PPO, including the use of phenol scavengers such as PVP, PEG, Amberlite XAD-2. In higher plants, the enzyme is localized to the tylakoid membranes of chloroplasts and other plastid organelles. Therefore, in most cases, full extraction of the enzyme requires the use of detergent such as Triton X-100.Citation[3,Citation6]
Several thiol containing compounds, sulfites, aromatic carboxylic acids, metal ion chelators have been shown to be effective in the control of enzymatic browning in plant products. Golan-Goldrich and WhitakerCitation[1] in same plant tissue (tuber, seed, fruit, and mushroom) showed the thiol reagent as the most effective inhibitors for those enzymes. Rosmarinus officinalis was originally from the Mediterranean region and it's used in traditional Turkish folk medicine for the treatment of hyperglycemia, is widely accepted as one of the medicinal herb with the highest antioxidant activity.Citation[7,Citation8] Besides the therapeutical application, the essential oil is widely applied in the cosmetic industry producing various cologne waters, bathing essences, hair lotions and shampoos and as a component of disinfectants and insecticides.Citation[9]
Polyphenol oxidase has been investigated extensively in a variety of tissues in many plants such as lettuce, field bean seed, orchid leaves and root, apple, sweet potato, Acanthophoenix rubra, Victoria grape, pear, Laurus nobilis, artichoke, and peppermint.Citation[10-Citation20] Little research has been reported about the PPO of Rosmarinus Officinalis. Therefore, characterization of the enzyme could help to develop more effective methods in controlling browning of plant products. The aims of this work were: (i) to optimize the extraction conditions of PPO (best PEG and Triton X-100 concentration), and (ii) to determine the characteristic properties of Rosemary PPO (optimum pH, KM, Vmax, number of isozymes, thermal stability, and effect of inhibitors).
MATERIALS AND METHODS
Chemicals
Catechol, 4-methylcatechol, gallic acid, L-dopa, DL-dopa, pyrogallol, thiourea, β-mercaptoethanol were obtained from Sigma Chemical co (St. Louis, MO). Triton X-100, L-ascorbic acid, (NH4)2SO4, Tris, HCl, L-cysteine, sodium azide, potassium cyanide were purchased from Merck, Germany. All other chemicals were of analytical grade.
Extraction and Partial Purification of PPO
Rosmarinus officinalis leaves tissue were homogenized in 0.1 M potassium phosphate buffer, pH 7.0 containing 0.5% Triton X-100 (w/v) and 0.5% PEG (w/v) in an ultraturax homogenizer. The suspension was filtered through four layers of cheesecloth and centrifuged at 14.0 g for 40 min. The supernatant was brought to 40% saturation with (NH4)2SO4. The precipitate was separated by centrifugation as described above and dissolved in 0.05 M potassium phosphate buffer at pH 7.0.
PPO Activity Assay
PPO activity was assayed according to the spectrophotometric procedure of Cosetang and Lee (1978).Citation[21] The assay mixture consisted of 2.95 ml of 20 mM catechol in 0.05 M potassium phosphate buffer pH 7.0, and 0.05 ml of enzyme. The increase in absorbance at 420 nm was measured as a function at time for 1 min. One unit of enzyme activity is defined as the amount of the enzyme that causes an increase in absorbance of 0.001 per min. at 25°C. PPO activity was assayed in triplicate measurements.
Effect of pH
PPO activity as a function of pH was determined using catechol, 4-methylcatechol, pyrogallol, DL-dopa, gallic acid and tyrosine as substrate. The buffers used were sodium acetate (pH 3.0–6.0), potassium phosphate (pH 6.0–8.0) and Tris HCl (pH 8.0–10.0) at 25°C. The pH stability was determined by incubating the enzyme in the above buffer at pH values ranging from pH 3.0 to 9.0 for 1 h at 25°C. At the end of the incubation period, samples were taken and assayed under standard conditions using catechol as substrate.
