Abstract
The aim of this study was to examine omega-3 LC PUFA content and oxidative stability of fish oil dietary supplements available in Poland. Nineteen brands of fish body oil and fish liver oil capsules were purchased over the counter and analyzed. Oil content, fatty acid composition and peroxide value were determined. The label claims for EPA and DHA for the majority of the products were presented with reasonable accuracy. However, it can be supposed that the oxidative stability of some fish oil products available on the market might not be sufficient to ensure health quality and safety during longer storage.
INTRODUCTION
Fish and other sea animals are the richest source of omega-3 long chain polyunsaturated fatty acids (LC PUFA) in human diet. Positive health effects of omega-3 LC PUFA, especially eicosapentaenoic acid C20:5 n-3 (EPA) and docosahexaenoic acid C22:6 n-3 (DHA), are well demonstrated. Omega-3 LC PUFA decrease the risk of cardiovascular diseases, some types of cancer and autoimmune disorders.Citation[1–Citation3] They are also important for proper development and function of the brain and retina.Citation[1 Citation–3] Omega-3 LC PUFA are structural components of neuronal and other cell membranes and desirably modulate the production of regulatory eicosanoids and inflammatory cytokines.Citation[2,Citation4] They are also used in prevention and treatment of many diseases like rheumatoid arthritis, cardiovascular diseases and some types of cancer. Desirable effects of omega-3 LC PUFA on human health were discovered in the seventies when Dyerberg and co-workers studied the health status of Greenland Eskimos in comparison to continental Danes. Very low incidence of cardiovascular diseases and cancer was found among Eskimos despite a diet extremely rich in saturated fats and cholesterol.Citation[5] This effect was associated with high fish and seal consumption, which contain high amounts of health protecting omega-3 LC PUFA in the lipid fraction.Citation[4 Citation,6]
Depending on fish species, age, season, and area of living, fish oils may contain 100 to 300 g kg−1 of EPA and DHA.Citation[7 Citation,8] However, there is a large gap between the actual consumption of fish and the recommended intake of omega-3 LC PUFA. Because of the low acceptance of oily fish in many societies—where the so-called Western-style diet is predominant—the average fish intake is currently far below the recommended two to three fish servings per person, per week.Citation[9] Currently, an average level of omega-3 LC PUFA intake in most developed Western countries is approximately 0.15 g per person per day, which is below recommended minimum. Also the ratio between omega-6 and omega-3 PUFA in the average diet is 15:1 instead of the recommended 4:1.Citation[9 Citation–12] The International Society for the Study of Fatty Acids and Lipids (ISSFAL) recommends an minimum intake of omega-3 LC PUFA to be 0.5 g of DHA plus EPA per person per day.Citation[13 Citation,14] The upper limit of omega-3 LC PUFA intake was established for the USA in the year 2000. The US Food and Drug Administration stated that daily intake of EPA and DHA should not exceed 3.0 g per person per day in the form of fish oil from food and dietary supplements.Citation[15] An adequate intake of omega 3 LC PUFA is particularly important for women of childbearing age. Higher maternal intake is required during pregnancy and lactation to support the development of the fetal and infant brain and may reduce the risk of allergic disease in the offspring. Women of childbearing age are recommended to eat two portions of oily fish per week (average 0.4–0.8 g of omega-3 LC PUFA per day).Citation[9 Citation,12 Citation,16] Besides fish consumption, alternative ways to ensure an optimal omega-3 LC PUFA intake is supplementation of the diet with fish oil capsules.Citation[2 Citation,17 Citation,18]
Due to low consumption of fish in many Western societies, supplementation of the diet with fish oil capsules seems to be the easiest way to elevate the level of omega-3 LC PUFA intake.Citation[15 Citation,16] This may result in better health protection. Such supplements usually contain well-refined, unhydrogenated fish oil from fish liver or whole fish body. Omega-3 LC PUFA is naturally present in to a greater extent in fish body oils than in fish liver oils. Fish liver oil is also a rich source of vitamins A and D. Some omega-3 LC PUFA supplements may contain algal oil (or other single cell oils), krill or seal oil, which are also a rich source of these fatty acids. However, the predominant source for omega-3 LC PUFA dietary supplements manufacturing is unhydrogenated fish oil, which may be used in natural or concentrated form, usually stabilized with antioxidants. Some pharmaceutical companies also produce free omega-3 LC PUFA concentrates, which are isolated from the triacylglycerol structure and subsequently esterified. Omega-3 LC PUFA rich oils are often encapsulated, which stabilizes oils and enables easy administration and dosage. Such products are sold only in drug stores in Poland.
