Abstract
Breadfruit starch samples were prepared after annealing at 45–60°C for 24 h and the relationship between the thermal properties and the retrogradation of starch was investigated using differential scanning calorimetry. The peak temperature (Tp) increased with increasing annealing temperature but enthalpy value was decreased with increasing annealing temperature. Twelve days after storage, according to the Avrami equation, the retrogradation rate of annealed breadfruit starch that estimated by the change of re-gelatinization enthalpy of starch was slowed when compared with the control. Furthermore, annealing treatments strongly affected the susceptibility of starch to enzymatic hydrolysis. These results showed that the increasing of Tp breadfruit starch by differences of annealing treatments could retard the retrogradation and resistant of breadfruit starch to enzymatic hydrolysis.
INTRODUCTION
Annealing is a process in which starch granules are heated in excess amounts of water at a temperature slightly below gelatinization temperature for a relatively long time. Tester et al.Citation[1] reported that annealing was restricted unless the moisture content exceeded 60% by weight of the mixture. Annealing decreases swelling power and solubility of starch,Citation2–4 delays gelatinization,Citation5–10 increases susceptibility to amylase,Citation[11] and changes pasting curves. Citation[10,Citation12]
Starch granules heated in excess water undergo an order-disorder phase transition, which is called gelatinization.Citation[13] This phase transition is associated with the diffusion of water into the granule, hydration and swelling of the starch granules, loss of crystallinity, and amylose leaching. As a consequence, swollen granules become embedded in a continuous matrix of entangled amylose molecules. On cooling, the complex composite sets as a viscoelastic gel when starch concentration is >6%. This change is called retrogradation. In this stage, amylose gel network develops relatively fast and remains unchanged during storage. Meanwhile gelation of amylopectin within the swollen granules is slow and progresses slowly during storage, resulting in increasing gel hardness.Citation14–16 However, reviewed the factors affecting starch retrogradation such as starch contains, temperature, sugar, lipids, salts, chemical and physical modification.Citation[13]
Retrogradation has been used to describe change in physical behavior following gelatinization. The changes due to association of starch chains as double helices, and variably ordered semi-crystalline arrays of these helices, as monitored by differential scanning calorimetry (DSC) to observe endothermic changes when structures are lost on heating.Citation17–19 Variation in molecular structure of starch can result in altered retrogradation behavior. Structural modification by means of chemical or physical modification of starch has been employed to alter the process of retrogradation.
Breadfruit (Artocarpus communis) starch is widely used to make a substitutes food called sukun in Indonesia. Sukun are eaten after roasting or boiling. Sukun can be served with cake and bread, and it requires a certain thermal properties for easy processing. The objective of this investigation was to determine of various annealing conditions, influence the gelatinization properties, retrogradation of breadfruit starch using differential scanning calorimetry (DSC) and susceptibility to enzymatic hydrolysis.
MATERIAL AND METHODS
Breadfruit starch was isolated from commercial breadfruit purchased in a local market, Malang, East Java, Indonesia. High-grade glucoamylase (glucoamylase activity: 41.9 units/mg) obtained from Rhizopus niveus and almost free of α-amylase was purchased from Amano Pharmaceutical Co., Ltd. (Nagoya, Japan). All chemicals were purchased from Wako Pure Chem. Ind. Ltd. (Osaka, Japan), unless otherwise noted.
Breadfruit starch was isolated from commercial breadfruit purchased in a local market by an alkaline steeping method. Breadfruits were slides and the slices were soaked in tap water for 3–4 h. The breadfruit slides were blended (1:4) with 0.02N NaOH solution using a Warring blender for 1 min. The slurry was filtered through the No. 400 sieve and the starch suspensions were stored at 4°C overnight. The supernatant and residue above the starch layer were discarded. The starch slurry was washed with distilled water until no alkali was detected and dried at 40°C in a drying oven. The starch passing through the No. 100 sieve was used in this work; its crude protein content (N × 6.25) was 0.32% on dry basis.
All starch slurries for annealing were prepared using water containing 0.02% sodium azide to prevent microbial contamination. Five gram of breadfruit starch was added to 50 ml of water and annealed at 45, 50, 55, and 60°C for 24 h. After cooling to room temperature, starch slurry was added to 1 L of anhydrous ethanol. The precipitated starch was collected on a G 3 glass filter pad, washed twice with anhydrous ethanol and once with acetone, and dried in a vacuum desiccator.
