Abstract
The effects of pectin and guar gum on rheology, microstructure and creaming stability of 1% (w/v) egg yolk granule stabilized emulsions were investigated. While the addition of low amount of pectin (0.1% (w/v)) had no effect on the emulsion viscosity, the addition of 0.5% (w/v) pectin greatly increased the viscosity. Granule-stabilized emulsion without hydrocolloids reflects the pseudoplastic behavior (shear-thinning behavior with flow behavior index, n < 1.0). Hydrocolloids, especially at high concentrations, affected the viscoelastic behavior of the emulsions and both storage (G′) and loss modulus (G′′) were regarded as frequency dependent. Emulsions behaved like a liquid with G′′ > G′ at lower frequencies, and like an elastic solid with G′ > G′′ at higher frequencies. Emulsion microstructure indicated that the presence of hydrocolloids induced flocculation. Creaming stability of emulsions was enhanced by the presence of hydrocolloids and increasing hydrocolloid concentration decreased the creaming by restricting the movement of oil droplets.
INTRODUCTION
Oil-in-water emulsions occur in many industrial processes and are the basis of many products. Emulsions are dispersions of one liquid in the form of fine droplets in another immiscible liquid. The two immiscible phases are usually oil and water. The change in the free energy during emulsion formation is usually positive and therefore emulsions are thermodynamically unstable.[Citation1] Emulsion stability is important for shelf-life, consumer acceptability and food structure required for pleasant feeling in the mouth. Therefore, the composition and the rheological conditions necessary to produce stable emulsions are of considerable industrial importance. Mainly proteins are employed to confer stability against coalescence in food emulsions in order to provide long shelf-life (kinetic stability). Shelf-life and consumer acceptability can also be related to creaming which is another important form of instability.[Citation1] Mostly, polysaccharides are used as thickening agents so as to modify the rheological behavior of the continuous phase and thus protect the emulsions against creaming. In a protein-stabilized oil-in-water emulsion containing polysaccharides, interactions between protein and polysaccharide can significantly affect the stability.[Citation2–6] The addition of non-adsorbing polysaccharides at low concentrations can speed up the process of creaming through the induction of flocculation by a depletion mechanism.[Citation1,Citation7,Citation8] However, the presence of a non-adsorbing polymer at higher concentrations may produce stabilization by conferring a very high apparent viscosity to the continuous phase and/or generating a strong emulsion network in the continuous phase. Tuinier and de Kruif[Citation6] have been studied the effect of exocellular polysaccharides in oil-in-water emulsions and reported that the rate of creaming decreased with increasing oil content due to hydrodynamic effects and this rate depended on the concentration of polysaccharides which was related to the strength of the depletion interaction and the viscosity of continuous phase.
The long-term physical stability of emulsions can be assessed through rheological studies. Steady state and oscillatory rheological measurements can give idea about the stability to creaming, flocculation, coalescence and phase inversion.[Citation9] Recently, several studies which have been performed on the rheological properties of emulsions,[Citation10–14] revealed that rheological properties may vary depending on the polysaccharide concentration, polysaccharide molecular weight, the type and the degree of interaction between protein and polysaccharide. Mostly, rheological behavior ranged between Newtonian and pseudoplastic.[Citation5]
Hen egg yolk associates appreciated organoleptic characteristics with very good emulsifying properties so that it has become a key ingredient for the preparation of a large variety of food emulsions such as mayonnaises, salad dressings and creams.[Citation15–17] Although it is a key ingredient in many food systems, physical properties of yolk emulsions are not entirely controlled because of the material's complicated composition and structure.[Citation17] Fractionation is therefore helpful to identify the proteins which play the major role in yolk functionality. However, individual constituents of yolk are difficult to prepare and only plasma (85% LDL (low density lipoproteins) and 15% livetin) and granules (70% HDL (high density lipoproteins), 16% phosvitin and 12% LDL) can be easily fractionated by simple dilution and centrifugation from yolk. Chemical and structural properties,[Citation16] adsorption kinetics,[Citation18] and emulsifying properties[Citation17,Citation19,Citation20] of egg yolk or egg yolk granule and plasma have been investigated previously. It has been reported that egg yolk proteins and lipoproteins form relatively strong film at the oil-water interface, which is largely responsible for the stability of emulsions against coalescence. Relatively limited studies have been performed on rheology of egg yolk and egg yolk fractions.[Citation15,Citation21] However, not many studies exist, which investigate the effect of hydrocolloids on the emulsifying and rheological properties of whole egg proteins or its invidual components (egg yolk proteins, egg white proteins). The aim of this study was to evaluate the effects of hydrocolloids on physicochemical properties of egg yolk granule stabilized emulsions by investigating the emulsion stability against creaming, the microstructure of emulsion, and the flow behavior and the dynamic rheological characteristics.