Encapsulated fish oil products are extensively commercialized in developed countries. Intake of fish oil capsules was shown to increase cardiovascular health, as well as body immunological defense.Citation[8 Citation,22 Citation–24] However, some consumer organizations indicate that the composition and quality of fish oil supplements available on the market might not reach quality requirements.Citation[25 Citation,26] The most important quality features of fish oil supplements and other fish oil products are concentration of omega-3 LC PUFA and stability against oxidation. Due to 5 and 6 unsaturated bounds in the carbon chain omega-3 LC PUFA are especially susceptible to oxidation resulting in the formation of peroxides and their byproducts, which can be harmful for humans.Citation[18,Citation19] Hence the objective of this study was to examine omega-3 LC PUFA content and oxidative stability of encapsulated fish oil dietary supplements available on the Polish pharmaceutical market.
MATERIALS AND METHODS
Samples
Nineteen brands of fish oil capsules, produced by different companies, were purchased over the counter in Warsaw drugstores and analyzed. The products were selected according to the market survey carried out in the years 2004–2006 and were characterized by significant rate of sale on the market.Citation[27] Eleven products containing fish body oils were marked as FBO (fish body oil) and numbered from 1 to 11. Eight fish liver oil products were marked as FLO (fish liver oil) and numbered from 1 to 8. All products were closed in thick gelatin capsule. All were in the middle of their shelf life. Identification of examined fish oil products by trade name, manufacturer, fish oil type, label claim for EPA, DHA content, and figures from our own analyses in mg per one capsule are presented in and .
Table 1 Claimed and experimental contents of fish oil, EPA and DHA in retail fish body oil products evaluated in the study, with product type
Table 2 Claimed and experimental contents of fish oil, EPA and DHA in retail fish liver oil products evaluated in the study, with product type
Oil Recovery
Fish oil content in the evaluated products was examined gravimetrically. Capsules of each brand were weighed, opened and oil was pressed out to a clean vial. The empty capsule cover was washed with hexane, wiped on a paper towel to recover any residual oil, and weighed again. Each measurement was done in triplicate.
Fatty acid analysis
Fatty acid composition was determined by gas chromatography (GC). To convert fish oil fatty acids to methyl esters (FAME) 25 μg of isolated oil was saponified by 0.5 N solution NaOH with methanol, covered with nitrogen, mixed and heated in a water-bath at boiling point for 40 min. The saponified sample was transmethylated with 14% BF3 in methanol reagent, covered with nitrogen, at boiling point for 3 min. After that, the mixture was cooled and 3 mL hexane added, covered with nitrogen and shaken vigorously for 30 seconds while still warm. Then 40 mL of saturated water solution of NaCl was added and shaken vigorously. After separation, the hexane layer was transferred by syringe to a thin glass tube and additionally dried over anhydrous Na2SO4 and decanted to clean a vial, covered with nitrogen, and capped. One μL of prepared FAME was injected into the chromatograph under appropriate conditions. The contents were determined with respect to methyl tricosanate (C23:0) internal standard (IS) (Sigma-Aldrich, Steinheim, Germany). FAME were prepared according to slightly modified AOCS method Ce 1b-89.Citation[28]
The analysis of FAME was performed using Agilent 6890N GC (Agilent, Böblingen, Germany) equipped with Rtx 2330 silica capillary column of 100 m length, 0.25 mm ID, df 0.1 μm (Restek Corp, Bellefonte, USA). Hydrogen was used as a carrier gas at flow rate 0.9 mL s−1. A split-splitless (50:1) injector at 235oC and flame-ionization detector (FID) at 250oC were used. Column temperature was programmed as follows: initial 155oC, time 55 min, next rate 1.5oC min−1, final temperature 210oC. Each sample was analyzed in triplicate. Results were collected in the Chem-station and transformed using software HP-Chem (Hewlett Packard, Palo Alto, USA). Peaks were identified by comparison with known standards: menhaden reference oil (Supelco, Bellefonte, USA) and Supelco 37 component FAME Mix (Supelco, Bellefonte, USA). Results were reported as peak area percentages and recalculated with respect to internal standard according to AOCS method Ce 1b-89. The EPA-IS-DHA factors of 0.99-1.00-0.97 were used for these omega-3 LC PUFA.Citation[28,Citation29]
Oxidative Stability
Peroxide value (PV) was measured as a primary oxidation indicator to determine the oxidative stability during storage. To accelerate the oxidation process capsules were stored at 43ºC in a Heareus B6 Function Line automatically controlled incubator (Kendro, Langenselbold, Germany). As controls fish oil capsules stored at 20ºC, limited light access were used. The measurements were done at the beginning, after 11 and after 22 days of storage. Each measurement was done in triplicate.
