Abstract
Tinctures of eleven plants used as spices (basil, celery, dill, horsetail, lovage, marjoram, milfoil, oregano, parsley, rosemary, and thyme) were tested for their antiradical properties by means of the DPPH (1,1-diphenyl-2-picrylhydrazyl) assay over a two year period. Seven of these plants (basil, lovage, marjoram, milfoil, oregano, rosemary and thyme) were selected to obtain a mixture, which was tested in situ as antioxidant on vegetable and animal fats by an accelerated oxidation test at 110°C. The herbal extract also showed antimicrobial activity against Staphylococcus aureus (ATCC 25923), Streptococcus pyogenes (ATCC 49399), Escherichia coli (ATCC 25922) and Candida albicans (ATCC 24433).
INTRODUCTION
One of the major problems in food is the formation of undesirable lipid oxidation products that reduce the quality of foods during processing and storage.Citation[1] Several synthetic and natural antioxidants with code numbers from E300 to E321 are used in food in the European Union. The E-code associated to a food additive indicates that it fulfills the European Scientific Committee for Food (SCF) safety requirements. However, some of these additives are suspected to be toxic and/or carcinogenic: propyl gallate (PG, E310) and its derivatives, tert-butylhydroquinone (TBHQ, E319), butylated hydroxyanisole (BHA, E320) and butylated hydroxytoluene (BHT, E321).Citation2–4
Aromatic plants and spices have great importance for food, cosmetics and pharmaceutical industries. Their use have taken place since ancient times, and despite many of them were substituted by synthetic ones, the demand for natural products is increasing.Citation[5] In recent years, different spice extracts have appeared in the market as antioxidants for the food industry. The antioxidant capacity of some of these compounds has been shown to be comparable to synthetic antioxidants like α-tocopherol and butylated hydroxytoluene.Citation6–8 The essential oils of basil, celery, dill, horsetail, lovage, marjoram, milfoil, oregano, parsley, rosemary and thyme, extracted via steam distillation from leaves and/or flowers, have proven remarkable antioxidant properties.Citation9–11 Some of these oils have also been shown to contain biologically-active constituents with antimicrobial properties, most of them with terpenic structure, like carvacrol, isoterpinolene, caryophyllene, camphene, pinene, and thymol.Citation12–14 The use of essential oils is still limited because of their susceptibility to oxidation, volatility and/or thermal instability.Citation[15] Some of these problems can be solved if, instead of oils, tinctures of the same plants are used. The purpose of this study was to prepare a natural extract from the spices used in the Romanian cuisine and to test its antioxidant and antimicrobial properties.
MATERIALS AND METHODS
Plant Material
Herbarium information of the eleven plant species which are individually numbered are as it follows: (1) basil (Ocimum basilicum), aerial part; (2) celery (Apium graveolens), leaves; (3) dill (Anethum graveolens), aerial part; (4) horsetail (Equisetum arvense), leaves; (5) lovage (Levisticum officinale), leaves; (6) marjoram (Maiorana hortensis), leaves; (7) milfoil (Millefoli flos), flowers; (8) oregano (Origanum vulgare), aerial part; (9) parsley (Petroselinum crispum), leaves; (10) rosemary (Rosmarinus officinalis), aerial part; and (11) thyme (Thymus vulgaris), aerial part. All these plants were acquired from an ecologic horticulture market, were washed with cold water and then dried at the room temperature.
Chemicals
The chemicals used were as it follows: DPPH (1,1-diphenyl-2-picrylhydrazyl) and indigo carmine (disodium-3-oxo-2-(3-oxo-5-sulfonato-1H-indol-2-ylidene)-1H-indole-5-sulfonate) from Merck (Darmstadt, Germany); ethanol (C2H5OH), methanol (CH3OH), glacial acetic acid (CH3COOH), propan-2-one (CH3-CO-CH3), ethoxyethane (CH3-CH2-O-CH2-CH3), potassium iodide (KI), potassium permanganate (KMnO4), hydrogen chloride (HCl) - solution 36%, ammonium sulfate ((NH4)2SO4) and sodium thiosulfate (Na2S2O3) from Chimopar (Bucureşti, România). All chemicals and solvents used were of analytical grade.