Effect of Temperature
For heat stability, the enzyme solution was heated at various temperatures between 20°C and 80°C for 60 min. The residual PPO activity was determined under the standard conditions.
Kinetic Study
The KM and Vmax values of Rosmarinus officinalis PPO towards 4-methylcatechol, catechol, and pyrogallol were determined from Lineweaver-Burk plots.Citation[22] The rate of the reaction was measured in terms of the increase in absorbance at the absorption maxima of the corresponding quinone products for each substrate at optimum conditions. (pH, temperature, and ionic strength).Citation[6]
Polyacrylamide Gel Electrophoresis
Polyacrylamide gels (7.5%) were prepared according to Laemmli (1970) without SDS (native condition).Citation[23] Runs were performed at constant current intensity (25 mA per plate), with cooling to 4°C for 3 h. After running, gel was incubated in 2.5 mM DL-dopa solution in 0.01 M phosphate buffer (pH 7.0) for 1 h and isoenzyme bands were developed.
Investigation of the Enzyme Inhibitors
The following compounds were used for the inhibition studies: sodium metabisulfite, dithiotretiol, thiourea, sodium azid, β-mercaptoethanol, glutathione, ascorbic acid, citric acid, sodium thiosulfate, EDTA and some amino acids. Inhibition of PPO activity was tested spectrophotometrically in a reaction mixture (3 ml) consisting of different concentration catechol and inhibitor in 0.05 M potassium phosphate buffer, pH 7.0. Lineweaver-Burk plots were applied to evaluate the type of inhibition and apparent Ki values for inhibitors. Five substrate concentrations were used for all kinetic studies. Percent activity graphs were drawn from these results to find I50 values, which show about 50% inhibition effect at four inhibitor concentrations using catechol as substrate.
Effect of Different Ions on Enzyme Activity
PPO activity was measured in the presence (final concentration 1.0 or 10 mm) and absence of various ionic compounds under the standard conditions.
RESULTS AND DISCUSSION
The optimal pH conditions for PPO extractions were investigated using different buffer in the pH range 4.0–9.0. PPO could be extracted by buffer of low molarities. The maximum yield was achieved at a concentration of 0.1 M phosphate buffer. The higher specific activity yields as a function of pH were obtained in pH 7.0, 0.1 M phosphate buffer. For the optimum extraction conditions, several buffer composition containing TritonX-100 and polyethylene glycol (PEG) were studied to extract PPO from Rosmarinus officinalis (). Addition of TritonX-100 and PEG produced an increase of PPO activity of extracts made from low and high concentration buffers. The increase of ionic strength by addition of sodium chloride only produced a slight decrease in PPO activity when extracts were made from low and high concentration buffers. Therefore, 0.1 M phosphate buffer, containing 0.5% (w/v) Triton X-100 and 0.5% (w/v) PEG was employed for all enzyme assays.
Table 1 Influence of extraction buffer composition on Rosemary PPO activity
Tanning reactions during enzyme extraction can cause partial inactivation of the enzymes. To avoid these reactions reducing agents are added during extraction, but must be removed before assay. Alternatively, phenolic substrates must be removed prior to the assay with phenol scavengers (like PVP and PEG).Citation[3] In this study, the cleavage of all phenolics could not be achieved by the use of PEG and consequently the extracted PPO activity was low (data not shown). When PEG used with a detergent (TritonX-100) the extracted PPO activity was improved by combining polar and non-polar binding capacity. An increase of enzyme activity by this detergent treatment has been reported.Citation[6]
Effect of pH and Temperature on Rosmarinus Officinalis PPO Activity
Optimum pH for Rosmarinus officinalis PPO activity was determined between pH 3.0 and 10.0. The optimum pH of the enzyme was found to be 7.0 for catechol (a), 4-methylcatechol, L-dopa and 6.0 for pyrogallol, and 5.0 for gallic acid. These results were in close agreement with the values reported for PPO from other sources. The optimum pH depends on genetic properties (variety), nature of phenolic substrates, and extraction methods.Citation[14] In general, most plants show maximum PPO activity at near neutral pH values.Citation[17] Optimum pH values for the PPO enzyme from different sources have been reported: 6.5 for potato, 7.0 for banana, 7.2 for guava with catechol as substrate, 6.5 for artichoke heads, 4.5 for green olive, 5.0 for potato, 7.2 for guava with pyrogallol as substrate, 6.0 for artichoke heads, 4.5 for sago log, and 4.0 sweet potato 4-methylcatechol as substrateCitation[14] MCitation.[17 ,Citation19,Citation24,25,Citation26] Rosemary PPO was stable in a broad pH range from 6 to 8 (), with more than 80% of the original activities retained at the extreme pH of 6 and 8 which is similar to that reported in the sunflower seeds with catechol as substrate.Citation[27]
Figure 1 (a) Effect of pH on Rosemary PPO activity; (b) pH stability of Rosemary PPO after incubating for 60 min. at pH's between 3.0 and 9.0; (c) Effect of temperature on Rosemary PPO; and (d) Heat-inactivation of Rosemary PPO at different temperatures, using catechol as substrate.