MATERIALS AND METHODS
Materials
Hen egg yolk powder were kindly supplied from NIVE (Nederlandse Industrie van Eiproducten, Holland). Low-methoxy pectin (P-9135) and guar gum (G-4129) were purchased from Sigma Chemical Company. Commercially available refined soybean oil was obtained from the local supermarket and used without further purification. Buffer salts, potassium dihydrogen orthophosphate, disodium hydrogen orthophosphate, and sodium chloride were purchased from Analar analytical reagent; BDH Chemical Ltd. All chemicals used were analytical grade.
Preparation of Hydrocolloids
Potassium dihydrogen orthophosphate and disodium hydrogen orthophosphate were dissolved in deionized water to prepare 0.05 M phosphate buffer solution (pH 7.0). Pectin (low methoxy) and guar gum stock solutions were prepared in 0.05 M phosphate buffer (pH 7.0) by stirring the dispersions vigorously for 30 min at room temperature, followed by heating at 50°C until the solution became clear.
Preparation of Egg Yolk Granule
Egg yolk powder was dissolved in phosphate buffer containing 0.17 M NaCl by stirring for 1 h and then centrifuged at 2100 g for 45 min. The plasma (supernatant) was separated from the precipitate (granule) by decantation. Precipitated granules obtained from first centrifugation were washed with 0.17 M NaCl containing phosphate buffer and then collected granules were redissolved in 0.5 M NaCl containing phosphate buffer. The protein content of granule was determined by Biuret method.
Emulsion Preparation
Oil-in-water emulsions were prepared with the egg yolk granule solutions with and without hydrocolloids and soybean oil. To prepare emulsions, 25% (v/v) soybean oil and 75% (v/v) of protein solution or protein-hydrocolloid mixtures were homogenized using lab scale homogenizer (Art-Miccra D-8, Germany) at 33,000 rpm for 5 min. The final composition of emulsion was 1% (w/v) protein and 0–0.5% (w/v) pectin/guar gum.
Determination of Emulsion Stability against Creaming
Immediately after preparation, a quantity of 10 ml of each emulsion was transferred into a graduated glass tube, and it is stored at an ambient temperature for 10 h. The stability of the emulsion was followed by monitoring the separation of cream layer and serum layer visually with time. The creaming was expressed as the percentage of the volume of the serum layer over the total volume of the emulsion in the cylinder:
Microscopic Observation
Microstructure of the emulsions was studied using Olympus BX51 light microscope (Tokyo, Japan) equipped with a Pixera PVC 100C camera. Approximately 50 μL of freshly prepared emulsion was placed on the microscope slide. A cover slip was placed on the sample by ensuring that no air gap or bubbles were trapped between sample and cover slip and the samples were examined with a 20X objective by taking images.