Microbiological media (tryptic soy agar and Müller-Hinton agar) were purchased from Merck (Darmstadt, Germany) and Sabouraud-2% (m/v) glucose agar from Biolife (Milan, Italy). Discs impregnated with antibacterial and antifungal substances were purchased from HiMedia Laboratoires, (Mumbai, India): Ofloxacin (5μg/disc), Penicilin (10 UI/disc), Cloramfenicol (10 UI/disc), Oxacilin (1 μg/disc), Itraconazole (10 μg/disc) and Nistatin (30 μg/disc); Bioanalyse Ltd. (Ankara, Turkey): Erytromycin (15 μg/disc) and Abtek Biologicals Ltd. (Liverpool, UK): Gentamicin (10 μg/disc). The McFarland Standard for turbidity was purchased from BioMérieux (Marcy l'Etoile, France). Reference bacterial strains were purchased from Microbiologics (Saint Cloud, USA) and included Staphylococcus aureus ATCC 25923, Streptococcus pyogenes ATCC 49399, Escherichia coli ATCC 25922 and Candida albicans ATCC 24433.
Preparation of the Extract
The dried plants were ground to a fine powder and 10 g of each were mixed with 100 ml ethanol (analytical grade) in dark glass vessel and left for 10 days at room temperature. The resultant extracts were collected through filtration on a 0.45 μm filter paper and the tinctures were kept in a refrigerator until analysis. For the final extract, a mixture formed from equal amounts of dried basil, lovage, marjoram, milfoil, oregano, rosemary, and thyme was subjected to the same treatment.
Main Phenolic Compounds (Antocianes and Tannins) Determination
The antocianes content was determined based on their property to present an absorption maximum at 550 nm in acid-alcoholic solutions.Citation[16] Briefly, each tincture was evaporated under vacuum at 30–40°C. The obtained residuum was dissolved in a mixture of cold water and ethanol (50:50) and kept for 24 h into the refrigerator (8°C). The obtained powder was dissolved in methanol 50% by a short heating at 60°C, than it was filtrated and concentrated under vacuum at 30–40°C. The last set of operations was repeated once again. The resulted powder was dissolved in hot water for quantitative determination. Separately an acid-alcoholic solution was obtained by adding 20 mL of concentrated HCl (density of 1.19 g/mL) to 980 mL ethanol 96%. Into a 100 mL flask were introduced 10 mL sample and 90 mL acid-alcoholic solution. The obtained sample solution was centrifuged at 3000 rpm. Absorbance of supernatant, at 550 nm, was read against the acid-alcoholic solution. The antocianes content in the sample solution, m, was determined with EquationEq. (1):
where D0 is absorbance value at 550 nm; and 0.015 is experimental coeficient.Citation[16] To obtain tannins with a high purity degree, each tincture was saturated with ammonium sulfate. The formed precipitate was separated by filtration through a 0.45 μm filter and dried. Then it was purified by distillation with propan-2-one. The obtained residuum was washed with ethoxyethane and dried again. For quantitative determination solid was resolved in cold water.Citation[16] Tannins were determined by cold titration with KMnO4 solution in the presence of indigo carmine as an indicator. Briefly, into a 100 mL flask were introduced 50 mL indigo carmine 0.15% and 2 mL sample. The mixture was titrated with potassium permanganate 0.01 N, until a yellow color appeared. The volume of potassium permanganate used for titration was noted V1. The same procedure was used for a tartaric acid solution (5 g/L, solved in ethanol 10%). The volume of potassium permanganate used for titration was noted V2. The tannins content, n, (mg/mL) was determined with EquationEq. (2):
Measurement of the DPPH Radical-Scavenging Activity
The DPPH radical-scavenging activity was assessed using an improved spectrophotometric method.Citation[17] Briefly, DPPH was dissolved in methanol (10-4M) and 2.9 mL of the solution was mixed with 0.1 mL sample (40 mg/mL) in 4 mL quartz vats (1-cm pathlength). The absorbance modifications were followed at 517 nm, by the means of an Ultrospec III (Pharmacia-LKB, Uppsala, Sweden) spectrophotometer, until a “plateau” was reached (1 min to 8 h, depending on the extract). Each experiment was repeated five times. The residual DPPH concentration was determined from the absorbance (A) values based on a calibration relation (CDPPH = (A-1.006)/10970). The half-life time (t1/2, s) of the DPPH solution was determined for each added tincture. The antiradical activity (AA, mol/L) expressed as BHT equivalent concentration was calculated using the following formulaCitation[18]: AA = (2.3/t1/2)0.8.
