Abstract
Angiotensin I-converting enzyme (ACE) inhibitory activities of commercial douchi were determined, and salt-fermented samples were found exhibiting higher activities than dandouchi. Douchi was prepared in laboratory scale by pure culture fermentation. Results showed that ACE inhibitory activity in bacteria-type douchi decreased during maturation, while it increased in fungi-type douchi, with the highest activity observed in Aspergillus oryzae fermented douchi after ripening for 30 days. These suggested that the type of starters affected significantly the generation of ACE inhibitors during douchi ripening. Sensory evaluation showed that A. oryzae produced more organoleptically acceptable douchi than other cultures used in this study.
INTRODUCTION
Hypertension is one of the major risk factors for the development of cardiovascular diseases. Angiotensin I-converting enzyme (ACE, EC 3.4.15.1) plays an important physiological role in the regulation of blood pressure. It can elevate blood pressure by converting the inactive angiotensin-I to the potent vasoconstrictor, angiotensin-II, and by catalyzing the degradation of bradykinin.Citation[1] Inhibiting ACE activity is considered a useful therapeutic approach to treat high blood pressure.
Although several synthetic ACE inhibitors have been currently used as clinical antihypertensive drugs,Citation[2] they cause adverse effects such as coughing, allergic reactions, taste disturbances and skin rashes. Therefore, in recent years, safer, innovative, and economical ACE inhibitors from food resources have been paid dramatic attention for the prevention and remedy of hypertension. Some literatures had reported that several fermented soybean foods, such as soy sauce,Citation[3] natto,Citation[4] Korean soybean paste,Citation[5] tofuyo,Citation[6] tempeh,Citation[7] and douchi,Citation[8] were also sources of ACE inhibitors. So far, the ACE inhibitory activities of fermented soybean foods were mainly attributed to the biophysiological properties of peptides derived from the fermentation of soybean proteins.
Douchi is a traditional fermented soybean product originating in China, which has been used as seasoning in foods and for medicinal purposes at least for 2000 years. It is still added to some traditional Chinese medicines nowadays. In general, douchi is produced by three steps: (1) pretreatment, (2) preparing douchi qu (also called ”koji,” a semi-finished product) by microbial solid-state fermentation, which is called pre-fermentation, and (3) ripening in dressing mixture. Except for common salt-fermented douchi, dandouchi, a special material for Chinese traditional medicine, could be thought of as a semi-product of douchi because it is produced without ripening.Citation[9]
Today, as populations who consume foods with added douchi are increasing, there is an increasing interest in its physiological properties. It had been reported that significant differences in biochemistry and microbiology among fermented foods could be raised up by using different microorganisms as starters.Citation[10 Citation,13] In commercial practice, Aspergillus spp., Mucor spp. and bacteria spp. are used for douchi preparation. Among these strains, Aspergillus spp. seemed to be the most frequently used for douchi production in China. So far, almost all the earlier investigations on Chinese douchi were related to the properties of the fungi-type, especially related to douchi fermented by Aspergillus species. It was reported that douchi started with Aspergillus egyptiacus possessed the improved ACE inhibitory activities over fermentation, and a peptide with potential blood pressure-lower-ing property was isolated.Citation[8] The antioxidant activities of douchi extracts were shown to increase significantly during fermentation when Aspergillus oryzae was used as starter culture.Citation[14,Citation15] In addition, douchi exhibited considerable α-glucosidase inhibitory activities, which were partly depended on the strains used.Citation[16] Little quantitative information on the diverse action of various starter cultures on the generation of ACE inhibitors in douchi is available.
Ripening is a crucial step for the formation of taste, flavor, and special texture as well as nutritional properties of fermented soybean food. In the present study, the ACE inhibitory activities of commercial douchi products, including thirty-six salt-fermented douchi and twenty-one dandouchi collected from different regions of China, and lab-made douchi produced by various pure cultures, namely Aspergillus oryzae, Mucor wutungkiao, Bacillus subtilis natto and Bacillus subtilis B1, were evaluated. In order to further investigate the effects of starter cultures on the ACE inhibitory activities of douchi, the microflora profiles and chemical parameters during ripening were analysed. Sensory attributes of douchi were also compared.