The applied iodometric method was based on ISO 3960 with chloroform and glacial acetic acid as solvents.Citation[30] Samples representing 1.0 g of fish oil, isolated from the capsules by pressing, were placed in the Erlenmeyer flask, and dissolved in 20 ml of chloroform. Then 30 ml of glacial acetic acid was added and the mixture was stirred for a few seconds to ensure complete mixing. After that, 0.5 ml of the potassium iodide (KI) solution was added. After 1 min, 30 ml of deionized water was added and the titration started. When the dark-yellow color changed to a pale-yellow, 0.5 ml of starch-solution was added. Titration was finished when the color disappeared. The mixture was stirred magnetically during the procedure. Results were calculated as micro equivalents of active oxygen per kg of oil (mEq O kg−1).
Data Analysis
The obtained results were statistically analyzed using an un-paired t-test to compare the claimed and measured omega-3 LC PUFA contents and one-way analysis of variance (ANOVA) to assess the oxidation over the three time periods.Citation[31] Results were analyzed using Statgraphics Plus version 4.1 software package (Statistical Graphic Corp., Herndon, VA, USA) at a significance level P < 0.05.
RESULTS AND DISCUSSION
Fish Oil Content
Fish oil content ranged from 350–1000 g in fish body oil products (FBOs) and from 250–570 g in fish liver oils (FLOs). The differences between the label claims and analyzed oil contents were not significant and ranged ± 1.8%, except FBO 4 where the experimental level was 2.8% higher than the label claim. ( and ). In all FBOs EPA and DHA contents were claimed on the labels. The determined EPA and DHA contents were similar or even higher by 2.5–24% than label claims, except FBO 10. In FBO 10 the measured EPA and DHA contents were respectively 5 and 7% lower than the claimed amounts. In five FLOs, the EPA and DHA contents were not claimed on the label. However, in three samples with EPA and DHA claims the determined contents were higher by 2.5 to 20%, depending on the sample.
Fatty Acid Analysis
In the analyzed products, over 40 different fatty acids were found. However, significant levels were shown for 15–22 fatty acids, depending on the product (, and ). The predominant fatty acid in most fish body oil products (except for FBOs 3, 6, and 7) was EPA C20:5 n-3 (19.1 to 24.5% of total fatty acids). Other major fatty acids were: palmitic acid C 16:0 (9.4–19.3%), DHA C22:6 n-3 (11.5–23.9%), oleic acid C 18:1 n-9 (7.4–10.1%), and palmitooleic acid C16:1 n-9 (3.9–9.9%). In FBO 6 the predominant fatty acid was oleic acid C18:1 n-9 (48.8%) and the EPA and DHA levels were much lower than in other fish body oil products (9 and 5.7%, respectively). However, FBO 6 was claimed to be a half/ half mixture of fish oil and olive oil. In contrast, FBO 3 and 7 contained much higher EPA and DHA level than other FBOs (40.7–40.9 and 23.3–23.4%, respectively) which suggested that these products contained fish oil concentrate. However, this was not claimed on the label.
Table 3 Fatty acids composition of fish body oil products evaluated in the study, % of total fatty acids
Figure 1 Chromatogram of FAME analysis, example of cod liver oil product FLO 6: 1 - C8:0, 2 - C14:0, 3 - C15:0, 4 - C16:0, 5 - C16:1 n-9, 6 - C18:0, 7 - C18:1t, 8 - C18:1 n-9, 9 - C18:1 n-7, 10 - C18:2 n-6, 11 - C18:3 n-3, 12 - C20:1 n-9, 13 - C18:4 n-3, 14 - C22:1 n-11 + n-13 + C20:3 n-3, 15 - C22:1 n-9, 16 - C20:5 n-3, 17 - C24:1c, 18 - C22:5 n-3, 19 - C22:6 n-3, IS – internal standard C23:0.