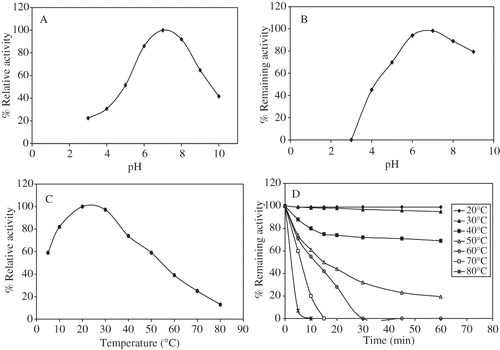
The optimum temperature for maximum PPO activity was at 25°C with catechol as substrate (). It is reported that optimum temperatures were 25°C for peppermint, 20°C for kiwi fruit, 25°C for plum, 30°C for Concord and Koshu grape, 37°C for peach PPO using catechol as substrate.[Citation20–32] Rosemary PPO enzyme is completely inactivated when heating at 80, 70 and 60°C for 7, 14, and 30 min, respectively (). Park and Luh (1985) showed that kiwi fruit PPO was completely inactivated at 65°C when heated for 5 min.Citation[28] PPO from peppermint was also reported to be completely inactivated in less then 3 min when heated at 70°C.[Citation20] At 50°C, even after heating for 60 min. completely inactivation of rosemary PPO was not achieved as it still retained about 20% of its maximum activity. Under these conditions, it appears that the heat inactivation of the enzyme is temperature-dependent.Citation[33]
Polyacrylamide Gel Electrophoresis
Electrophoresis under non-denaturing conditions of rosemary PPO showed four bands on when stained for activity with DL-dopa (). Most of the studies have reported 2 to 4 isoenzymes for PPO from different fruits.Citation[32,Citation34] However, Galeazzi et al. (1981) reported 10 isoenzymes for crude banana PPO.Citation[35]
Kinetic Study
The kinetic study was conducted at optimum pH and temperature conditions for each substrate. A number of monohydroxy, dihydroxy, and trihydroxy phenols were used to test substrate specificity (). The PPO extracted from different sources has been shown to have varying substrate specificity as reported by Jen and Kahler,Citation[32] and Wong et al.Citation[34] The KM and Vmax values of the enzyme were determined according to the method of Lineweaver-Burk and found to be 14.3 mM and 14261 U/ml.min for catechol, 17.0 mM and 11720 U/ml.min for 4-methylcatechol, 20.0 mM and 8700 U/ml.min for pyrogallol as substrates. This is similar to the value of 10.5 mM from field bean seed, 10.2 mM artichoke heads using catechol as substrate.Citation[11,Citation19] In this study, the rosemary PPO KM value obtained for catechol is lower than those of sweet potato (96 mM) and concord grape (67 mM) PPO.Citation[14,Citation30]
Table 2 Substrate specificity of Rosemary PPO
Inhibitors
PPO activity may be inhibited by heat or removal of one of its necessary components: O2, enzyme, Cu+2 or substrate.Citation[2,Citation36,Citation37,Citation38] The effect of various inhibitors on Rosemarinus officinalis PPO obtained after (NH4)2SO4 fractionation and dialysis with catechol as substrate was determined (). Several compound reported as PPO inhibitors were also shown to have inhibitors effect on the rosemary PPO.Citation[27] The results from inhibitor studies in other plant tissues showed thiol reagents as the most effective inhibitors for those enzymes.Citation[27,Citation38] Reducing agents, antioxidants, and enzymatic inhibitors prevent browning chemically reducing the o-quinones. The effect of these reducing agents can be considered temporary because these compounds are oxidized irreversibly by reaction with pigment intermediates, endogenous enzymes and metals such as