MATERIALS AND METHODS
Materials
Thirty-six commercial salt-fermented douchi and twenty-one dandouchi were sampled from various parts of China ( and ). For lab-made douchi producing, Aspergillus oryzae was provided by the Institute of Microbiology from Chinese Academy of Sciences (Beijing, China), Mucor wutungkiao was kindly donated by China Center of Industrial Culture Collection (Beijing, China), Bacillus subtilis natto was purchased from Yuzo Takahashi Laboratory (Yamagata, Japan), and Bacillus subtilis B1 was previously isolated in our laboratory from douchi qu provided by Weiyizhai Babao Douchi Manufacturer (Linyi, China).Citation[17] Soybeans were purchased from the Center of Soybean Research, Agricultural Academy of Jilin Province (Jilin, China).
Table 1 Brands, origins, types of soybean used, classification and ACE inhibitory activities of commercial salt-fermented douchi samples
Table 2 Origins, types of soybean used and ACE inhibitory activities of commercial dandouchi samples
Hippuryl-His-Leu (HHL), angiotensin I-converting enzyme (ACE, from rabbit lung), o-phthaldialdehyde (OPA) and 2,4,6-trinitrobenzenesulfoinc acid (TNBS) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). All other reagents were of analytical grade.
Starter Cultures and Culture Conditions
Fungal strains of A. oryzae and M. wutungkiao were grown individually on a sterilized substrate consisting of wheat bran, wheat flour and distilled water (5:1:5, w/w) in an incubator (HPS-250, Dongming Medical Instrument Co., Harbin, China) at 28°C. After 3 d of incubation, homogenous conidia suspension was obtained by washing the substrate with sterilized water and filtration. The conidia were enumerated using a thrombocytometer. B. subtilis natto and B. subtilis B1 were cultured respectively using Luria-Bertani liquid medium for 12 h at 37°C with shaking at 150 rpm in an incubation shaker (HZQ-F160, Donglian Electron Technology Co., Ltd., Harbin, China), and suspension was used for the subsequent inoculation.
Douchi Preparation
Douchi was prepared according to the method described by Li et al.Citation[18] The steps and production conditions were as follows:
| 1. | Soybeans were washed and soaked in tap water (1:3, w/w) at 28°C for 8 h. After draining, the soybeans were steamed at 121°C for 40 min in a retort (YXQ–SG46–280A, Beijing Boxun Medical Instrument Co., Ltd., Beijing, China), and then cooled to 30°C. | ||||
| 2. | Pre-fermentation. The cooked soybeans were inoculated with conidia (107 spores/mL) of either A. oryzae or M. wutungkiao (1 mL conidia/100 g cooked soybeans), and then incubated at 28°C and 90% relative humidity (RH) for 72 h in an incubator (EYELA KCL-1000, Tokyo Rikakikai Co., Ltd., Tokyo, Japan). Another series of experiments were conducted by inoculating cooked soybeans with B. subtilis natto or B. subtilis B1 (1 mL/100 g cooked soybeans), which were fermented at 37°C for 24 h under 90% RH. The semi-products were called douchi qu. | ||||
| 3. | Ripening of douchi. Douchi qu were matured in glass bottles after supplementation with 10% salt (w/w, based on the unsoaked soybeans) and 2% ethanol (v/w, based on the unsoaked soybeans). Douchi were obtained after ripening for 30 d at 37°C. | ||||
Fresh douchi were sampled at 10 d intervals during ripening. Three replicate trials were conducted for each preparation of douchi.
Preparation of Douchi Extracts
Samples were lyophilized with a freeze dryer (EYELA FDU-540, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and ground. One gram of douchi powder was suspended in 10 mL of distilled water, followed by homogenization (T25 basic, IKA Labortechnik, Staufen, Germany) at 15,000 rpm for 2 min and sonication (KQ-100E, Ultrasonic Instrument Co., Ltd., Kunshan, China) for 5 min. The mixture was extracted in an orbital shaker (WSZ-100A, Yiheng Technical Co., Ltd., Shanghai, China) for 1 h at room temperature. After boiling for 15 min, the suspension was centrifuged (TGL-16C, Anting Scientific Instrument Co., Shanghai, China) at 12,000 × g for 15 min. The resulted supernatant was collected after filtration by a 0.45 μm membrane and then used for assaying. The concentration was labeled as 100 mg/mL.