The DSC measurements were done using a Shimadzu DSC apparatus (Model DSC-60, Kyoto, Japan) that was controlled by TA-60 WS software and connected to a thermal analysis. The calorimeter was calibrated with indium (melting point = 156.7°C, ΔH = 27.6 J/g) and the reference used was liquid paraffin as reported by Morita et al.Citation[19] The sample (4–5 mg) in aluminum DSC pans were weighed, and deionized water was added to starch sample (dry solid) to make the ratio of water to starch of 2:1. After sealing, the pan was left for 1 h to allow the sample to mix and equilibrate at room temperature before heating. Then the sample was scanned (first scan) at a rate of 10°C per min from 30 to 125°C under nitrogen gas.
The sample pans of gelatinized starch were storage for 0 to 12 days at 22°C. Subsequently, the sample were equilibrated at room temperature for 1 h, and then rescanned (second scan) in the calorimeter from room temperature to 125°C at a rate of 10°C per min to measure the retrogradation temperature and enthalpy. Onset temperature (To ), peak temperature (Tp ) and the enthalpy for starch (ΔHg ) were measured to characterize the thermal properties of starch. Retrogradation of starch during storage was estimated from a DSC thermogram. The Avrami model was employed to describe the kinetics of starch retrogradation in the starch. The model can be expressed as Citation[20]:
where θ: is the fraction of uncrystallized starch at time t; nH0 and nHt are the enthalpy changes at time 0 and time t, respectively; nH∞ is the limiting enthalpy change; k is the rate constant; and n is the Avrami exponent. nH∞ was taken to be the limiting enthalpy change at infinite time (t → ∞) obtained from the plot of 1/nHt against 1/t.Citation[21] The rate constants (k) and exponents (n) for the starch (where nH0 was zero in this study) were obtained from linear regression of the retrogradation enthalpy data as:
Five hundred mg of a sample was immersed in 5 ml of acetate buffer and treated with the glucoamylase solution, which was at a final concentration of 200 units/ml. In the control, the same volume of buffer replaced the glucoamylase solutions. The samples were incubated at 37oC for various times with gentle stirring on shaker bath at 8 rpm and the reaction was stopped by the addition of 0.2 N HCl. The residual sample was separated by centrifugation at 2500 g for 10 min. The reducing sugar concentration of supernatant was assayed by the glucose oxidase-peroxidase method. Degree of hydrolysis (D.H.) was as follows:
Reducing sugar was assayed by the glucose oxidase-peroxidase method using glucose as a standard.Citation[22] Acid hydrolysis was carried out by treating starch with HCl (1 g starch mixed with 20 ml 1 N HCl) at 100oC for 2 h.
RESULTS AND DISCUSSIONS
The annealed of breadfruit starch were investigated by analyzing the gelatinization properties of the samples using DSC. The DSC curves of sample showed a main endothermic peak gelatinization (Tp ) at around 73.8–75.5oC. After annealed at 45, 50, 55, and 60oC for 24 h, the Tp of sample was increased compared to the control. After annealed at 55oC, the Tp became stationary until 75.5oC (). StuteCitation[10] and Kiseleva et al.Citation[23] reported that the increment and narrow peaks indicate melting of the crystallites and hydration of the granules are more homogenous in an annealed starch.
Figure 1 Thermal properties of gelatization of breadfruits starch. (○): Peak Temperature; and (●): gelatinization enthalpy value. Data are mean ± SD of three replication.
