Abstract
The relationship between elevated raw milk somatic cell count (SCC) and casein micelle dimension was investigated by transmission electron microscopy (TEM). Milk samples collected from the dairy cattle with three different levels of SCC (<200,000, 200,000 to 800,000, and >800,000 cells/ml) were studied by TEM. The results indicated that an increase in SCC resulted in a decrease in the casein micelle size with an increase in their aggregation. The present research supported the hypothesis that elevated proteolytic activity, reduced secretary ability of the mammary glands, lower electrostatic and steric repulsion as well as different mineral contents of mastitic milk could affect casein micelle properties.
INTRODUCTION
Mastitis is the inflammation of the mammary glands, which results in an elevation of leukocytes (somatic cells) to engulf and destroy invasive bacteria, hence counting the somatic cells is an effective way of monitoring subclinical mastitis.Citation[1 Citation,2] During mastitis because of the lower synthetic ability of mammary glands, increased permeability of the blood-milk barrier and higher enzymatic activity, the concentration of different components of milk such as proteins, lactose, fat, α-lactalbumin, β-lactoglobulin, calcium and potassium decrease, whereas serum albumin, lactoferrin, immunoglobulins, sodium, and chloride, which lead to increased electrical conductivity as a diagnostic tool for intramammary infection increase.Citation[1 Citation[3 Citation[4 Citation[5 Citation6]
The lysosome of somatic cells contains a wide range of active hydrolytic enzymes like cathepsin B, cathepsin D, cathepsin G, elastase, which aid in destruction of bacteria.Citation[2 Citation,7] Mastitis can also lead to an increase in the concentration of plasmin and plasminogen from 0.18 to 0.37 mg/l and from 0.85 to 1.48 mg/l, respectively, as SCC increases from less than 250000 to more than 1000000 cells/ml.Citation[8 Citation,9] Plasmin hydrolyzes αs1-, αs2-casein and β-casein; therefore, elevated proteolytic activity is associated with a decrease in casein as a percentage of total protein and results in a deterioration of the coagulation properties of milk as well as decreasing milk's ability to withstand thermal processing.Citation[10 Citation–12]
The plasmin system may have two additional adverse effects on dairy processing. First, the activity of the enzyme continues considerably during cold storage. This leads to changes in the coagulation properties of milk; second, plasmin activity remains even after severe thermal processing.Citation[10]
Higher level of proteolytic enzymes and other compositional changes in mastitic milk can deteriorate the quality of dairy products,Citation[7] for instance, in UHT milk they may lead to age gelation as the consequence of disaggregation of casein micelles and undesirable bitterness due to released hydrophobic peptides from casein. Most researchers indicated the effect of high SCC milk on higher casein loss into whey, slower rate of curd formation, lower cheese yield and inferior textural properties of final product, which play a key role in consumer acceptance.Citation[13 Citation[14 Citation15]
Casein micelles, the colloidal particles of milk, are stabilized from aggregating by steric and electrostatic repulsion, and they are essential for the production of flocculated and gelled products such as yogurt, cheese, and ice-cream.Citation[16 Citation[17 Citation18] Although the properties of casein micelles largely determine rheological properties of dairy products and their stability during commercial processing operations such as pasteurization, sterilization, concentration, cheese making, there is negligible reported article on the effects of the health status of animals on casein micelle properties. Since the average size and distribution of casein micelles is a major factor determining textural properties and water holding capacity of fermented dairy products, factors that affect the casein micelle size should be carefully characterized.Citation[17 Citation,19 Citation,20] Therefore, the main purpose of this research was to study the relationship between elevated SCC and casein micelle size by TEM.
MATERIALS AND METHODS
Milk Collection
Individual quarter milk samples with three different somatic cell levels (Low: SCC < 200,000, Medium: 200,000 < SCC < 800,000 and High: SCC > 800,000 cells/ml) were collected.Citation[21] The SCC was analyzed, using a Fossomatic cell counter (Foss Electric, Denmark).Citation[22]
Specimen Preparation
Whole milk samples were skimmed using a centrifuge (Kokusan Co., Japan) at 6190 × g for 20 min at 4°C followed by separation of casein micelles by centrifuge (Beckman Inst, Inc, Palo Alto, Ca., USA) at 48,000 × g for 20 min at 2°C.