ACE Inhibitory Activity Assay
ACE inhibitory activity assay was measured by using the modified method described by Horie.Citation[19] A 20 μL of douchi extract was mixed with 40 μL of ACE solution (12.5 munits/mL) and 40 μL of substrate solution (4.66 mM HHL in 400 mM phosphate buffer containing 600 mM NaCl, pH 8.5) and then incubated at 37°C for 1 h. The reaction was terminated by adding 150 μL of 1.2 M NaOH solution. The mixture was incubated for 20 min at room temperature after the addition of 40 μL of 2% OPA dissolved in methanol. The derivation reaction was terminated by adding 40 μL of 6 M HCl solution. The fluorescence intensity was measured using a spectrofluorophotometer (RF-5300PC, Shimadzu Co., Kyoto, Japan) under the following conditions: excitation wavelength, 340 nm; emission wavelength, 455 nm; slit width, 5 nm. ACE inhibitory activity was calculated as follows:
where a is the fluorescence intensity of the ACE solution with the addition of ACE inhibitors to the buffer; b is the fluorescence intensity of the ACE solution in the buffer; c is the fluorescence intensity of the ACE inhibitors in the buffer; d is the fluorescence intensity of the buffer. IC50 value was defined as the concentration of inhibitor required to inhibit 50% of the ACE activity.
Microbiological Analysis
Five gram portions of fresh douchi sample was aseptically weighted and homogenized using a mortar and pestle, and then it was mixed with 45 mL of sterile water. One milliliter of the homogenate was mixed with 9 mL of sterile 0.1% peptone solution. Further decimal dilutions were conducted and duplicate counting plates were prepared for appropriate dilutions. Mould and yeast populations were determined in pour-plates of Rose Bengal agar. Total count of mesophilic aerobic bacteria (TMAB) was enumerated in pour-plates of Plate Count Agar (PCA, CM325, Oxoid, England). Lactic acid bacteria (LAB) were enumerated in pour-plates of de Man, Rogosa and Sharpe medium (MRS, catalogue no. 1.10661, Merck, Germany). Samples were pasteurized at 80°C for 10 min and bacterial endospores were enumerated in pour-plates of PCA.
Chemical Analysis
A one gram sample was suspended in 10 mL of distilled water. After shaking for 1 h, the suspension was centrifuged (GL-20B, Anting Scientific Instrument Co., Shanghai, China) at 4°C and 5000 × g for 10 min. The supernatant was used as crude enzyme extract. Protease activity was measured using the modified method described by Yang et al.Citation[20] A 2 mL of crude enzyme extract was mixed with the same volume of 2% casein dissolved in 0.2 M phosphate buffer (pH 7.5). The mixture was incubated at 37°C for 10 min. The reaction was terminated by the addition of 4 mL of 0.4 M trichloroacetic acid solution, and the mixture was centrifuged. The soluble peptide in the supernatant was assessed with tyrosine as the reference compound. Amounts of free amino groups in douchi extracts were measured according to the method of Haynes et al.Citation[21] using TNBS, the specific reagent for amino groups, on a spectrophotometer (UV 1240, shimadzu, Co., Kyoto, Japan).
The color values of cooked soybeans and douchi samples were determined by using a chroma meter (CR-300, Minolta Co., Ltd., Osaka, Japan). Total color difference, ΔE* ab , was calculated to express the change in douchi color during fermentation according to the following equation:
where L*t , a*t and b*t represent color values of soybeans before fermentation; L*, a* and b* represent color values of douchi during ripening.