Emulsion Rheology
Rheological measurements were performed at 25 ± 0.01°C, with a RheoStress RS-1 controlled stress rheometer (HAAKE, Karlsruhe, Germany), using a cone and plate geometry (cone diameter 35 mm, angle 2°) by assuming slip effects were not significant. All emulsions were prepared immediately before being loaded into the rheometer. For each measurement, 2 ml of emulsion was carefully deposited over the plateau of the rheometer. After the plateau has been contacted with the cone, the exposed surface of sample was covered with a thin layer of silicone oil to prevent evaporation during the measurement. Stress sweep tests (1 Hz at 25°C) were made to determine the linear viscoelastic region of all samples; a stress value of 1 Pa was chosen for all the frequency tests. Oscillatory (dynamic) tests were performed inside the linear viscoelastic region in a frequency range of 0.001 to 10 Hz.and the storage (G′) and loss (G′′) moduli were recorded versus frequency. Data analysis software (Reowin Pro Data Manager Version 2.64) was used to obtain the experimental data (loss modulus, storage modulus, phase angle, etc.).
Steady state flow measurements were carried out in the range of 0–200 s−1 for 5 min and rheological parameters (shear stress, shear rate, apparent viscosity) were obtained from the software. Experimental flow curves were fitted to the power law model, τ = kγ n , where τ is the shear stress (Pa), γ is the shear rate (s −1), k is the consistency index and n is the flow behavior index.
Statistical Analysis
All experiments were performed in duplicate and the data were analyzed using analysis of variance (ANOVA). The level of confidence was 95%. Significant difference between means were identified by the LSD test using the SPSS 8.0 system software.
RESULTS AND DISCUSSION
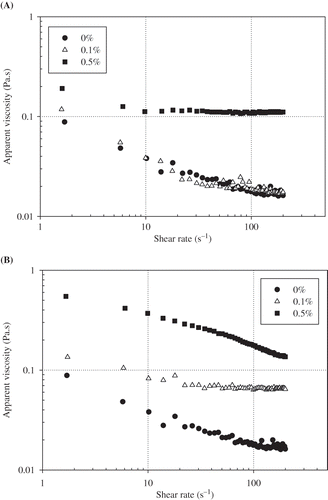
Emulsion Viscosity
and show the effects of different pectin and guar gum concentrations on viscosity of egg yolk granule-stabilized emulsions, respectively. Addition of pectin at a concentration of 0.1% (w/v) had approximately no effect on emulsion viscosity. But addition of 0.5% (w/v) pectin greatly increased emulsion viscosity. In guar gum containing emulsions increasing hydrocolloid concentration significantly (p < 0.05) increased the emulsion viscosity. At γ = 22 s−1 a 10-fold increase in viscosity was observed with 0.5% (w/v) guar gum compared with the emulsion without guar gum. This increase in the viscosity can be explained by the thickening effect of polysaccharide in the bulk phase.[Citation7,Citation22–24] As shown from and , viscosity of all emulsions decreased with increasing shear rate. As the shear rate sufficiently increases to overcome the Brownian motion, the emulsion droplets become more ordered along the flow field and offer less resistance to flow and hence lower the viscosity.[Citation13]
Flow Behavior of Emulsions
and represent shear stress versus shear rate data of egg yolk granule-stabilized emulsions, fitted to power law (τ = kγ n ) model, at different pectin and guar gum concentrations, respectively. The shear stress showed a linear dependence on the shear rate for all emulsions with and without hydrocolloids. The power law rheological parameters were given in , k the consistency index and n the flow behavior index. The flow behavior index indicates the pseudoplastic (n < 1.0), dilatant (n > 1.0) or Newtonian (n = 1.0) behavior.[Citation20] Granule-stabilized emulsion without hydrocolloids reflects the pseudoplastic behavior. The flow behavior index of the emulsion containing 0.1% (w/v) pectin was not very different from that of the emulsions containing no hydrocolloid but it was observed to be increasing with pectin concentration (0.5% (w/v)) and exhibiting Newtonian behavior.
Figure 2 Shear stress versus shear rate curves of 1% (w/v) egg yolk granule-stabilized emulsions at different (A) pectin and (B) guar gum concentrations.