Transmission Electron Microscopy
Specimens were prepared for transmission electron microscopy by routine negative staining method. Milk samples were fixed by adding glutaraldehyde to milk (1:7, V/V) followed by diluting in a ratio of 1:50 with 0.01 M calcium chloride. For negative staining, a drop of diluted suspension was placed on formvar-coated electron microscopy grids for 1 min and a drop of phosphotungstic acid (2% at pH 7.2) was added to the milk. The grids were air dried and subsequently examined by electron microscope.Citation[23 Citation[24 Citation[25 Citation26] TEM images were obtained using a Phillips EM-208 TEM with an accelerating voltage of 100 kV at a magnification of 200,000 x. Exposure times for the bright field TEM images were 0.6–0.7 sec.
Statistical Analysis
Data were analyzed by one-way Analysis of Variance (ANOVA) followed by Duncan's New Multiple Range Test to determine significant differences among the mean values. Statistical analysis was conducted using SPSS 12.0 software (SPSS INC., Chicago, IL, USA) to determine significant effect (p < 0.05 and p < 0.01) of the different treatments.Citation[27]
RESULTS AND DISCUSSION
The microstructure of casein micelles for three different somatic cell levels was examined by TEM as shown in . The casein micelles in low SCC samples (SCC < 200,000 cells/ml) observed in individual state and somehow spherical, and diameter of micelles was 65 ± 3 nm (). The casein micelle size of medium SCC samples (200,000 < SCC < 800,000 cells/ml) was 29 ± 0.7 nm ().
Figure 1 Transmission electron micrographs of casein micelles from milk with 200,000 < SCC cells/ml. Bar: 100 nm (A, B).
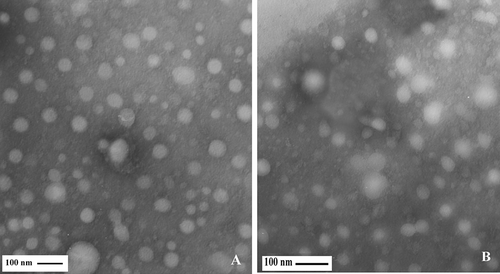
Figure 2 Electron micrograph of casein micelles in milk with 200,000 < SCC < 800,000 cells/ml. Bar in (A, B, C): 100 nm, Bar in D: 50 nm.
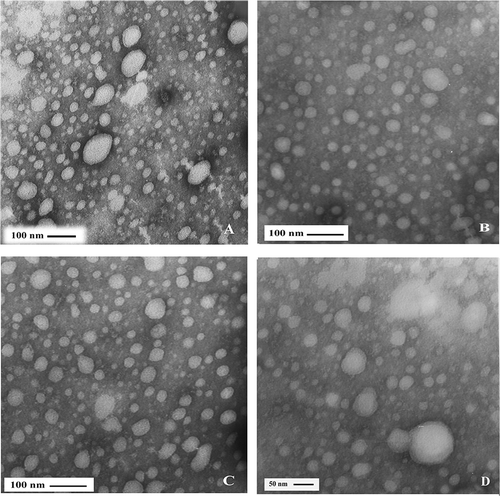
Figure 3 Electron micrograph of casein micelles in milk with more than 800,000 somatic cell counts cells/ml. Bar (A, B):100 nm.