Sensory Evaluation
Douchi samples matured for 30 d were evaluated organoleptically alongside several commercial available douchi products, including TPQ, TC, YJQ, and WYZ produced in Hunan, Sichuan, Guangdong and Shandong province, respectively. Samples were boiled in water (1:5, w/w) for 10 min and the douchi soup were assessed randomly by eight panelists (students familiar with Chinese fermented soy-products) on a 5-point hedonic scale in which 5, 4, 3, 2, and 1 indicated much better (pleasant smell, pleasant taste, good flavour and no bitterness), better (sour but pleasant taste, good flavour and a little bitter), good (moderate smell, moderate flavor and moderately sour taste), average (less smell, less flavour and less taste), and poor (off smell, putrefactive flavor, and bitter taste), respectively, on the basis of comparison with the reference products. The evaluation was performed in duplicate on two different days.
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) using the general linear model (Version 8.0, SAS Institute Inc., NC, USA). Duncan's multiple range tests were used to determine the differences among samples. Significant levels were defined as probabilities of 0.05 or less.
RESULTS
ACE Inhibitory Activities of Commercial Douchi Samples
ACE inhibitory activities of commercial salt-fermented douchi and dandouchi samples, expressed as IC50 values, are shown in and . All the aqueous douchi extracts exhibited in vitro ACE inhibitory activities. While there was no significant difference in the ACE inhibitory activities between Aspergillus-type douchi and Mucor-type douchi, significant differences were observed in douchi started by fungi from that started by bacteria (p < 0.05). The higher ACE inhibitory activities were found in two Aspergillus-type douchi, namely sample Nos. 15 and 17 with IC50 values of 0.3130 and 0.3995 mg/mL, respectively, and in three Mucor-type douchi, namely sample Nos. 21, 25 and 27 with IC50 values of 0.3897, 0.2509, and 0.3756 mg/mL, respectively. Dandouchi showed weaker values of ACE inhibitory activities in comparison with salt-fer-mented douchi, with the highest ACE inhibitory activity being recorded as an IC50 value of 0.4800 mg/mL in sample No. 5.
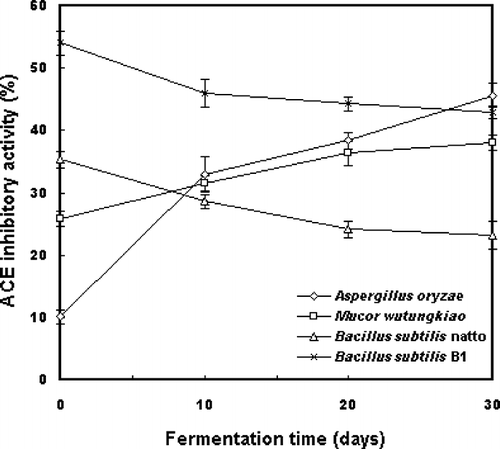
ACE Inhibitory Activities of Pure-cultured Douchi During Ripening
depicts the changes in the ACE inhibitory activities during the ripening of douchi. Although the ACE inhibitory activity of B. subtilis-type douchi decreased during salting and maturation, it increased in fungi fermented douchi. The fast increase of ACE inhibitory activity from 10.1% to 45.6% was observed in douchi fermented with A. oryzae at a concentration of 0.2 mg/mL. Significant differences of the ACE inhibitory activities among final products were observed when various strains were selected as starter cultures (p < 0.05). Douchi started by A. oryzae exhibited the highest activity with an IC50 value of 0.2591 mg/mL. The IC50 value of douchi was 0.3150 mg/mL for B. subtilis B1, and it was 0.4378 mg/mL for M. wutungkiao. The lowest activity was observed in B. subtilis natto fermented douchi with an IC50 value of 0.5122 mg/mL.
Chemical Parameters of Douchi During Ripening
The changes in protease activities during douchi ripening are shown in . Although no significant change in protease activities of samples was observed during ripening, protease activities in douchi fermented with B. subtilis natto and B. subtilis B1 were significantly higher than that in douchi fermented with A. oryzae and M. wutungkiao. The highest protease activity was recorded as 652.0 U/g dry matter in B. subtilis natto fermented douchi, whereas protease activity was only 80.0 U/g dry matter in M. wutungkiao fermented douchi at the end of maturation.