copper. Among sulfur containing agents, L-cycteine is an effective compound to prevent enzymic browning. Direct inhibition of polyphenol oxidase by cystein through the formation of stable complexes with copper has also been proposed.Citation[39] Halim and Montgomery (1978) showed in a series of publications that Cys can inhibit enzymic browning of pear juice concentrate more effectively than sulfite.Citation[2] Kahn (1985) used Cys to inhibit browning of cut or pureed avocados and bananas.Citation[17]
Table 3 Ki values and inhibition types of polyphenol oxidase with different inhibitors
Among the tested anti-browning reagent, the most effective ones were dithiotreithol and sodium metabisulfite. Historically, enzymatic browning was controlled by the application of sulfites but its use has been banned in raw fruits and vegetables since it can lead to health problems in some individuals.Citation[38] The action of sulfite in the prevention of enzymatic browning can usually be explained by several processes. One is the action on o-quinones. The formation of quinone-sulfite complexes prevents the quinone polymerization.Citation[40] A further action of metabisulfite on PPO is directly on the enzyme structure leading to the inactivation of PPO. Golan-Goldhirsch and Whitaker and Embs and Markasis found that during pre-incubation of PPO with sulfite (dithiothreitol, glutathione), there was a gradual loss in the ability of the enzyme to cause browning.Citation[1,Citation40] It has been suggested that sulfite reacts with disulphide bonds with PPO. This leads to the change in tertiary structure of enzyme and inactivation. The third process leading to PPO inhibition by bisulphate is via reduction of the intermediate quinones as described for ascorbic acid.Citation[40] The enzyme also seemed to be sensitive to thiourea since PPO contains copper as a co-factor, the irreversible inactivation of this enzyme can be effected by substances (such as thiol compounds thiourea, -hydroxyquinoline, etc.), which remove copper from the active site of the enzyme.Citation[41]
Acidulants, such as citric acid can inhibit PPO activity by reducing pH and/or chelating Cu in a food product.Citation[37,Citation38] Ascorbic acid can also be considered as an effective compound at higher concentrations. The mechanism of ascorbic acid inhibition involves the reduction of quinones generated by PPO.Citation[42-Citation43] It is believed that EDTA can form a complex with Cu+2 in PPO. This formation leads to a decrease in enzyme activity. The inhibitory effect of EDTA would depend on the binding constant for the complex formed between metal ion and EDTA compared to the binding constant for the complex metal ion with enzyme.Citation[41] It was reported by several workers that EDTA is not good PPO inhibitor. Luh and Phitakpol found that the effect of EDTA on cling peaches PPO activity was quite low.Citation[44] Several amino acids were investigated as inhibitor of PPO with catechol as substrate. The I50 values of these amino acids were found to be 6.3 × 10−3 M, 12.8 × 10−3M, 12 × 10−3 M, 24 × 10−3, and 25 × 10−3 M for arginin, glycine, phenylalanin, glutamic acid, and aspartic acid respectively ().