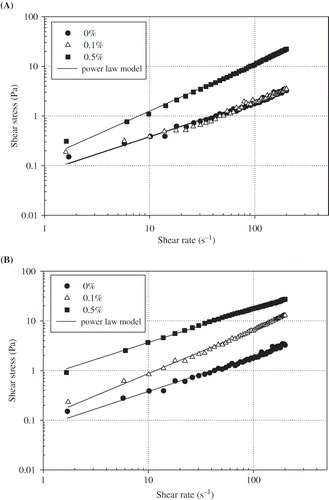
Table 1 Flow behavior index and consistency index of the power law model to describe the rheological behaviour of 1.0% (w/v) egg yolk granule stabilized emulsions with and without hydrocolloids
The emulsion with 0.1% (w/v) guar gum behaved like a Newtonian fluid since flow behavior index was 0.978. However, the emulsion showed a pseudoplastic (shear-thinning) behavior in the presence of 0.5% (w/v) guar gum as evidenced by the value of the flow behavior index of 0.627. While this value approximated unity in presence of low amounts of guar gum, a progressively smaller value was obtained at increased guar gum concentration. Guar gum is known to exhibit pseudoplastic behavior and hence induces a shear-thinning behavior.[Citation7] Shear thinning character is associated with the flocculation of fat droplets. This is usually the case with weakly flocculated emulsions or those to which a thickener is added.[Citation25] Flocculation increases the apparent volume of dispersed phase and leads to the formation of nonspherical aggregates. Both factors contribute to change the flow behavior of emulsion.[Citation26] The values of consistency index of guar gum containing emulsions were given in as a function of hydrocolloid concentration. Guar gum concentration had a significant effect (p < 0.05) on consistency index and also on flow behavior index. Increasing guar gum concentrations increased the viscosity and hence the consistency index of emulsions and this was correlated with the results of many authors.[Citation5,Citation7,Citation13,Citation27,Citation29]
Viscoelastic Properties of Emulsions
Dynamic frequency sweep tests were performed in the linear viscoelastic range. Oscillatory rheological measurements of storage and loss modulus indicated whether the emulsion system is strongly or weakly flocculated.[Citation9] In this later category the system is essentially liquid-like. Mechanical spectra of the emulsions with and without pectin or guar gum obtained by the frequency sweep measurements represented that these emulsions behaved like a viscous liquid at low frequencies, with the loss modulus (G′′) higher than the storage modulus (G′). However, both moduli showed a frequency dependency ( and ) and it was also observed that two moduli crossed each other at higher frequencies, G′ became greater than G′′ and the system behaved like an elastic solid. At both pectin concentrations similar G′ values were obtained as with the emulsion containing no pectin (). But addition of 0.5% (w/v) pectin decreased G′′ and cross-over frequency for the two moduli moved to lower frequency value. This was the indicative of a strong structure.[Citation1] This strong structure in the presence of a polysaccharide can be attributed either to the formation of a highly flocculated droplet network structure that gives this pronounced viscoelastic behavior or to the formation of a gel-like structure in the continuous phase by the polysaccharide itself. Addition of guar gum caused the increase of both moduli, G′ and G′′ (). This effect was more pronounced in 0.5% (w/v) guar gum containing emulsions. Increase of G′ indicated the formation of more elastic structure with the addition of guar gum. The increase of the elastic character of these emulsions due to the presence of guar gum suggested that the presence of guar gum induced more extensive aggregation of the proteins during shearing. It could also be resulted from changes in the viscoelastic profile which mean that the macromolecular mobility within the network was decreased due to the presence of the neutral polysaccharide.[Citation30]
Figure 3 Viscoelastic moduli (G′-filled symbols; G′′-open symbols) of egg yolk granule stabilized emulsions containing different concentrations of pectin.
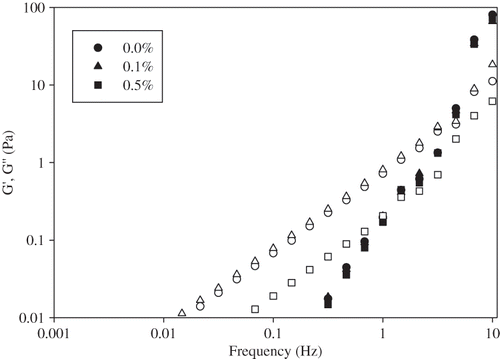
Figure 4 Viscoelastic moduli (G′-filled symbols; G′′-open symbols) of egg yolk granule stabilized emulsions containing different concentrations of guar gum.