Figure 2 Changes in the (A) protease activities, (B) amounts of free amino groups, and (C) colors during the ripening of douchi fermented by various starter cultures, namely Aspergillus oryzae (—◊—), Mucor wutungkiao (—□—), Bacillus subtilis natto (—▵—), and Bacillus subtilis B1 (—∗—). The error bars indicated the standard deviation of three replicates.
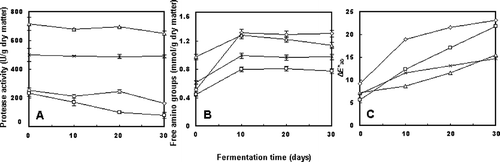
shows variation in the amounts of free amino groups during the ripening of douchi. Rapid increases were observed in all of the samples during the first 10 d of ripening, with the largest increment of 0.801 mmol/g dry matter being observed in douchi started by A. oryzae. After 30 d of maturation, the amount of free amino groups reached the highest level of 1.303 mmol/g dry matter in A. oryzae started douchi. represents the differences of douchi colors, which are expressed as ΔE* ab . Generally, douchi became increasingly brown over ripening. It was found that douchi fermented by fungal strains turned colors more rapidly than those fermented by bacterial strains.
Microbial Changes During Douchi Ripening
shows the changes in microbial populations of pure-cultured douchi being subjected to 30 d maturation. Although considerable decline of moulds took place in douchi started by A. oryzae during ripening, 104 cfu/g of moulds were detected at the end of maturation, and TMAB and bacterial endospore populations remained in the range of approximately 103 cfu/g. With significant increases of TMAB and bacterial endospores in douchi fermented by M. wutungkiao, mould populations decreased from around 107 cfu/g to non-detectable level after 10 d of maturation. Moulds could not be detected in douchi fermented by both B. subtilis natto and B. subtilis B1, while bacterial endospores and TMAB kept at high levels of 108 cfu/g and 109 cfu/g in these two B. subtilis fermented samples. The counts of LAB increased to 104 cfu/g in douchi fermented by fungal strains, while it remained unchanged at the level of 105–106 cfu/g in bacteria-type douchi. Yeasts proliferated to approximately 104 cfu/g in douchi prepared during 30 d of maturation.
Table 3 Microbial changes during douchi ripening with various starter cultures
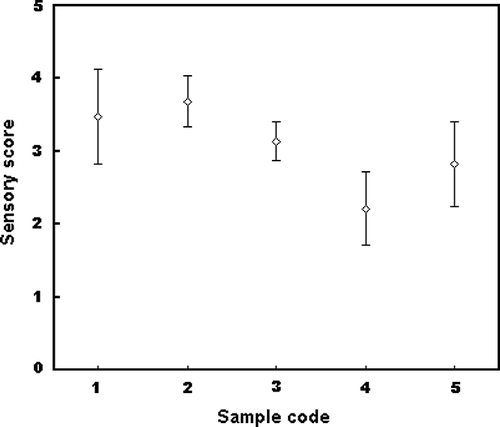
Sensory Evaluation of Douchi
shows the sensory attributes of douchi, in which commercial available products were used as references. Although there was no significant difference (P > 0.05) in the organoleptic attribute scores among all of the samples, the best organoleptically acceptance was recognized as the product fermented by A. oryzae and the lowest score was recorded in douchi when B. subtilis natto was used as starter culture.
DISCUSSION
The ACE inhibitory activities of fifty-seven commercial douchi samples including twenty-one dandouchi were investigated, which can be regarded as representative of the major types of douchi found in China. They could be further distinguished according to the production origins, the starter cultures, and the types of soybean. As compared to dandouchi, commercial salt-fermented douchi extracts showed stronger ACE inhibitory activities. The ACE inhibitory activities in most Aspergillus-type and Mucor-type douchi seemed to be significantly higher than those in bacteria-type douchi (p < 0.05). Whereas the ACE inhibitory activities of douchi extracts were influenced by the starter cultures used, there was no clear regional and directional tendency between the production origins and high ACE inhibitory activities.