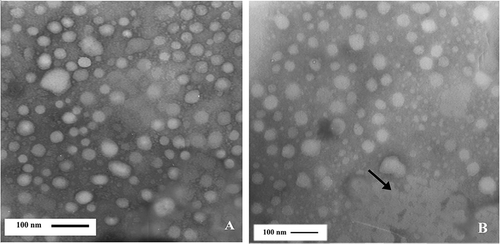
TEM micrographs revealed that the diameter of casein micelles in high SCC milk (SCC > 800,000 cells/ml) was 28 ± 0.9 nm. The aggregation of casein micelles in high SCC specimens was more than medium and low SCC milk (). The size of casein micelles in low SCC milk was significantly different (p < 0.01) from medium and high SCC milk samples, but no significant differences (p > 0.05) were found between the high and medium samples.
The elevation of somatic cell count of milk affected casein micelle microstructure and properties due to a destruction in the ability of secretory tissue to synthesize milk components, increased permeability of the blood-milk barrier, which may cause the reduction of different kinds of minerals (calcium, potassium, zinc), and hydrolysis of casein particles by increasing proteolytic enzymes such as plasmin, neutral, and acidic proteases.Citation[1 Citation,9]
The previous studies revealed that milk contains a complete plasmin system: plasmin, plasminogen, plasminogen activators (PAs) and inhibitors of PAs, and of plasmin. This system enters through blood and plasmin activity increases during mastitis. Plasmin is highly specific for Lys-x and Arg-x bonds, which cleaves Lys-x much faster than Arg-x bonds and the preferred substrates for plasmin are αs2-casein and β-casein as well as αs1-casein.Citation[9 Citation,28]
Higher contents of neutral and acidic proteases such as cathepsin G, cathepsin D, cathepsin B, elastase, and collagenase have been reported in mastitic milk. Citation[7 Citation,15] Cathepsin G is a neutral serine proteinase, which cleaves αs1-casein and β-casein at least at 16 and 21 sites, respectively.Citation[29] Cathepsin D (EC 3.4.23.5) as an aspartic proteinase and lysosomal enzyme hydrolyses αs1-, αs2 - and β–caseins. It can also produce para-κ-casein from κ-casein and coagulates milk at high concentrations.Citation[30] Lysosomes of somatic cells contain many cysteine proteinases like cathepsin B. The proteolytic specificity of bovine cathepsin B against αs1 and β–casein has been determined and enzyme cleaves β-casein and αs1-casein at sites 32 and 35, respectively.Citation[31] The other protease from polymorphonuclear leukocytes is elastase. It is one of the predominant enzymes in somatic cells of mastitic milk, which hydrolyses milk proteins. Elastase cleaves twenty-five sites of αs1-casein.Citation[32] It has a broad cleavage on β-casein at several sites.Citation[2]
Hydrolysis of casein may reduce some of the repulsive forces like electrostatic and steric repulsion. Previous studies have reported plasmin as the most important protease in mastitic milk; therefore, it is likely that plasmin causes most of the changes in casein micelle properties. Although plasmin did not appear to degrade κ-casein and it is unlikely that the enzyme removes any of the κ-casein “hairs,” it can affect other caseins present on the micelle surface, as it has been estimated that κ-casein may only cover one-third of the casein surface. The action of plasmin could remove negatively charged peptides, which would reduce charge repulsion between caseins.Citation[11] Hydrolysis of casein by plasmin reduces the zeta-potential of the casein micelles (from approximately −19 to −16 mV), which may promote micellar aggregation or changes in casein micelle microstructure.Citation[33]
CONCLUSION
The results indicated that mastitis or elevated SCC is associated with a decrease in casein micelle size and an increase in their aggregation especially in high SCC milk samples. It could be hypothesized that the decrease of casein micelle size can also affect aggregation of casein micelles; since previous studies suggested that zeta-potential decreased along with decreasing casein micelle size and smaller micelles could aggregate more readily than larger casein micelles.[34] Additionally, the origin of changes in casein micelle microstructure might be due to the reduced secretory ability of mammary glands, different mineral contents, higher enzymatic activity and lower electrostatic and steric repulsion in medium and high SCC milk samples, which may result in a lower size and a higher tendency to aggregate in mastitic milk.
ACKNOWLEDGMENTS
The authors would like to thank Iran Dairy Industries Company (Pegah) for financial support of this research and Dr. Kambiz Shamsi for his valuable guidance.