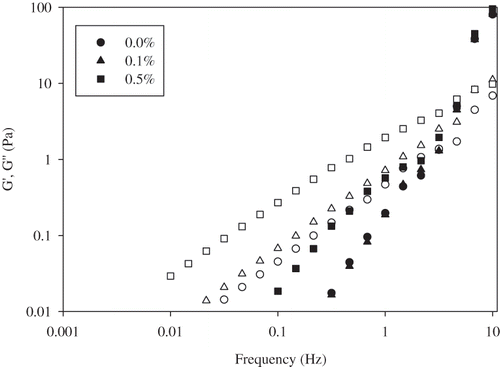
In addition to storage modulus and loss modulus, phase angle values of egg yolk granule stabilized emulsions with and without hydrocolloids were also determined by using dynamic oscillatory measurements to have a direct view on whether the emulsions behave as liquid or solid. Phase angle value (tan δ = G″/G′ can provide information about the nature of the viscoelastic response of the emulsion system and measures the energy loss compared to energy stored in cyclic deformation. A phase angle of 90° indicates the material is fully viscous while an elastic material is characterized by phase angle value approaches to 0°.[Citation31] In the range of studied frequency, phase angle values of granule-stabilized emulsions in the absence and presence of pectin or guar gum were decreased with increasing frequency and found to be changing between 89°–10°. At lower frequencies, phase angle (δ) values of emulsions were around 89° at all hydrocolloid concentrations and the system showed a viscous response. This value reduced with increasing frequency and approached to 10° and the system behaved like an elastic solid at high frequencies. Therefore, it was concluded that egg yolk granule-stabilized emulsions with and without pectin/guar gum had a viscoelastic property and it could be characterized as weak gels as a typical behavior of dressings and emulsions as reported previously.[Citation5]
Microstructure of Emulsions
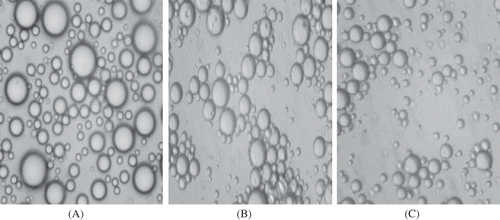
Microscopic images of emulsions was represented in . Microscopic images showed that egg yolk granule stabilized emulsions without hydrocolloids had homogeneously distributed individual droplets. No flocculation was observed in these emulsions () As can be seen from figure, larger droplets occured in the emulsions containing no hydrocolloids. It was observed that emulsions containing pectin or guar gum had small droplets and oil droplets appear to be flocculated; more droplet free areas appeared between the droplets ( and ).
Creaming Stability of Emulsions
In protein stabilized emulsions, creaming of oil droplets become apparent during storage for a relatively short time period and a serum layer develops at the bottom of the sample, while a cream layer develops at the top of the sample. The effects of pectin and guar gum addition on creaming behavior of egg yolk granule stabilized emulsions were shown in and , respectively. The emulsion without hydrocolloids creamed readily within 4 h and the creaming percentage reached at a plateau value which was determined as 50%. The addition of low amount of pectin (0.01–0.05% (w/v)) caused a slight change in creaming. At a pectin concentration of 0.1% (w/v) or higher, a significant decrease in the creaming was observed. Increasing the hydrocolloid concentration decreased the creaming and significantly increased the emulsion stability against creaming. This effect was more pronounced in the emulsions containing guar gum. Emulsions containing 0.2% (w/v) guar gum creamed only 10% after 10 h. No creaming was observed in the emulsion containing 0.5% (w/v) guar gum even after 24 h, which can be attributed to the dominant role of a rise in the emulsion viscosity. It was reported that the creaming process could be related to the rheological properties of continuous phase in the presence of a non-adsorbing polysaccharide.[Citation6,Citation23] The inhibition of creaming at high hydrocolloid concentrations was probably due to the immobilization of dispersed oil phase in a weak gel-like network, which was resulted from depletion flocculation.[Citation3,Citation8]
Figure 6 Effect of pectin concentration on creaming behaviour of 1% (w/v) egg yolk granule stabilized emulsions.• 0% (w/v), □ 0.01% (w/v), ▴ 0.05% (w/v), ∇ 0.1% (w/v), Δ0.2% (w/v), and ♦0.5% (w/v) pectin.