Although this work did not aim to investigate the influence of pre-fermentation on the ACE inhibitory activities of douchi in this work, bacteria-type douchi qu obtained from pre-fermentation was evident to exhibit higher ACE inhibitory activities as compared with fungi-type samples, because the ACE inhibitory activities of douchi qu could be represented by the experimental data at 0 d of ripening (). This result suggested that pre-fermentation was more favorable for the production of ACE inhibitory activities in bacteria-type douchi. The present work showed that the ripening stage was beneficial for the subsequent increases of ACE inhibitory activities in fungi-type douchi, however, it brought forth undesirable loss of ACE inhibitory activities in bacteria-type douchi, suggesting that ripening possessed a different influence on the generation of ACE inhibitors from the pre-fermentation. After ripening, the largest ACE inhibitory activity was found in douchi fermented by A. oryzae, followed by B. subtilis B1 fermented douchi, while the lowest ACE inhibitory activity was observed in douchi fermented by B. subtilis natto. Dandouchi, on the other hand, showed weaker ACE inhibitory activities compared to salt-fermented douchi products under the present investigation. This phenomenon was probably due to insufficient generation of ACE inhibitors in dandouchi, because it was commonly produced by fermentation with fungi, but without ripening stage conducted in China.
The ACE inhibitory activities are dependent on the conditions of processing including the type of starter cultures. Han et al.Citation[22] reported that Actinomucor elegans, the mould starter for tofu fermentation, was not active metabolically after salting, and that the generated enzyme was also inactive during the ripening and storage steps because of the high levels of salt and/or ethanol in the dressing mixture. Using the same strain as starter culture, Shi and FungCitation[23] also pointed out that no moulds were detected in fermented tofu after ripening. In addition, Chen et al.Citation[16] reported that Actinomucor elegans and Rhizopus arrhizus had lower abilities to survive in the presence of salt than Aspergillus oryzae during douchi fermentation. The present work showed that mould populations were at a higher level in douchi started by A. oryzae, even though that moulds were undetectable in douchi when M. wutungkiao was used as starter culture. On the contrary, bacterial populations remained at a much higher level in M. wutungkiao fermented douchi than in douchi started by A. oryzae. Therefore, A. oryzae exhibited stronger viability to inhibit the growth of other microbes and to resist salt and ethanol compared with M. wutungkiao under experimental conditions. The special microbiological properties could presumably contribute to generate higher ACE inhibitory activity in douchi produced by fermentation with A. oryzae.
So far, the ACE inhibitors were principally recognized as peptides in fermented soybean foods, which were resulted from the hydrolysis of soybean protein by proteases derived from the microorganisms.Citation[4 Citation,7] The amounts of free amino groups were able to reflect, to a certain extent, the degrees of protein hydrolysis. In this work, the amounts of free amino groups in douchi increased rapidly in the earlier period of ripening and reached a constant value with slight change over 10 d of maturation. The maximum amount of free amino groups was observed in douchi fermented by A. oryzae, which was due to the special properties of proteases derived from A. oryzae, and its corresponding ACE inhibitory activity was also the largest. Chiang and co-workersCitation[24] and Fan and co-workersCitation[25] reported that higher hydrolysis degree of soy protein did not guarantee a higher ACE inhibitory activity due to the different properties of the enzymes used. Several literatures depicted that different inoculants involved in fermentation could induce variation in protein degradation, and thus produce various bioavailability.Citation[12,Citation13] These phenomena implied that proteases derived from various starter cultures were able to act on different catalytic sites of soybean protein, and consequently, generate peptides with different structures. Li et al.Citation[26] reported that the ACE inhibitory activities fairly depended on the structures of the peptides. The apparent superiority of A. oryzae could be therefore presumably attributed to its active metabolism, which facilitated the generation of bioactive peptides at higher levels than other tested cultures, although the properties of these strains were not analyzed in details. On the other hand, the decreases of ACE inhibitory activities in two B. subtilis fermented douchi might be resulted from inordinate hydrolysis of the ACE inhibitory peptides, largely due to the higher protease activities, which was also evident from more ammoniac flavor released in these samples.