In Situ Antioxidant Activity Testing
From the DPPH measurements, the quantity of the extract expected to have the same antioxidant activity as 75 mg BHT/kg fat was evaluated (Romanian legislation allows maximum 100 mg BHT/kg fat) and it was found to be about 0.5 mL/kg fat. Corresponding amounts were introduced in unrefined sunflower oil and in lard, provided directly by the producer without any addition of synthetic antioxidants. To ensure fluidity, lard was heated at 40oC. Portions of 100 mL samples were placed in 150 mL identical Berzelius flasks and then stirred for five minutes in an ultrasonic bath for better dispersion. Then they were exposed for five hours of daylight, exposure in order to initiate oxidation.
The antioxidant activity was tested on vegetable and animal fats using an accelerated oxidation test at 110°C for 100 hours, in a ventilated oven. The peroxide value (AOCS Cd 8b-90 (02)) and the specific UV absorbance (AOCS Ch 5–91 (01)) were evaluated. Samples were taken after 12, 36, 60, and 100 hours. For absorbance determination, an Ultrospec III spectrophotometer from Pharmacia-LKB (Uppsala, Sweden) was used. For UV spectra, 10 μL sunflower oil and respectively 3 μL lard were diluted in 3 mL isooctane in a quartz vat (1 cm pathlenghth). The results were compared to those for 75 mg BHT/kg added fat. Samples of unrefined sunflower oil and lard with no antioxidants were submitted to the same procedure.
Antibacterial Activity Testing
Before the analysis, bacterial strains were cultured on tryptic soy agar under aerobic conditions for 24 h at 37°C, except for Streptococcus pyogenes ATCC 49399, which was cultivated on tryptic soy agar with addition of 5% sterile defibrinated sheep blood. The yeast-like fungal strain Candida albicans was cultivated on Sabouraud 2% (m/v)-glucose agar under aerobic conditions for 48 h at 37°C.
The antibacterial activity of the extract was estimated using the disk agar diffusion method according to Kirby-Bauer test.Citation[19] Briefly, inocula of bacterial cells and yeast-like fungal blastospores were suspended in sterile physiological saline until the density of the test suspension matched the turbidity standard, which was equivalent to a concentration of 3.0 × 108/mL (McFarland Standard, BioMérieux, Marcy l'Étoile, France). Petri dishes (d = 9 cm) with Müller-Hinton agar (for bacterial species) or Sabouraud-agar (for yeast-like fungi) were inoculated with 1 mL of microbial suspension. The suspension was spread over the surface of the agar plates using a sterile 1 mL syringe to ensure complete coverage. Plates were left for 5 min before excess fluid was removed using a sterile pipette. Then, the inoculated Petri dishes were dried at room temperature for a maximum of 20 min.
Sterile paper discs impregnated with 80 μL of undiluted herbal extract were then aseptically applied to the surface of each of the inoculated plates, in a central position. Sterile forceps were used to place the antimicrobial discs on the plates and each disc was gently pressed to ensure even contact with the surface of the medium. Control discs impregnated with antimicrobial substances were also placed into the inoculated Petri dishes, at well-spaced intervals. Gentamicin, ofloxacin, cloramfenicol, penicillin, erytromycin and oxacillin were used as reference antibacterial and nistatin and itraconazole as reference antifungal substances. Inoculated plates were incubated for 20 h at 37°C for bacterial species, and 48 h for C. albicans. Inhibition zones were expressed in mm as the diameters of clear zones around discs. The results of antimicrobial activity were expressed as the mean value of three independent analyses.