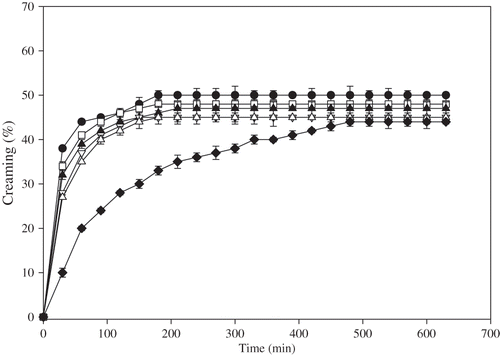
Figure 7 Effect of guar gum concentration on creaming behaviour of 1% (w/v) egg yolk granule stabilized emulsions • 0% (w/v),□ 0.01% (w/v), ▴ 0.05% (w/v),∇ 0.1% (w/v), and ♦0.2% (w/v) guar gum.
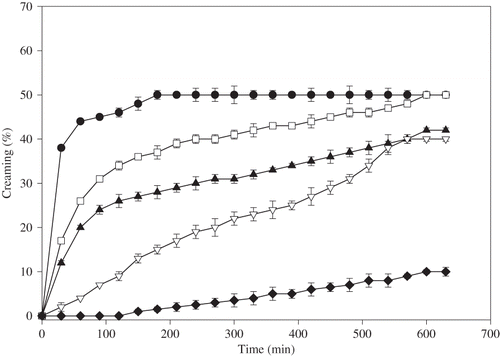
At low pectin/guar gum concentrations (0.01–0.05% (w/v)), there were diffuse boundaries between the two separating phases, the cream and the serum phase, and the serum phase remained turbid. This behavior is characteristics of polydispersed emulsions in the presence of little or no polysaccharide, where individual droplets or small aggregates move independently to the top of the container.[Citation32] At higher hydrocolloid concentrations (0.1–0.5% (w/v)), the boundary lines between the serum and cream phase were sharp and a clear serum phase was observed which indicated that the emulsion was completely flocculated. The boundary line gradually became sharper but the height of boundary did not change with time. The same behavior was observed previously in oil-in-water emulsions containing hydrocolloids.[Citation7,Citation8,Citation12,Citation13,Citation32]
CONCLUSION
Egg yolk granule stabilized emulsions without hydrocolloids showed pseudoplastic behavior. With the addition of pectin flow type approached to Newtonian behavior while with the addition of guar gum flow type became more pseudoplastic. Addition of hydrocolloids affected emulsion viscosity, viscoelastic properties, microstructure, and emulsion stability against creaming. The presence of hydrocolloids decreased the creaming percentage by increasing the viscosity of aqueous phase and also they keep droplets apart and protect them against creaming. The effect of guar gum was more pronounced than that of pectin because of its higher thickening ability. The presence of 0.5% (w/v) guar gum significantly inhibited creaming. It was concluded that stability and rheology of emulsions can be controlled by the addition of hydrocolloid gums.
ACKNOWLEDGMENTS
NIVE (Nederlandse Industrie van Eiproducten) is acknowledged for providing the egg yolk sample. The financial support from The Scientific & Teechnological Research Council of Turkey (TUBITAK) is gratefully acknowledged.