In the present work, douchi colors turned darker in douchi started by fungi. Douchi discoloration was mainly resulted from Maillard reactions occurred during fermentation. Okamoto et al.Citation[27] reported that colored compounds in soy sauce as well as fish sauce were responsible, to a certain extent, for their ACE inhibitory activities. Rufián-Henares and MoralesCitation[28] reported that melanoidins resulted from aqueous Maillard reaction model systems showed some ACE inhibitory activities. Therefore, the total ACE inhibitory activities of douchi may occur from the combined function of various bioactive substances produced in douchi by fermentation. It is necessary to carry out further investigation to clarify the materials contributing to the ACE inhibitory activities in douchi.
The findings showed that significant variation in the ACE inhibitory activities of douchi during ripening was observed according to various starter cultures used. Ripening had certain advantage to fungi-type douchi over bacteria-type douchi in terms of the generation of ACE inhibitory activities. The primary mechanism can be attributed to the subsequent generation of ACE inhibitors as a result of microorganism and/or enzyme generated being active metabolically during salting and maturation. It was also interesting to note that, although the ACE inhibitory activity in douchi fermented by B. subtilis B1 tended to decrease during ripening, a similar ACE inhibitory activity as that in douchi fermented by A. oryzae could be reached after ripening, indicating that B. subtilis B1, except for A. oryzae, may have the potential to produce douchi with higher ACE inhibitory activities. Sensory attributes, however, showed that A. oryzae was able to produce better organoleptically acceptance of douchi as compared with B. subtilis B1 under the present observation. It will now be of interest to evaluate if the sensory attributes as well as consumer acceptability could be improved by the supplementation with other spices when B. subtilis B1 was employed as starter culture.
ACKNOWLEDGMENTS
This work was supported by the National Key-technologies R&D Program (2006BAD27B09 & 2007BAD83B03) of the 11th 5-year Plan of the People's Republic of China. This study was also conducted within the framework of the collaborative research project between Japan and China entitled “Development of sustainable production and utilization of major food resources in China” supported by Japan International Research Center for Agricultural Sciences (JIRCAS).
REFERENCES
- Erdos , E.G. 1975 . Angiotensin-I converting enzyme . Circulation Research , 36 : 247 – 255 .
- Kuster , D.J. and Marshall , G.R. 2005 . Validated ligand mapping of ACE active site . Journal of Computer-Aided Molecular Design , 19 ( 8 ) : 609 – 615 .
- Kinoshita , E. , Yamakashi , J. and Kikuchi , M. 1993 . Purification and identification of an angiotensin I-converting enzyme inhibitor from soy sauce . Bioscience, Biotechnology, Biochemistry , 57 ( 7 ) : 1107 – 1110 .
- Okamoto , A. , Hanagata , H. , Kawamura , Y. and Yanagida , F. 1995 . Anti-hypertensive substances in fermented soybean, natto . Plant Foods for Human Nutrition , 47 : 39 – 47 .
- Shin , Z.I. , Yu , R. , Park , S.A. , Chung , D.K. , Ahn , C.W. and Nam , H.S. 2001 . His-His-Leu, an angiotensin I converting enzyme inhibitory peptide derived from Korean soybean paste, exerts antihypertensive activity in vivo . Journal of Agricultural and Food Chemistry , 49 : 3004 – 3009 .
- Kuba , M. , Tanaka , K. , Tawata , S. , Takeda , Y. and Yasuda , M. 2003 . Angiorensin I-converting enzyme inhibitory peptides isolated from tofuyo fermented soybean food . Bioscience, Biotechnology, Biochemistry , 67 ( 6 ) : 1278 – 1283 .
- Gibbs , B.F. , Zougman , A. , Masse , R. and Mulligan , C. 2004 . Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food . Food Research International , 37 : 123 – 131 .
- Zhang , J.H. , Eizo , T. , Ding , C.H. and Li , L.T. 2006 . Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product . Food Chemistry , 98 : 551 – 557 .
- Chinese Pharmacopoeia Commission (CPC) . 2005 . Chinese Pharmacopoeia , 230 Beijing : CPC . (in Chinese)
- Mateo , J.J. , Jiménez , M. , Pastor , A. and Huerta , T. 2001 . Yeast starter cultures affecting wine fermentation and volatiles . Food Research International , 34 : 307 – 314 .