Table 4 Effect of amino acids on the activity of PPO from Rosemary
PPO was weakly inhibited some metal ions such as Ca++ and Mn++ (). NaCl, a browning inhibitor and observed to be the weak lest PPO inhibitor in several plant tissues was also inhibited rosemary PPO at 10 mM concentration.[Citation20,Citation27] It is believed the action of NaCl is due to its interaction with the cooper at the active center of the enzyme.Citation[45 Citation,46] Some works have been reported on the effect of NaCl on PPO activity. It was shown that the inhibition of PPO from d'Anjou pears by 10 Mm NaCl was 12% similar bindings were also reported for cherry PPO.[Citation2,Citation33]
Table 5 Effect of various compounds on the activity of PPO from Rosemary
CONCLUSION
A partial characterization of PPO activities in Rosemary was described. The enzyme was effectively extracted from Rosemary using combination Triton X-100 and PEG. The optimum pH varies from about 6.0 to 7.0, depending on the substrate. The enzyme seemed to have the highest affinity (lowest KM) for catechol, 4-methylcatechol, and pyrogallol. L-tyrosine was also tested but was not oxidized by PPO. Heat inactivation studies showed temperatures >40°C in loss of enzyme activity. Native PAGE analysis of the enzyme revealed four activity bands when stained DL-dopa. Among various PPO inhibitors tested, dithiotreithol was the most effective noncompetitive inhibitor of the enzyme with a Ki of 1.86 × 10−6 M. The possible PPO inhibitory effect of three nontoxic compounds, ascorbic acid, citric acid and NaCl were studied. These results showed that L-ascorbic acid are good inhibitor of Rosemary PPO. The enzyme was markedly inhibited by NaF and Hg(CH3COOH)2. Various amino acids such as L-csyteine, L-glycine, L-arginine, L-phenyl alanin, L-glutamic acid, and L-aspartic acid have been investigated and the results showed that L-cysteine (I50 value 5.80 × 10−5 M) was the most effective inhibitors. Due to its nontoxic properties, Cys may be promising alternatives to sulfite in preventing browning of plant foods.
REFERENCES
- Golan-Goldhirsh , A. and Whitaker , J.R. 1984 . Effect of ascorbic acid, sodium bisulfite and thiol compounds on mushroom polyphenol oxidase . Journal of Agricultural and Food Chemistry , 32 : 1003 – 1008 .
- Halim , D.H. and Montgomery , M.W. 1978 . Polyphenol oxidase of d'Anjou pears (Pyrus communis L.) . Journal of the Science , 43 : 603 – 610 .
- Mayer , A.M. and Harel , E. 1979 . Polyphenol oxidase in plants . Phytochemistry , 18 : 193 – 215 .
- Vamos-Vigyazo , L. 1981 . Polyphenol oxidase and peroxidase in fruits and vegetables. CRC. Critical . Rev. in Food Sci. Nutr. , 15 : 49 – 127 .
- Mathew , A. and Parpia , H.A.B. 1971 . Food browning as a polyphenol reaction . Advance. Food Research , 19 : 75 – 145 .
- Zhou , H. and Feng , X. 1991 . Polyphenol oxidase from yali pears (Pyrus bretschneideri) . Journal of the Science of Food and Agriculture , 57 : 307 – 313 .
- Ensminger , A.H. , Ensminger , M.E. , Kondale , J.E. and Robson , J.R.K. 1983 . Foods & Nutrition Encyclopedia , Clovis, CA : Pegasus Press .
- Ibanez , E. , Kubatova , A. , Senorans , F.J. , Cavero , S. , Reglero , G. and Hawthorne , S.B. 2003 . Subcritical water extraction of antioxidant compounds from rosemary plants . Journal of Agricultural and Food Chemistry , 51 : 375 – 382 .
- Boelens , M.H. 1985 . Essential oils and aroma chemicals from Eucalyptus globulus Labill . Perfumer and Flavorist , 9 : 1 – 14 .
- Castner , M. , Gil , M.I. , Artes , F. and Barberan , F.A.T. 1996 . Inhibition of browning of harvested head lettuce . Journal of the Science , 61 : 314 – 316 .