REFERENCES
- Kontogiorgos , V. , Biliaderis , C. G. , Kiosseoglou , V. and Doxastakis , G. 2004 . Stability and rheology oof egg-yolk-stabilized concentrated emulsions containing cereal β-glucans of varying molecular size . Food Hydrocolloids , 18 : 987 – 998 .
- Akhtar , M. and Dickinson , E. 2003 . Emulsifying properties of whey protein-dextran conjugates at low pH and different salt concentrations . Colloids and Surfaces B: Biointerfaces , 31 : 125 – 132 .
- Dickinson , E. 2003 . Hydrocolloids at interfaces and influence on the properties of dispersed systems . Food Hydrocolloids , 17 : 25 – 39 .
- Erçelebi , E.A. and Ibanoğlu , E. 2007 . Influence of hydrocolloids on phase separation and emulsion properties of whey protein isolate . Journal of Food Engineering , 80 : 454 – 459 .
- Mandala , I.G. , Savvas , T.P. and Kostaropoulos , A.E. 2004 . Xanthan and locust bean gum influence on the rheology and structure of a white model-sauce . Journal of Food Engineering , 64 : 335 – 342 .
- Tuinier , R. and de Kruıif , C.G. 1999 . Phase separation, creaming, and network formation of oil-in-water emulsions induced by an exocellular polysaccharide . Journal of Colloid and Interface Science , 218 : 201 – 210 .
- Neirynck , N. , Van lent , K. , Dewettinck , K. and Van der Meeren , P. 2007 . Influence of pH and biopolymer ratio on sodium caseinate-guar gum interactions in aqueous solutions and in O/W emulsions . Food Hydrocolloids , 21 : 862 – 869 .
- Paraskevopoulou , A. , Boskou , D. and Kiosseoglou , V. 2005 . Stabilization of olive oil-lemon juice emulsion with polysaccharides . Food Chemistry , 90 : 627 – 634 .
- Torres , L.G. , Iturbe , R. , Snowden , M.J. , Chowdhry , B.Z. and Leharne , S.A. 2007 . Preparation of o/w emulsions stabilized by solid particles and their characterization by oscillatory rheology. Colloids and Surfaces A . Physicochemical & Engineering Aspects , 302 : 439 – 448 .
- Diftis , N.G. , Biliaderis , C.G. and Kiosseoglou , V.D. 2005 . Rheological properties and stability of model salad dressing emulsions prepared with a dry-heated soybean protein isolate-dextran mixture . Food Hydrocolloids , 19 : 1025 – 1031 .
- Dolz , M. , Hernadez , M.J. , Delegido , J. , Alfaro , M.C. and Munoz , J. 2007 . Influence of xanthan gum and locust bean gum upon flow and thixotropic behaviour of food emulsions containing modified starch . Journal of Food Engineering , 81 : 179 – 186 .
- Drakos , A. and Kiosseoglou , V. 2008 . Depletion flocculation effects in egg-based model salad dressing emulsions . Food Hydrocolloids , 22 : 218 – 224 .
- Sun , C. , Gunasekaran , S. and Richards , M.P. 2007 . Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions . Food Hydrocolloids , 21 : 555 – 564 .
- Valdez , M.A. , Acedo-Carrillo , J.I. , Rosas-Durazo , A. , Lizardi , J. , Rinaudo , M. and Goycoolea , F.M. 2006 . Small-deformation rheology of mesquite gum stabilized oil in water emulsions . Carbohydrate Polymers , 64 : 205 – 211 .
- Anton , M. , Le Denmat , M. , Beaumal , V. and Pilet , P. 2001 . Filler effects of oil droplets on the rheology of heat-set emulsion gels prepared with egg yolk and egg yolk fractions. Colloids and Surfaces B . Biointerfaces , 21 : 137 – 147 .
- Anton , M. , Martinet , V. , Dalgalarrondo , M. , Beaumal , V. , David-Briand , E. and Rabesona , H. 2003 . Chemical and structural characterization of low-density lipoproteins purified from hen egg yolk . Food Chemistry , 83 : 175 – 183 .