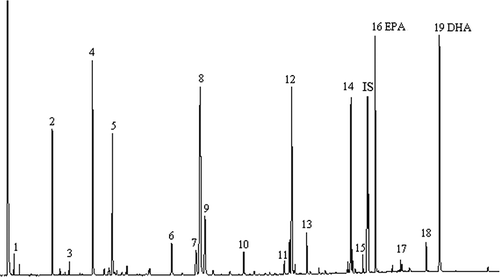
Table 4 Fatty acids composition of fish liver oil products evaluated in the study, % of total fatty acids
In the majority of fish liver oil products, except FLO 2, the predominant fatty acid was oleic acid C18:1 n-9 (14.6–31.7% of total fatty acids). Other main fatty acids in FLOs were: C16:0 (8.2–17.7%), C22:1 n-9 (6–14.3%) and C20:1 n-9 (7–11.7%). EPA was the predominant fatty acid in FLO 2 (35.9%) which suggests that this product contained liver oil with an elevated EPA level. In typical fatty acid profile of liver oil, the EPA level is low, like it was shown in FLO 5, 7, and 8. In other FLOs, the EPA and DHA levels ranged 2.4–10.3% and 2.3–15.1% of total fatty acids, respectively.
Generally, the EPA and DHA contents were significantly higher in FBOs than in FLOs, except for FBO 6 and FLO 2. However, the EPA and DHA levels in FBOs, as well as in FLOs, were significantly differentiated. FBO capsules contained from 67.9 to 374 mg of EPA and from 45.1 to 213 mg of DHA, except FBO 6, which contained much less amount of these fatty acids—45.3 and 28.7 mg, respectively. FLO capsules contained from 5.9 to 58.3 mg of EPA and from 5.7 to 85.3 mg of DHA, except FLO 2, which contained much higher level of EPA (198.9 mg). The results showed that the label claims for EPA and DHA for the majority of the examined products were presented with reasonable accuracy.
The amounts of total saturated fatty acids (SFA) in FBOs ranged from 3.3 to 32.1% of total fatty acids, monounsaturated (MUFA)—from 15.2 to 55.9% and polyunsaturated—from 5.7 to 23.7% of total fatty acids (). In FLOs SFA ranged from 10.8 to 23.9%, MUFA—from 43.9 to 71.2%, PUFA—from 5.0 to 38.9% of total fatty acids concentration (). In the majority of FBOs the total SFA and PUFA contents were significantly higher than in FLOs, though MUFA content was significantly lower. This general proportion of main fatty acids groups in FBOs was typical for fish body and in FLOs—for fish liver oils. Exceptions from these results were FBO 6 (mixture of fish oil and olive oil) as well as FBO 3, 7, and FLO 2 containing concentrates.
Oxidative Stability
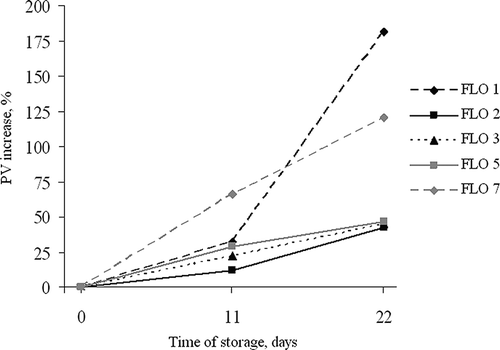
The PV of FBOs and FLOs at the beginning of the storage test at 43ºC were significantly differed ranging from 1.0–5.5 or even 9.8 (FBO 5) mEq O kg−1, depending on the sample. During the accelerated storage test at elevated temperature PV significantly increased in all products reaching at the end of the test from 2.2–12.5 mEq O kg−1, depending on the sample ( and However, PV of control samples stored at 20ºC were stable during all the storage time and differed from the initial values only by 10–20%. Nevertheless, in all products, except for FBO 5, the PV did not reach the upper tolerable limit for fish oil, which is estimated as 10 mEq O kg−1.Citation[32 Citation,33] Formation of the primary oxidation products was the highest in FBO 8 and FBO 10, among all evaluated products. In these samples the PV increased by 230 and 241%, respectively compared to the initial values (). In FBO 1 and 9 PV increased by 53 and 75%, respectively. The rest of FBO samples showed smaller increases—from 27 to 39%. In FLO 1 and FLO 7 the PV increased by 181 and 122%, respectively (). In FLOs 2, 3 and 5 the PV increase was by 45–47%. In the rest of the FLOs (4, 6, and 8) the increase was much lower—from 36 to 50% of the initial value. However, the PV increase in control samples stored at 20ºC was not significant reaching up to 20% of the initial value.