- Paul , B. and Gowda , L.R. 2000 . Purification and characterization of a polyphenol oxidase from the seeds of field bean (Dolichos lablab) . Journal of Agricultural Food Chemistry , 48 : 3839 – 3846 .
- Ho , K. 1999 . Characterization of polyphenol oxidase from aerial roots of an orchid, Aranda ‘Christine 130’ Plant Physiol . Biochem. , 37 : 841 – 848 .
- Murata , M. , Kurokami , C. and Homma , S. 1992 . Purification and properties of chlorogenic acid oxidase from apple (Malus pumula) . Bioscience, Biotechnology and Biochemistry , 56 : 1705 – 1710 .
- Lourenco , E.J. , Neves , A.V. and Da Silva , M.A. 1992 . Polyphenol oxidase from sweet potato and properties . Journal of Agricultural Food Science , 40 : 2369 – 2373 .
- Robert , C.M. , Rouch , C. , Richard-Forget , F. , Pabion , M. and Cadet , F. 1996 . Partial characterisation of Acanthophoenix rubra polyphenol oxidase . Plant Physiology and Biochemistry , 34 : 369 – 375 .
- Rapeanu , G. , Loey , A.V. , Smout , C. and Hendrickx , M. 2006 . Biochemical characterization and process stability of polyphenoloxidase extracted from Victoria grape (Vitis vinifera ssp. Sativa) . Food Chemistry , 94 : 253 – 261 .
- Kahn , V. 1985 . Effect of proteins, protein hydrolyzates and amino acids on o-dihydroxy phenolase activity of polyphenol oxidase of mushroom, avocado and banana . Journal of the Science , 50 : 111 – 115 .
- Aydemir , T and Cinar , S. 2006 . Partial purification and some properties of polyphenol oxidase from Laurus nobilis L . Agrochimica , 50 : 238 – 254 .
- Aydemir , T. 2004 . Partial purification and characterization of polyphenol oxidase from artichoke (Cynara scolymus L.) heads . Food Chemistry , 87 : 59 – 67 .
- Kavrayan , D. and Aydemir , T. 2001 . Partial purification and characterization of polyphenol oxidase from peppermint (Mentha piperita) . Food Chemistry , 74 : 147 – 154 .
- Cosetang , M.Y. and Lee , C.Y. 1978 . Changes in apple polyphenol oxidase and polyphenol concentrations in relation to degree of browning . Journal of the Science , 52 : 985 – 988 .
- Lineweaver , H. and Burk , D. 1934 . The determination of enzyme dissociation constants . Journal of American Chemistry Society , 56 : 658 – 661 .
- Laemmli , U. 1970 . Cleavage of structural proteins during assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Augustin , M.A. , Ghazali , H.M. and Hashim , H. 1985 . Polyphenol oxidase from guava (Psidium guajara L.) . Journal of the Science of Food and Agriculture , 36 : 1259 – 1265 .
- Ben-Shamol , N. , Kahn , V. , Harel , E. and Mayer , A.W. 1977 . Catechol oxidase from green olives: properties and partial purification . Phytochemistry , 16 : 1153 – 1158 .
- Onsa , G.H. , Saari , N. , Selamat , J. and Bakar , J. 2004 . Purification and characterization of membrane bound peroxidases from Metroxylon sagu . Food Chemistry , 85 : 365 – 376 .
- Raymond , J. , Rakariyatham , N. and Azanza , J.L. 1993 . Purification and some properties of polyphenol oxidase from sunflower seeds . Phytochemistry , 34 : 927 – 931 .
- Park , E.Y. and Luh , B.S. 1985 . Polyphenol oxidase of kiwi fruit . Journal of Food Science , 50 : 679 – 684 .
- Siddig , M. , Sinha , N.K. and Cash , Y.N. 1992 . Characterization of a polyphenol oxidase from stanley plums . Journal of Food Science , 57 : 1177 – 1179 .