RESULTS AND DISCUSSION
DPPH Radical-Scavenging Activity and Phenolic Compounds Content
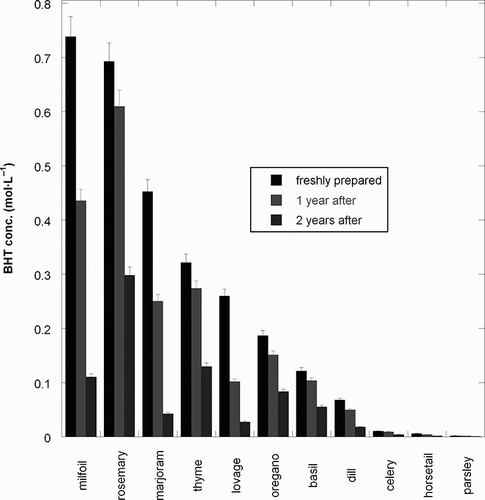
The DPPH assay is based on the reaction between the stable free radical of DPPH (1,1-diphenyl-2-picrylhydrazyl) and molecules that can donate hydrogen atoms (such as most antioxidants). As a result a stable non-radical form of the DPPH was obtained (1,1-diphenyl-2-picrylhydrazine), with simultaneous change of the violet color to pale yellow due to the picryl group present in solution. The higher the antiradical activity of an extract, the faster would be the discoloration of DPPH solution.Citation[18] The evolution of the antiradical activity for the studied extracts, over a two year period (which is exactly the shelf life of tinctures), is shown in .
Due to the complex composition of plant extracts, a single/substance/single-assay produces relative results.Citation[20] Therefore, antioxidant activities of those studied in this paper were also determined using the chemiluminescence method involving luminol. The measurements showed similar antioxidant properties.Citation[21] In the freshly prepared tinctures, the most effective was that of milfoil one, followed by rosemary, marjoram, thyme, lovage, oregano, and basil. Dill, celery, horsetail and parsley had very low antiradical activities.
The antocianes and tannins content for the eleven freshly prepared tinctures are given in . The highest antocianes content was found in celery tincture and the smallest in dill and milfoil tinctures. The tannins content in rosemary tincture was almost double compared with oregano and marjoram tinctures and much higher than for the other tinctures. Parsley tincture had the smallest quantity of tannins. High values of antioxidant activity can be correlated with high phenolic compounds concentration.
Table 1 Antocianes and tannins content
After one year, the antiradical activity of the tinctures decreased more or less depending on the type of plant. Rosemary, thyme, oregano and basil exhibited little variation. The highest variation of the antiradical activity was noted for milfoil, followed by thyme and marjoram.
After two years, the activity of all tinctures decreased to less than a half comparing to the initial ones. The most active was the rosemary tincture followed by thyme, milfoil, and oregano.
The purpose of this study was to find a mixed tincture from spices with an aromatic effect, and with high antioxidant/antiradical activity. Based on the results shown in , tinctures of dill, celery, horsetail, and parsley were eliminated. A mixture of tinctures from milfoil, rosemary, marjoram, thyme, lovage, oregano and basil was further studied. These tinctures were chosen because of their high antioxidant activity (milfoil and rosemary tinctures), or due to their time constancy, especially during the first year.
The mixture was prepared in two ways and checked: (a) mixing equal amounts of tinctures of the seven selected plants; (b) obtaining directly a tincture from equal parts of the seven dried plants. The antiradical activity of the two mixtures, determined with the DPPH method as described previously, is summarized in . Both mixtures had rich and pleasant aromas, but the antiradical activity of the mixture obtained by method (b) was twice that of the mixture (a). Thus, further research was done on the mixture obtained by method (b).
Table 2 Antioxidant activity (AA) of the extracts obtained from milfoil, rosemary, marjoram, thyme, lovage, oregano and basil
In Situ Antioxidant Activity
Peroxide value (PV)
The peroxide value provides indication about the first oxidation step, the formation of hydroperoxides.Citation[15] The change in PV of sunflower oil and lard, submitted to 110°C heating, with and without added antioxidant is given in . Significant variations in PV values were noticed even during the first 12 h. During the first period, the PV is expected to increase because of fatty acids peroxidation. After that, the PV fluctuated because of the instability of the formed hydroperoxides at high temperatures. From is clear that both the mixture and the BHT favorably influenced the peroxide formation in sunflower oil during the first hours.