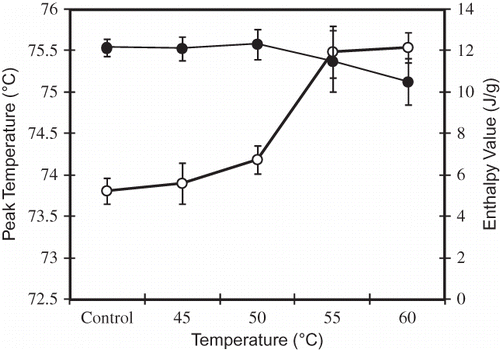
Breadfruits starch annealed at 45 and 50°C for 24 h had similar gelatinization enthalpy value (ΔHg ) than the breadfruit starch control (12 J/g) (). The decrease in enthalpy with annealing implies that the molecular order in the treated starch granules had increased. Starch annealed at 55 and 60°C had a lower enthalpy values, 11.51 and 10.52 J/g, respectively, than those of the starches annealed at <55°C, indicating increasing molecular rearrangement and partial melting may have occurred simultaneously. It could be found that the annealing did not only shift the peak melting temperature (higher) but also induced a shaper distribution of the DSC endothermic. It is believed that the rise of melting temperature, as well as the narrowing of the endothermic value, is related to enhancement of crystalline order.Citation23–25
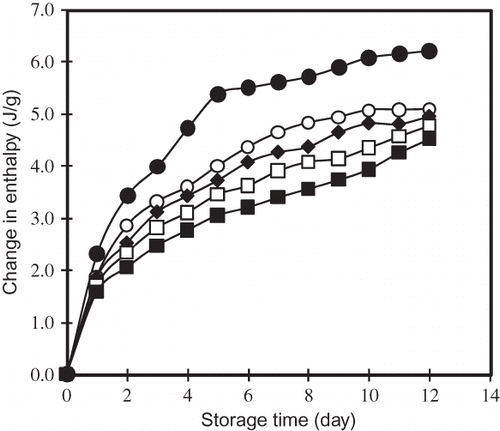
The enthalpy value of re-gelatinization of starch stored at 22°C during 0–12 days was observed in the range from 50 to 75°C. The peak is identified as the melting peak of amylopectin crystallites.Citation[26,Citation27] The ΔHt at 0 day of all the samples tested was undetectable after heating, because starch was completely gelatinized during heating. The enthalpy for melting of starch increased logarithmically with storage time (). The starch control showed a rapid increase in ΔH commonly at the first day of storage, resulting in stationer ΔH by the 6th day but after second day storage, the annealed starch showed a gradually increase in ΔH during storage. The annealed starch showed less ΔH than that of the control after 12 days of storage. In the course of storage, annealing treatment reduced greatly the value in the ΔH than that of the control. This result suggests that the retrogradation of starch was retarded after the annealing treatments.
Figure 2 Gelatinization enthalpy of retrogradated breadfruits starch after annealing as a function of storage at 22°C. (●): Control; (○): 45°C; (r): 50°C; (□): 55°C; and (▄): 60°C.
The Avrami model was found to give a reasonable description of starch retrogradation during the starch gelatinization, with a regression coefficient of 0.94–0.99 (), and ΔH∞ values were in the ranges of 4.44–7.40 (). The Avrami exponent (n) for retrogradation kinetics had a range of 0.68–0.74, but those different with the n value of wheat starch, which is in the range of 0.78–1.26.Citation[26] The difference in values between the breadfruits and wheat starch suggests that the mechanism for recrystallization of starch might be different.Citation[20] For the rate constant (k), the annealing of starch control increased the retrogradation rate by value of 0.4 day−1. The retrogradation rate of annealed starch was less than the retrogradation rates of the control and increased with the increasing of annealing treatment. This result suggests that the annealed starch is better at retarding the retrogradation of starch during storage than is the control. The increasing molecular rearrangement as discussed above might prevent the reorganization of amylopectin molecules during storage.
Figure 3 Plot of logarithmic fraction of cystallization [Log {−lnΔH ∞−ΔHt/ΔH ∞}] against logarithmic time (log t) during retrogradation of breadfruits starch. A: Control; B: 45°C; C: 50°C; D: 55°C; and E: 60°C.
![Figure 3 Plot of logarithmic fraction of cystallization [Log {−lnΔH ∞−ΔHt/ΔH ∞}] against logarithmic time (log t) during retrogradation of breadfruits starch. A: Control; B: 45°C; C: 50°C; D: 55°C; and E: 60°C.](/cms/asset/a2c7a065-cef5-499e-b2e6-38f2620c549a/ljfp_a_371486_o_f0003g.gif)
Table 1 Effect of differences annealing of breadfruit starch on temperature and enthalpy of retrogradation and Avrami parameters
shows the susceptibility of annealed breadfruits starch to hydrolysis of starch by glucoamylase. About 33% of breadfruits starch control in solution was hydrolyzed by the enzyme and hydrolysis did not proceed further. The limitation of hydrolysis may be due to the existence of phosphate groups attached to the glucosyl residue.Citation[28] The breadfruits starch prepared in this study contained about 165 μg of phosphorus per 100 mg of breadfruits starch.
Figure 4 Susceptibility of breadfruits starch after annealing to hydrolysis of starch by glucoamylase at 37°C. (●): Control; (○): 45°C; (◊): 50°C; (□): 55°C; and (▄): 60°C.