Table 5 Peroxide values of fish body oil products evaluated during storage, mEq O kg−1
Table 6 Peroxide values of fish liver oil products evaluated during storage, mEq O kg−1
Figure 2 Percentage of peroxide value increase in fish body oil products of the lowest oxidative stability during storage test.
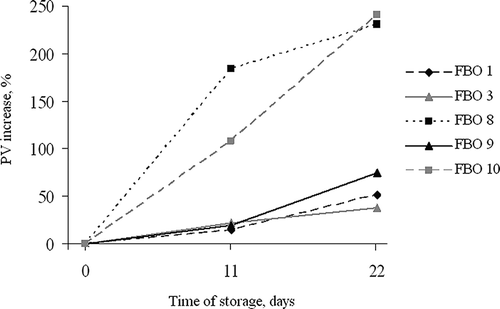
Figure 3 Percentage of peroxide value increase in fish liver oil products of the lowest oxidative stability during storage test.
The significant PV increase in some evaluated samples during the accelerated storage test suggest that despite thick gelatin capsule cover, the oxidative stability of some fish oil products might be limited. This could be a result of the type, level and combination of antioxidants substances added, as well as the initial purity and stability of fish oil and conditions during manufacturing or concentration. Presence of conditions promoting oxidation of PUFA, like oxygen permeability, elevated temperature, light access, iron and copper ions presence during processing may impair the stability of encapsulated fish oil products.Citation[19 Citation,24 Citation,36] Fish oil tends to be unstable during processing due to the high susceptibility to oxidation of the omega-3 LC PUFA. During oxidation of fish oil the fishy off flavor significantly increases, which is the natural and strong indicator of fish oil rancidity.Citation[8 Citation, 35,36] However, it cannot be detected in fish oil capsules covered by thick gelatin coat prior to being swallowed. Our results showed that the oxidative stability of some of the evaluated fish oil products was significantly impaired by the accelerated storage test. However, only one of the evaluated products reached the upper PV tolerable limit.
In recent years, encapsulated fish oil supplements have been strongly commercialized in many developed countries. However, some consumer organizations have indicated that the content of omega-3 LC PUFA, as well as oxidative stability of fish oil products in some cases did not meet quality guidelines. Nevertheless, as a result of the improvement in technology, the purity and stability of fish oil products is increasing. Unfortunately, published data about the composition and quality of fish oil products is still lacking. In the study of Fantoni and co-workers,Citation[37] the oxidative stability of fish oil products was shown to be much lower than in the present study.Citation[37] In 1989, Ackman and co-workers showed that in the tested fish oil products the EPA and DHA label claims for the majority of tested fish oil products were reasonably accurate.Citation[29] Some recent evaluations conducted by ConsumerLab in USA or Consumer in New Zealand indicated that some of the fish oil products available on the market did not contain claimed amounts of EPA and DHA or were oxidized, which could be harmful for consumers health.Citation[25,Citation26] This problem was also reported in 1989, by Shukla and Perkins, as well as in 1992 by Sagredos.Citation[38,Citation39] Sufficient oxidative stability is the most important concern related to safety of all fish oil products. Nevertheless, because of the low level of omega-3 LC PUFA in the Western-style diet, supplementation with good quality fish oil capsules seems to be advisable for health protection.Citation[40] Moreover, due to good processing technology, the food and pharmaceutical grade fish oil used for dietary supplements production is free from toxic contamination such as mercury or PCB, which in the recent years have often been detected in many fish species and seafood.Citation[41]
CONCLUSION
The results obtained in this study showed that the label claims for EPA and DHA for the majority of the evaluated fish oil supplements were presented with reasonable accuracy. However, oxidative stability of some fish oil products available on the market might be not sufficient to ensure health quality and safety during longer storage. Increasing omega-3 LC PUFA intake is a challenge not only for the pharmaceutical, but also for food industry. The possibility of food fortification with omega-3 LC PUFA by fish oil addition should be also explored. For individuals with low fish consumption regular intake of good quality fish oil supplements or fish oil fortified foods can help to ensure adequate dietary level of omega-3 LC PUFA, thus decreasing the risk of many diseases.