Table 3 PV for the analyzed fats
The antioxidant effect of BHT is manifested only in the late stages of the oxidation. In the case of lard, both the mixture and BHT were useful in retarding the oxidation process. The mixture was displaying even a better effect than the synthetic antioxidant during the last stages of oxidation. The small PV after prolonged oxidation period is due to the fact that most of the hydroperoxides had already been transformed in advanced oxidation products.Citation[22]
Specific UV absorbance
By monitoring the UV spectra, useful information can be obtained regarding the fatty acids oxidation. Some products of the oxidation process showed maximum absorption at a certain wavelength. Based on the absorbance values (Eλ), the specific absorbance (Kλ), at 232 and 270 nm, corresponding to conjugated dienes (K232), and trienes (K270) were calculated using the following formulaCitation[23]:
where “c” is the concentration (g/100 mL); and “l” is the path length. The Kλ values at 232 and 270 nm are shown in . It can be noticed that in the case of the sunflower oil, both BHT and the herbal extract showed little influence on the formation of conjugated dienes and trienes. However, their influence was significant in the case of lard. High values for K232 and K270 were obtained after 60 h, which were followed by small values at the end of the experiment (after 100 h). The decrease of the specific absorbance values may be correlated with polymeric film formation.
Table 4 K232 and K270 values
Antibacterial activity
The product so formulated exerted bactericidal activity against bacterial species Staphylococcus aureus, Streptococcus pyogenes and Escherichia coli, and also fungicidal activity against the yeast-like fungus Candida albicans (). The largest inhibition zones were noticed against S. pyogenes (from 18 to 20 mm), and against S. aureus (from 17.5 to 19 mm). Smaller inhibition zones were recorded for the studied extract against E. coli (from 13 to 16). Strong fungicidal activity was shown by products against C. albicans, with the inhibition zones similar to S. aureus (from 17.5 to 19 mm).
Table 5 Antimicrobial activity of the herbal extract compared to antibacterial and antifungal substances
The results suggested that antibacterial activity expressed against the Gram-positive pathogenic bacterial strains Staphylococcus aureus, Streptococcus pyogenes was comparable to the antimicrobial activity of the reference antibacterial substances. Antibacterial activity against Escherichia coli, a Gram-negative bacterium, was also detected, but at a much reduced level (half of the antibacterial activity exerted by the reference substances). Also, the strong fungicidal activity of the herbal extract was found to be comparable to that of the reference substances.
CONCLUSION
The herbal extract obtained from equal amounts of milfoil, rosemary, marjoram, thyme, lovage, oregano and basil displayed an antioxidant activity comparable to BHT, both on saturated and unsaturated fats. The plant mixture also rendered a remarkable antimicrobial activity against Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Candida albicans. Taking also into account its rich and pleasant aroma, this extract might be recommended as a natural additive with antioxidant, antimicrobial and aromatic properties that may be used to replace synthetic antioxidants in the food products.
REFERENCES
- Yamazaky , E. , Inagaki , M. , Kurita , O. and Inoue , T. 2007 . Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit . Food Chemistry , 100 : 171 – 177 .
- Ssonko , U.L. and Wenshui , X. 2005 . Food Phenolics, Pros and Cons: A Review . Food Reviews International , 21 ( 4 ) : 367 – 388 .
- Mizutani , T. , Nomura , H. , Nakanishi , K. and Fujita , S. 1987 . Hepato-toxicity of butylated hydroxytoluene and its analogs in mice depleted of hepatic glutathione . Toxicology and Applied .Pharmacology , 87 : 166 – 176 .
- Williams , G.M. , Maeura , Y. and Weisburger , J.H. 1983 . Simultaneous inhibition of liver carcinogenicity and enhancement of bladder carcinogenicity of N-2-fluorenylacetamide by butylated hydroxytoluene . Cancer Letters , 19 : 55 – 60 .
- Guillen , M.D. , Cabo , N. and Burillo , J. 1996 . Characterisation of the essential oils of some cultivated aromatics. Plants of industrial interest . Journal of the Science of Food and Agriculture , 70 : 359 – 363 .
- Cuvelier , M.E. , Berset , C. and Richard , H. 2001 . Use of a new test for determining comparative antioxidant activity of BHA, BHT, α- and γ-tocopherols and extracts from rosemary and sage . Science des Aliments , 10 : 797 – 806 .
- Baratta , M.T. , Dorman , H.J. and Deans , S. 1998 . Antimicrobial and antioxidant properties of some commercial essential oils . Flavor and Fragrance Journal , 13 : 235 – 244 .
- Tang , S.Y. , Whiteman , M. , Peng , Z.F. , Jenner , A. , Yong , E.L. and Halliwell , B. 2004 . Characterization of antioxidant and antiglycation properties and isolation of active ingredients from traditional Chinese medicines . Free Radical Biology and Medicine , 36 ( 12 ) : 1575 – 1587 .