- Beaumont , M. 2002 . Flavouring composition prepared by fermentation with Bacillus spp . International Journal of Food Microbiology , 75 : 189 – 196 .
- Scannell , A.G.M. , Kenneally , P.M. and Arendt , E.K. 2004 . Contribution of starter cultures to the proteolytic process of a fermented non-dried whole muscle ham product . International Journal of Food Microbiology , 93 : 219 – 230 .
- Nora , N.T. , Esther , S.D. and Wisdom , K.A. 2006 . The comparative ability of four isolates of Bacillus subtilis to ferment soybeans into dawadawa . International Journal of Food Microbiology , 106 : 145 – 152 .
- Wang , L.J. , Li , D. , Zou , L. , Chen , X.D. , Cheng , Y.Q. and Yamaki , K. 2007 . Antioxidative activity of Douchi (a Chinese traditional salt- fermented soybean food) extracts during its processing . International Journal of Food Properties , 10 : 385 – 396 .
- Wang , D. , Wang , L.J. , Zhu , F.X. , Zhu , J.Y. , Chen , X.D. , Zou , L. , Saito , M. and Li , L.T. 2008 . In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese slat-fermented soybean food) . Food Chemistry , 107 : 1421 – 1428 .
- Chen , J. , Cheng , Y.Q. , Yamaki , K. and Li , L.T. 2007 . Anti-α-glucosidase activity of Chinese traditionally fermented soybean (douchi) . Food Chemistry , 103 : 1091 – 1096 .
- Fan , J. F. 2006 . Antioxidant activity and gel-forming ability of douchi peptides and soy peptides , Beijing : China Agricultural University . Ph.D. Thesis
- Li , L.T. , Li , Z.G. and Yin , L.J. 2003 . Soybean processing and application , 415 Beijing : Chemical Industry Press .
- Horie , H. 1999 . Handbook of functional evaluation of food. Japanese Ministry of Agriculture, Forestry and Fisheries. Agriculture, Forestry and Fishery Research Council . National Food Research Institute: Tokyo , : 117 – 121 . (in Japanese)
- Yang , J.K. , Shih , I.L. , Tzeng , Y.M. and Wang , S.L. 2000 . Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes . Enzyme and Microbial Technology , 26 : 406 – 413 .
- Haynes , R. , Osuga , D.T. and Feeney , R.E. 1967 . Modification of proteolytic enzyme . Biochemistry , 6 : 541 – 547 .
- Han , B.Z. , Cao , C.F. , Rombouts , F.M. and Nout , M.J.R. 2004 . Microbial changes during the production of Sufu-a Chinese fermented soybean food . Food Control , 15 : 265 – 270 .
- Shi , X. R. and Fung , D.Y.C. 2000 . Control of foodborne pathogens during sufu fermentation and aging . Critical Reviews in Food Science and Nutrition , 40 ( 5 ) : 399 – 425 .
- Chiang , W.D. , Tsou , M.J. , Tsai , Z.Y. and Tsai , T.C. 2006 . Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor . Food Chemistry , 98 : 725 – 732 .
- Fan , J.F. , Saito , M. , Tatsumi , E. and Li , L.T. 2003 . Preparation of Angiotensin I-converting enzyme inhibiting peptides from soybean protein by enzymatic hydrolysis . Food Science and Technology Research , 9 ( 3 ) : 254 – 256 .
- Li , G.H. , Le , G.W. , Shi , Y.H. and Shrestha , S. 2004 . Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects . Nutrition Research , 24 : 469 – 486 .
- Okamoto , A. , Hanagata , H. , Matsumoto , E. , Kawamura , Y. , Koizumi , Y. and Yanagida , F. 1995 . Angiotensin I converting enzyme inhibitory activities of various fermented foods . Bioscience, Biotechnology, Biochemistry , 59 ( 6 ) : 1147 – 1149 .
- Rufián-Henares , J.A. and Morales , F.J. 2007 . Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities . Food Research International , 40 : 995 – 1002 .