REFERENCES
- Harmon , R.J . 1994 . Physiology of Mastitis and Factors Affecting Somatic Cell Count . Journal of Dairy Science , 77 : 2103 – 2112 .
- Considine , T. , Healy , A. , Kelly , A.L. and McSweeney , P.L.H. 1999 . Proteolytic Specificity of Elastase on Bovine β-casein . Food Chemistry , 66 : 463 – 470 .
- Bruckmaier , R.M. , Ontsouka , C.E. and Blum , J.W. 2004 . Fractionized Milk Composition in Dairy Cows with Subclinical Mastitis . Veterinarni Medicina , 49 : 283 – 290 .
- Henningsson , M. , Ostergren , K. and Dejmek , P. 2005 . The Electrical Conductivity of Milk-The Effect of Dilution and Temperature . International Journal of Food Properties , 8 : 15 – 22 .
- Moslehishad , M. , Ezzatpanah , H. and Aminafshar , M. October 24–26 2007 . Effects of Somatic Cell Count on Chemical and Microbial Quality of Raw Milk , 2nd , October 24–26 , Istanbul, , Turkey : International Congress on Food and Nutrition .
- Therdthai , N. and Zhou , W. 2002 . Hybrid Neural Modeling of the Electrical Conductivity Property of Recombined Milk . International Journal of Food Properties , 5 ( 1 ) : 49 – 61 .
- Roux , Y.L.E. , Laurent , F. and Moussaoui , F. 2003 . Polymorphonuclear Proteolytic Activity and Milk Composition Change . Veterinary Research , 34 : 629 – 645 .
- Bastian , E.D. and Brown , R.J. 1996 . Plasmin in Milk and Dairy Products: An Update . International Dairy Journal , 6 : 435 – 457 .
- Chen , L. , Daniel , R.M. and Coolbear , T. 2003 . Detection and Impact of Protease and Lipase Activities in Milk and Milk powders . International Dairy Journal , 13 : 255 – 275 .
- Ballou , L.U. , Pasquin , M. and Bremel , R.D. 1995 . Factors Affecting Herd Milk Composition and Milk Plasmin at Four Levels of Somatic Cell Counts . Journal of Dairy Science , 78 ( 10 ) : 2186 – 2195 .
- Srinivasan , M. and Lucey , J.A. 2002 . Effects of Added Plasmin on the Formation and Rheological Properties of Rennet-induced Skim Milk Gels . Journal of Dairy Science , 85 : 1070 – 1078 .
- Theodorou , G. , Kominakis , A. and Rogdakis , E. 2007 . Politis, I. Factors Affecting the Plasmin-Plasminogen System in Milk Obtained from Three Greek Dairy Sheep Breeds with Major Differences in Milk Production Capacity . Journal of Dairy Science , 90 : 3263 – 3269 .
- Fernandes , A.M. , Bovo , F. , Moretti , T.S. , Rosim , R. E. , Lima , C.G. and Oliveira , C.A.F. 2008 . Casein Fractions of Ultra High Temperature Milk with Different Somatic Cell Counts . Pesquisa Agropecuária Brasileira , 43 ( 1 ) : 149 – 152 .
- Guizani , N. , Al-Attabi , Z. , Kasapis , S. and Gaafar , O.M. 2006 . Ripening Profile of Semi-Hard Standard Goat Cheese Made From Pasteurized Milk . International Journal of Food Properties , 9 ( 3 ) : 523 – 532 .
- Verdi , R. J. and Barbano , D.M. 1991 . Properties of Proteases from Milk Somatic Cells and Blood Leukocytes . Journal of Dairy Science , 74 : 2077 – 2081 .
- Karlsson , A.O. , Ipsen , R. , Schrader , K. and Ardo , Y. 2005 . Relationship between Physical Properties of Casein Micelles and Rheology of Skim Milk Concentrate . Journal of Dairy Science , 88 : 3784 – 3797 .