- Cash , J.N. , Sistrunk , W.A. and Stutge , C.A. 1976 . Characteristic of Concord grape polyphenol oxidase involved in juice color loss . Journal of the Science , 41 : 1398 – 1402 .
- Nakamura , K. , Amano , Y. and Kagami , M. 1983 . Purification and some properties of a polyphenol oxidase from Koshu Grapes. Am . J. Enol. Vitic. , 34 : 122 – 127 .
- Jen , J.J. and Kahler , K.R. 1974 . Characterization of polyphenol oxidase in peaches grown in southeast, Horticultural Science . 9 : 590 – 598 .
- Benjamin , N.D. and Montgomery , M.W. 1973 . Polyphenol oxidase of Royal Ann cherries: purification and characterization . Journal of Science , 38 : 799 – 804 .
- Wong , T.C. , Luh , B.S. and Whitaker , J.R. 1971 . Isolation and characterization of polyphenol oxidase isozymes of cling stone peach . Plant Physiology , 48 : 19 – 25 .
- Galeazzi , M.A.M. , Scarbieri , V.C. and Constantinides , S.M. 1981 . Isolation purification and physicochemical characterization of polyphenol oxidase (PPO) from a dwarf variety of banana (Musa cavendishii, L.) . Journal of the Science , 46 : 150 – 155 .
- Lambrecht , H.S. 1995 . “ Sulfite substitutes for the prevention of enzymatic browning in foods ” . In Enzymatic browning and its Prevention , Edited by: Lee , C.Y. and Whitaker , J.R . 313 – 323 . Washington, D.C. : ACs Symposium series 600. American Chemical Society Prevention .
- Richard-Forget , F.C. , Groupy , P.M. and Nicholas , J.J. 1992 . Cysteine as an inhibitor of enzymatic browning. 2. Kinetic studies . Journal of Agricultural and Food Chemistry , 40 : 2108 – 2113 .
- Sayaverde-Soto , L.A. and Montgomery , M.W. 1986 . Inhibition of polyphenol oxidase by sulfite . Journal of Food Science , 51 : 1531 – 1535 .
- Nicholas , J.J. , Richard-Forget , F.C. , Goupy , P.M. , Amiot , M.J. and Aubert , S.Y. 1994 . Enzymatic browning reactions in apple and apple products . CRC Critical Reviews in Food Science and Nutrition , 34 : 109 – 157 .
- Embs , R.J. and Markakis , P. 1965 . The mechanism of sulphite inhibition of browning caused by polyphenol oxidase . Journal of the Science , 30 : 753 – 758 .
- Schwimmer , S. 1981 . Source Book of Food Enzymology , Edited by: Schwimmer , S. 267 – 274 . Westport : AVI Publishing .
- Whitaker , J.R. and Lee , C.Y. 1995 . “ Recent advances in chemistry of enzymatic browning: An overview. In Enzymatic Browning and its Prevention ” . In ACS Symposium Series 600 , Edited by: Chang , Y.L. and Whitaker , J.R. 2 – 7 . Washington, DC : American Chemical Society .
- Whitaker , J.R. 1994 . “ Enzyme inhibitors ” . In Principles of Enzymology for the Food Sciences , 2nd , 241 – 270 . New York : J.R. Whitaker, Marcel Dekker, Inc .
- Luh , B.S. and Phithakpol , B. 1972 . Characteristic of polyphenol oxidase related to browning in cling peaches . Journal of the Science , 27 : 264 – 268 .
- Sappers , G.M. , Hicks , K.B. , Phillips , J.G. , Garzarella , L. , Pondih , D.L. , Matulaitis , R.M. , McCormack , T.J. , Sondy , S.M. , Seib , P.R. and El-Ataway , Y.S. 1989 . Control of enzymatic browning in apple with ascorbic acid derivatives, polyphenol oxidase inhibitors and complexing agents . Journal of Food Science , 54 : 997 – 1002 .
- Martinez , M. and Whitaker , J.R. 1995 . The biochemistry and control of enzymatic browning . Trends Food Science Technology , 6 : 195 – 200 .