- Le Denmat , M. , Anton , M. and Beaumal , V. 2000 . Characterisation of emulsion properties and of interface composition in O/W emulsions prepared with hen egg yolk, plasma and granules . Food Hydrocolloids , 14 : 539 – 549 .
- Mel'nikov , S.M. 2002 . Effect of pH on the adsorption kinetics of egg yolk at the triacylglycerol-water interface and viscoelastic properties of interfacial egg yolk films: a drop tensiometry study. Colloids and Surfaces B . Biointerfaces , 27 : 265 – 275 .
- Aluko , R.E. and Mine , Y. 1998 . Characterization of oil-in-water emulsions stabilized by hen's egg yolk granules . Food Hydrocolloids , 12 : 203 – 210 .
- Anton , M. , Chapleau , N. , Beaumal , V. , Delepine , S. and de Lamballerie-Anton , M. 2000 . Effect of high-pressure treatment on rheology of oil-in-water emulsions prepared with hen egg yolk . Innovative Food Science and Engineering Technologies , 2 : 9 – 21 .
- Raikos , V. , Campbell , L. and Euston , S.R. 2007 . Rheology and texture of hen's egg protein heat-set gels as affected by pH and the addition of sugar and/or salt . Food Hydrocolloids , 21 : 237 – 244 .
- Leng , X.J. and Turgeon , S.L. 2007 . Study of the shear effects on the mixture of whey protein/polysaccharides-2: Application of flow models in the study of the shear effects on WPI/polysaccharide system . Food Hydrocolloids , 21 : 1014 – 1021 .
- Velez , G. , Fernandez , M.A. , Munoz , J. , Williams , P.A. and English , R.J. 2003 . Role of hydrocolloids in the creaming of oil in water emulsions . Journal of Agriculture and Food Chemistry , 51 ( 1 ) : 265 – 269 .
- Ye , A. , Hemar , Y. and Singh , H. 2004 . Influence of polysaccharides on the rate of coalescence in oil-in-water emulsions formed with highly hydrolyzed whey proteins . Journal of Agriculture and Food Chemistry , 52 ( 17 ) : 5491 – 5498 .
- Tadros , T. 2004 . Application of rheology for assessment and prediction of the long-term physical stability of emulsions . Advances in Colloid Science and Interface Science , 108 : 227 – 258 .
- Boutin , C. , Giroux , H.J. , Paquin , P. and Britten , M. 2007 . Characterization and acid-induced gelation of butter oil emulsions produced from heated whey protein dispersions. International Dairy Journal . 17 : 696 – 703 .
- Kayacier , A. and Doğan , M. 2006 . Rheological properties of some gums-salep mixed solutions . Journal of Food Engineering , 72 : 261 – 265 .
- Lizarraga , M.S. , de Piante Vicin , D. , Gonzalez , R. , Rubiolo , A. and Santiago , L.G. 2006 . Rheological behaviour of whey protein concentrate and ı-carrageenan aqueous mixtures . Food Hydrocolloids , 20 : 740 – 748 .
- Mothe , C.G. and Rao , M.A. 1999 . Rheological behavior of aqueous dispersions of cashew gum and gum Arabic: effect of concentration and blending . Food Hydrocolloids , 13 : 501 – 506 .
- Tavares , C. and Lopes da Silva , J.A.L. 2003 . Rheology of galactomannan-whey protein mixed systems . International Dairy Journal , 13 : 699 – 706 .
- Ahmed , J. and Ramaswamy , H. S. 2006 . Viscoelastic properties of sweet potato puree infant food . Journal of Food Engineering , 74 : 376 – 382 .
- Santiago , L.G. , Gonzalez , R.J. , Fillery-Travis , A. , Robins , M. , Bonaldo , A.G. and Carrara , C. 2002 . (2002). The influence of xanthan and i-carrageenan on the creaming and flocculation of an oil-in-water emulsion containing soy protein. Brazilian . Journal of Chemical Engineering , 19 ( 4 ) : 411 – 417 .