REFERENCES
- Bucher , H.C. , Hengstler , P. , Schindler , C. and Meier , G. 2002 . N-3 polyunsaturated fatty acids in coronary heart disease . A meta-analysis of randomized controlled trials. Am. J. Med. , 112 : 298 – 304 .
- Harris , W. 2004 . Fish oil supplementation: Evidence for health benefits . Cleveland Clin. J. Med. , 71 : 208 – 221 .
- Von Schacky , C. , Angerer , P. , Kothny , W. and Mudra , H. 1999 . The effect of dietary omega 3 fatty acids on coronary atherosclerosis . A randomized, double-blind placebo-controlled trial. Ann. Int. Med. , 130 : 554 – 562 .
- Nestel , P.J. 2000 . Fish oil and cardiovascular disease: lipids and arterial function . Am. J. Clin. Nutr. , 71 : 228 – 231 .
- Dyerberg , J. , Bang , H.O. and Hjorne , N. 1975 . Fatty acid composition of the plasma lipids in Greenland Eskimos . Am. J. Clin. Nutr. , 28 : 958 – 966 .
- Connor , W.E. 2000 . Importance of n-3 fatty acids in health and disease . Am. J. Clin. Nutr. , 71 ( Suppl ) : 171 – 179 .
- United States Department of Agriculture. USDA national nutrient database for standard release. 2007 http://www.nal.usda.gov (Accessed: 3 May 2008 ).
- Shahidi , F. 2007 . “ Omega-3 oils: some applications and health effects ” . In Marine nutraceuticals and functional foods , Edited by: Barrow , C. and Shahidi , F. 23 – 62 . CRC Press: Bocca Raton .
- Scientific Advisory Committee on Nutrition, Committee on Toxicity. Advice on fish consumption: benefits and risks. The Stationery Office: London, Great Britain, 2004 http://www.food.gov.uk/multimedia/pdfs/fishreport2004full.pdf (Accessed: 12 January 2007 ).
- Kris-Etherton , P.M. , Taylor , D.S. , Yu-Poth , S. , Huth , P. , Moriarty , K. , Fishell , V. , Hargrove , R.L. , Zhao , G. and Etherton , T.D. 2000 . Polyunsaturated fatty acids in the food chain in the United States . Am. J. Clin. Nutr. , 71 ( Suppl ) : 179 – 188 .
- Saldeen , T. , Wallin , R. and Marklinder , I. 1998 . Effects of a small dose of stable fish oil substituted for margarine in bread on plasma phospholipids fatty acids and serum triglycerides . Nutr. Res. , 18 : 1483 – 1492 .
- Simopoulos , A.P. 2002 . The importance of the ratio of omega-6/omega-3 essential fatty acids . Biomed. Pharmacother. , 56 : 365 – 379 .
- International Society for Study on Fatty Acids and Lipids. Recommendations for intake of polyunsaturated fatty acids in healthy adults. 2005 http://www.issfal.org.uk/index.php?option= com_content&task=view&id=23&Itemid=8 (Accessed: 3 May 2007 ).
- Simopoulos , A.P. , Leaf , A. and Salem , N. 1999 . Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids . Ann. Nutr. Metab. , 43 : 127 – 131 .
- US Food and Drug Administration. Letter regarding dietary supplement health claim for omega-3 fatty acids and coronary heart disease. 2004 http://www.cfsan.fda.gov/~dms/ds-ltr11.html/ (Accessed: 12 December 2006 ).
- Laidlaw , M. and Holub , B.J. 2003 . Effects of supplementation with fish oil-derived n-3 fatty acids and γ-linolenic acid on circulating plasma lipids and fatty acid profiles in women . Am. J. Clin. Nutr. , 77 : 37 – 42 .
- Anonymous . Omega-3 oil: fish or pills? . Consumer Reports , 68 30 – 32 .
- Trautwein , E.A. 2001 . N-3 fatty acids - physiological and technical aspects for their use in food . Eur. J. Lipid Sci. Technol. , 103 : 45 – 55 . 2003
- Kolanowski , W. and Laufenberg , G. 2006 . Enrichment of food products with polyunsaturated fatty acids by fish oil addition . Eur. Food Res. Technol. , 222 : 472 – 477 .