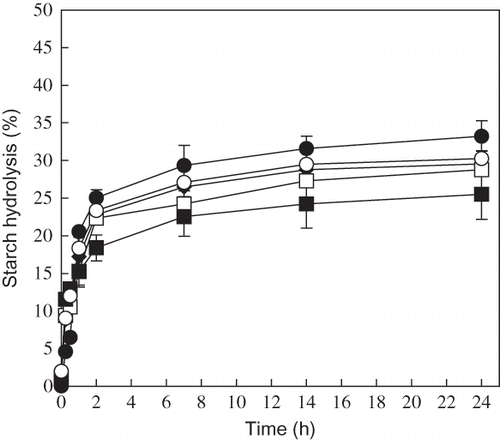
The hydrolysis of the annealed rapidly during the early stages of the reaction, and then was nearly stationary after 7 h. At 14 h of the reaction, the extent of hydrolysis ranged from 28–24% for 45–60°C. Among the sample annealed, enzymes susceptibilities of these samples rank in the order: control >45 > 50 > 55 > 60°C. The extent of hydrolysis was correlated negatively with annealing temperature (r2 = 0.85), suggesting that the extent of hydrolysis of the starch primarily depends on the annealing temperature of starch.
Annealed breadfruits starch affected enzyme susceptibility of starch at two levels: first, starch granules are restricted to swell during incubation at 60°C; second, re-organized of breadfruits starch during anneal more resistant to enzyme digestion than free amylose. Such behavior probably arises from structural characteristics that limit the access of the enzyme to glycosidic bonds.Citation[11] Susceptibility to enzymes may be related to characteristics of starch that affect accessibility to enzymes. Morrison et al.Citation[29] suggested that resistant starch granules have a more rigid crystalline layer than non-resistant wheat starch granules at the granular surface, therefore only the external glucosyl chain residues of amylopectin are accessible. During mild heating in water, starch granules swell somewhat at amorphous zones, so pores on the granule surface become soluble. Subsequently, amylose leaches from starch granules, and an enzyme can access to the granule interior. O'Brien and WongCitation[30] reported that the high resistance of amylolysis of potato starch was ascribed to its high percentage of double-helical chains formed by amylase and amylopectine, whereas that of amylomaize was attributed to a high percentage of inter-double-helical chain association.Citation[31] The high amylose content probably hindered the enzyme action by interaction among them and/or with amylopectine during hydrolysis.
CONCLUSION
The breadfruits starch annealed by different temperatures showed distinct changes on their thermal characteristics. The results indicated that annealed by temperature caused the increase of peak temperature, retarded the retrogradation of breadfruits starch during storage and retarded the enzyme hydrolysis of breadfruits starch. These findings can be important for elucidating the complicated thermal in modified breadfruits starch under various thermal processes.
REFERENCES
- Tester , R. F. , Debon , S. J. J. and Karkalas , J. 1998 . Annealing of wheat starch . Journal of Cereal Science , 28 : 259 – 272 .
- Eerlingen , R. C. , Jacobs , H. , Block , K. and Delcour , J. A. 1997 . Effects of hydrothermal treatments on the rheological properties of potato starch . Carbohydrate Research , 297 : 347 – 356 .
- Adebowale , K. O. and Lawal , O.S. 2002 . Effect of annealing and heat moisture conditioning on the physicochemical characteristics of Bambarra groundnut (Voandzeia subterranea) starch . Nahrung/Food , 46 : 311 – 316 .
- Gomes , A. M. M. , Mendes da silva , C. E. and Ricardo , N.M.P.S. 2005 . Effects of annealing on the physicochemical properties of fermented cassava starch (polvilho azedo) . Carbohydrate Polymers , 60 : 1 – 6 .
- Fisher , D. K. and Thompson , D. B. 1997 . Retrogradation of maize starch after thermal treatment within and above the gelatinization temperature range . Cereal Chemistry , 74 : 344 – 351 .
- Mahesh , G. , Balmeet , S. G. and Amarinder , S. B. 2008 . Gelatinization and X-ray Crystallography of Buckwheat Starch: Effect of Microwave and Annealing Treatments . International Journal Food Properties , 11 : 173 – 185 .
- Knutson , C. A. 1990 . Annealing of maize starches at elevated temperature . Cereal Chemistry , 67 : 376 – 384 .
- Krueger , B. R. , Walker , C. E. , Knutson , C. A. and Inglett , G. E. 1987 . Differential scanning calorimetry of raw and annealed starch isolated from normal and mutant maize genotypes . Cereal Chemistry , 64 : 187 – 190 .
- Seow , C. C. and Teo , C. H. 1993 . Annealing of granular rice starches interpretation of the effect on phase transitions associated with gelatinization . Starch/Staerke , 45 : 345 – 351 .
- Stute , R. 1992 . Hydrothermal modification of starches: The difference between annealing and heat/moisture-treatment . Starch/Staerke , 44 : 205 – 214 .