- Srilaorkul , S. , Ozimek , L. , Ooraikul , B. , Hadziyev , D. and Wolfe , F. 1999 . Effect of Ultrafiltration of Skim Milk on Casein Micelle Size Distribution in Retentate . Journal of Dairy Science , 74 : 50 – 57 .
- Tuinier , R. and de Kruif , C.G. 2002 . Stability of Casein Micelles in Milk . Journal of Chemical Physics , 117 ( 3 ) : 1290 – 1295 .
- Fox , P.F. 2003 . “ The major constituents of milk ” . In Dairy Processing- Improving Quality , Edited by: Smit , G. 24 – 25 . Boca Raton : CRC Press and Woodhead Publishing Limited .
- Harte , M. , Luedecke , L. O. , Swanson , B.G. and Barbosa-Cánovas , G. V. June 2002 . Disrupting effect of high hydrostatic pressure on casein micelle isolates , June , California : Annual Meeting and Food Expo-Anaheim .
- Grace , V. , Houghtby , G. A. , Rudnick , H. , Whaley , K and Lindamood , J. 1992 . Standard Methods for the Examination of Dairy Products , Edited by: Marshall , R.T. 59 – 84 . Washington, DC : American Public Health Association .
- Association of Official Analytical Chemists . 1990 . Official Methods of Analysis , 15th , Arlington, VA : AOAC Int .
- Morr , C.V. 1967 . Effect of Oxalate and Urea upon Ultracentrifugation Properties of Raw and Heated Skim Milk Casein Micelles . Journal of Dairy Science , 50 : 1744 – 1751 .
- Kalab , M. 1981 . Electron Microscopy of Milk Products: A Review of Techniques . Scanning Electron Microscopy , III : 453 – 472 .
- Harwalkar , V.R. , Allan-Wojtas , P. and Kalab , M. 1989 . Effect of Heating to 200°C on Casein Micelles in Milk: A Metal Shadowing and Negative Staining Electron Microscope Study . Food Microstructure , 8 : 217 – 224 .
- Needs , E.C. , Capellas , M. , Patricia Bland , A. , Manoj , P. , Macdougal , D. and Paul , G. 2000 . Comparison of Heat and Pressure Treatment of Skim Milk, Fortified with Whey Protein Concentrate, for Set Yogurt Preparation: Effect on Milk Proteins and Gel Structure . Journal of Dairy Research , 67 : 329 – 348 .
- SPSS . 2002 . Statistical Package for Social Science , Chicago, IL : SPSS Advanced Statistics .
- Fox , P.F. and Kelly , A.L. 2006 . Indigenous Enzymes in Milk: Overview and Historical Aspects - Part 1 . International Dairy Journal , 16 : 500 – 516 .
- Considine , T. , Healy , A. , Kelly , A.L. and McSweeney , P.L.H. 2002 . Proteolytic Specificity of Cathepsin G on Bovine α- and β-caseins . Food Chemistry , 79 : 59 – 67 .
- Hurley , M.J. , Larsen , L.B. , Kelly , A. L. and McSweeney , P.L.H. 2000 . The Milk Acid Proteinase Cathepsin D: a review . International Dairy Journal , 10 : 673 – 681 .
- Considine , T. , Healy , A. , Kelly , A. L. and McSweeney , P.L.H. 2004 . Hydrolysis of Bovine Caseins by Cathepsin B, a Cysteine Proteinase Indigenous to Milk . International Dairy Journal , 14 : 117 – 124 .
- Crudden , A. , Afoufa-Bastien , D. , Fox , F.P. , Brisson , G. and Kelly , A.L. 2005 . Effect of Hydrolysis of Casein by Plasmin on the Heat Stability of Milk . International Dairy Journal , 15 : 1017 – 1025 .
- Park , S.Y. , Niki , R. and Sano , Y. 1999 . Size Effects of Casein Micelles on Rennet Gels in the Presence of β-lactoglobulin . International Dairy Journal , 9 : 379 – 380 .