- Kris-Etherton , P.M. , Harris , W.S. and Appel , L.J. 2002 . Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease . Circulation , 106 : 2747 – 2757 .
- Kromhout , D. , Feskens , E.J. and Bowles , C.H. 1995 . The protective effect of a small amount of fish on coronary heart disease mortality in an elderly population . Int. J. Epid. , 24 : 340 – 345 .
- Stenson , W.F. , Cort , D. and Beeken , W. 1992 . Dietary supplementation with fish oil in ulcerative colitis . Ann. Intern. Med. , 116 : 609 – 614 .
- Volker , D. , Fitzgerald , P. , Major , G. and Garg , M. 2000 . Efficacy of fish oil concentrate in the treatment of rheumatoid arthritis . J. Rheumatol. , 27 : 2343 – 2346 .
- Holub , D.J. and Holub , B.J. 2004 . Omega-3 fatty acids from fish oils and cardiovascular disease . Mol. Cell Biochem. , 263 : 217 – 225 .
- ConsumerLab. Omega-3 supplements test. 2007 http://www.consumerlab.com/results/omega3.asp/ (Accessed: 1 June 2007 ).
- Anonymous . 2007 . Something fishy? Omega-3 supplements test . Consumer , 469 : 12 – 15 .
- Kolanowski , W. 2008 . Seasonal variability of encapsulated fish oil dietary supplements intake among inhabitants of Warsaw . Nutr. Res. , 28 : 245 – 250 .
- American Oil Chemists’ Society . 1997 . “ Official method Ce 1b-89 Fatty Acid Composition by GLC - Marine Oils (modified) ” . In Official Methods and Recommended Practices of the AOCS , 5th , 1 – 3 . AOCS Press: Champaign, IL .
- Ackman , R.G. , Ratnayake , W.M.N. and Macpherson , E.J. EPA . 1989 . DHA contents of encapsulated fish oil products . J. Am. Oil Chem. Soc. , 66 : 1162 – 1163 .
- International Standard Organization . 2001 . ISO 3960—Animal and vegetable fats and oils – determination of peroxide value , 7 Geneva, , Switzerland : ISO .
- Christensen , R. 1998 . Analysis of variance, design and regression: applied statistical methods , 123 – 129 . London, , Great Britain : Chapman & Hall .
- Bimbo , A.P. 1998 . Guidelines for characterisation of foodgrade fish oil . Inform , 5 : 180 – 188 .
- Codex Alimentarius Commission . 1999 . Codex standard for edible fats and oils not covered by individual standards (CAC/RS 19-1981, Rev. 2-1999) , 4 Rome : FAO/WHO .
- Fournier , V. , Destaillats , F. , Lambelet , P. , Dionisi , F. , Sebednio , J.L. and Berdeaux , O. 2007 . Degradation products formed from long-chain PUFA during deodorization of fish oil . Lipid Technol. , 19 : 9 – 11 .
- Jacobsen , C. 1999 . Sensory impact of lipid oxidation in complex food systems . Fett/Lipid. , 101 ( Suppl ) : 484 – 492 .
- Kolanowski , W. , Jaworska , D. and Weißbrodt , J. 2007 . Importance of instrumental and sensory analysis in the assessment of oxidative deterioration of omega-3 LC PUFA-rich foods . J. Sci. Food Agric. , 87 : 181 – 191 .
- Fantoni , C.M. and Cuccio , A.P. 1996 . Barrera-Arellano, D. Brazilian encapsulated fish oils: oxidative stability and fatty acid composition . J. Am. Oil. Chem. Soc. , 73 : 251 – 253 .
- Sagredos , A.N. 1992 . The quality and contamination of fish oil capsules . Fat Sci. Technol. , 94 : 101 – 111 .
- Shukla , V.K.S. and Perkins , E.G. 1998 . Rancidity in encapsulated health-food oils . Inform , 9 : 955 – 961 .
- Anonymous . 2006 . Oily fish and omega 3 fat supplements. Health recommendations conflict with concerns about dwindling supply. Br . Med. J. , 332 : 739 – 740 .
- Nesheim , M.C. and Yaktine , A.L. 2007 . Seafood choices: balancing benefits and risks , 121 – 128 . Washington, DC : The National Academies Press .