- Wang , W. J. , Powell , A. D. and Oates , C. G. 1997 . Effect of annealing on the hydrolysis of sago starch granules . Carbohydrate Polymers , 33 : 195 – 202 .
- Jacobs , H. , Eerlingen , R. C. , Clauwaert , W. and Delcour , J. A. 1995 . Influence of annealing on the pasting properties of starches from varying botanical sources . Cereal Chemistry , 72 : 480 – 487 .
- Hoover , R. 1995 . Starch retrogradation . Food Review International , 11 : 331 – 346 .
- Morris , V. J. 1990 . Starch gelation and retrogradation . Trends Food Science and Technology , 1 : 2 – 6 .
- Biliaderis , C. G. 1992 . Structures and phase transitions of starch in food systems . Food Technology , 46 : 98 – 109 .
- Koo , Min C. , Tae , Wha M. and Jae , Kun C. 2000 . Influence of annealing on gel properties of mung bean starch . Cereal chemistry , 77 : 567 – 571 .
- Aikawa , R. , Kawabata , A. and Nakamura , K. 2002 . Studies on Gelatinization and Retrogradation of Katakuri Starch (Erythronium Japonicum DECEN) by Differential Scanning Calorimetry (DSC) . Food Preservation Science , 28 : 299 – 305 .
- Kaoru , K. , Junko , M. , Takeshi , Y. and Tomoko , S. 2004 . A differential thermal analysis of the gelatinization and retrogradation of wheat starches with different amylopectin chain lengths . Carbohydrate Polymer , 58 : 71 – 77 .
- Morita , N. , Nakata , K. , Hamauzu , Z. and Oyosawa , I. 1996 . Effect of α-glucosyl rutin as improvers for wheat dough and breadbaking . Cereal Chemistry , 73 : 99 – 104 .
- McIver , R. G. , Axford , D. W. E. , Colwell , K. H. and Elton , G. A. H. 1968 . Kinetic study of the retrogradation of gelatinized starch . Journal of Science Food Agricultural , 19 : 560 – 563 .
- Mita , T. 1992 . Structure of potato starch paste in the aging process by the measurement of their dynamic moduli . Carbohydrate Polymers , 17 : 269 – 276 .
- Karkalas , J.J. 1985 . An improved enzymatic method for the determination of native and modified starch . Journal of the Science of Food and Agriculture , 36 : 1019 – 1027 .
- Kiseleva , V.I. , Tester , R.F. , Wasserman , L.A. , Krivandin , A.V. , Popov , A.A. and Yuryev , V.P. 2003 . Influence of growth temperature on the structure and thermodynamic parameters of barley starches . Carbohydrate Polymers , 51 : 407 – 415 .
- Genkina , K. N. , Noda , T. , Koltisheva , G.I. , Wasserman , L.A , Tester , R.F and Yuryev , V.P. 2003 . Effect of growth temperature on some structural properties of crystalline lamellae in starches extracted from sweet potatoes (Ayamurasaki and Sunnyred cultivars) . Starch/Starke , 55 : 350 – 357 .
- Genkina , K.N. , Wasserman , L.A. , Noda , T. , Tester , R.F. and Yuryev , V.P. 2004 . Effects of annealing on the polymorphic structure of starches from sweet potatoes (Ayamurasaki and Sunnyred cultivars) grown at various soil temperatures . Carbohydrate Research , 339 : 1093 – 1098 .
- Zhang , W. and Jackson , D.S. 1992 . Retrogradation behavior of wheat starch gels with differing molecular profiles . Journal of Food Science , 57 : 1429 – 1432 .
- Lai , V.M.F. , Lu , S. and Lii , C. 2000 . Molecular characteristic influencing retrogradation kinetics of rice amylopectins . Cereal Chemistry , 77 : 272 – 278 .
- Abe , J. , Takeda , Y. and Hizukuri , S. 1982 . Action of glucoamylase from Aspergillus niger on phosphorylated substrate . Biochimica et Biophysica Acta , 703 : 26 – 33 .
- Morrison , W.R. , Tester , R.F. and Gidley , N.J. 1994 . Properties of damaged starch granules. II. Crystallinity, molecular order and gelatinisation of ball-milled starches . Journal of Cereal Science , 19 : 209 – 217 .
- O'Brien , S. and Wang , Y.J. 2008 . Susceptibility of annealed starches to hydrolysis by α-amylase and glucoamylase . Carbohydrate Polymers , 72 : 597 – 607 .
- Kimura , A. and Robyt , J. F. 1995 . Reaction of enzymes with starch granules: Kinetics and products of the reaction with glucoamylase . Carbohydrate Research , 222 : 87 – 107 .