- Szabo , M.R. , Idiţoiu , C. and Dincă , N. 2005 . Evaluation of antiradical activity in natural aqueous extracts by chemiluminescence . Proceedings of the 12th Symposium on Analytical and Environmental Problems . September 26 2005 , Szeged, Hungary. Edited by: Galbacs , Z . pp. 58 – 62 . Szeged : SZAB .
- Szabo , M.R. , Idiţoiu , C. , Dincă , N. and Chambre , D. 2005 . Antioxidant activity analysis of some biocolloids from plants . Proceedings of the 8th Symposium of Colloid and Surface Chemistry . June 2–4 2005 , Galaţi, România. pp. 40 – 44 . Galaţi : Academica .
- Çelik , S.E. , Özyürek , M. , Altun , M. , Bektaçoălu , B. , Güçlü , K. , Berker , K.I. , Özgökçe , F. Apak’ , R. 2008 . Antioxidant Capacities of Herbal Plants Used in the Manufacture of Van Herby Cheese: ‘Otlu Peynir’ . International Journal of Food Properties , 11 ( 4 ) : 747 – 761 .
- Sartoratto , A. , Machado , A.L.M. , Delarmelina , C. , Figueira , G. M. , Duarte , M.C.T. and Rehder , V.L.G. 2004 . Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil . Brazilian Journal of Microbiology , 35 : 275 – 280 .
- Tantatoui-Elaraki , A. and Beraoud , L. 1994 . Inhibition of growth and aflatoxin production in Aspergillus parasiticus by essential oils of selected plant materials. Journal of Environmental Pathology . Toxicology and Oncology , 13 : 67 – 72 .
- Rahman , M. S. , Al-Sheibani , H. I. , Al-Riziqi , M. H. , Mothershaw , A. , Guizani , N. and Bengtsson , G. 2006 . Assessment of the anti-microbial activity of dried garlic powders produced by different methods of drying . International Journal of Food Properties , 9 ( 3 ) : 503 – 513 .
- Banu , C. 2000 . “ Aromatizanţi naturali ” . In Aditivi Şi ingrediente pentru industria alimentară , 413 Bucureşti : Tehnică .
- Ianculov , I. and Modoran , D. 1997 . “ Caracterizarea polifenolilor existenţi în unele specii vegetale ” . In Cercetări Ştiinţifice, Seria III: Procese Şi Tehnologii Alimentare , 102 – 110 . Timisoara : Agroprint .
- Szabo , M.R. , Idiţoiu , C. , Chambre , D. and Lupea , A.X. 2007 . Improved DPPH determination for antioxidant activity spectrophotometric assay . Chemical Papers , 61 ( 3 ) : 214 – 216 .
- Szabo , M.R. , Iditoiu , C. and Chambre , D. 2006 . New method for the calculus of the antioxidant activity in the DPPH spectrophotometric asssay. Scientific and Technical Bulletin of Aurel Vlaicu University, Series: Chemistry . Food Science & Engineering , 11 : 122 – 125 .
- Bauer , A.W. , Kirby , W.M.M. , Sherris , J.C. and Turck , M. 1966 . Antibiotic susceptibility testing by a standardized single disc method . American Journal of Clinical Pathology , 45 : 493 – 496 .
- Eminagaoglu , O. , Tepe , B. , Yumrutas , O. , Akpulat , H.A. , Daferera , D. , Inouye , S. , Takizawa , T. and Yamaguchi , H. 2001 . Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact . The Journal of Antimicrobial Chemotherapy , 47 : 565 – 573 .
- Szabo , M.R. , Idiţoiu , C. , Chambre , D. and Lupea , A.X. 2008 . Antiradical activity of some plants used in the Romanian cuisine . International Journal of Food Properties , 2 : 330 – 338 .
- Farag , S.R. , Mahmoudi , E.A. and Basuny , A.M. 2007 . Use crude oil leaf juice as a natural antioxidant for the stability of sunflower oil during heating . International Journal of Food Science and Technology , 42 : 107 – 115 .
- Vieira , T. and Regitano-D'Arce , M. 1998 . Stability of oils heated by microwave: UV-spectrophotometric evaluation . Ciência e Tecnologia de Alimentos , 18 ( 4 ) : 433 – 437 .