Abstract
This study was aimed to investigate the antioxidant capacity and antioxidant components in fresh, powder and fiber products prepared from Mangifera foetida fruit, locally known as bacang. The antioxidant capacity and components (reducing, flavonoid, carotenoid, and ascorbic acid contents) were in the order of fresh > fiber > powder, whereas based on β-carotene bleaching method, order of antioxidant activity was fiber > powder > fresh. Pearson correlation coefficient showed that there was positive and significant correlation (r > 0.9) between antioxidant capacity and certain antioxidant components.
INTRODUCTION
Antioxidants are micro-constituents present in the diet that can delay or inhibit lipid oxidation by inhibiting the initiation or propagation of oxidizing chain reactions. In general, antioxidants can be categorized into two basic groups, namely, synthetic and natural. Natural antioxidants that found in fruits and vegetables have gained increasing interest among consumers because epidemiological studies have consistently shown that there is an abundant health beneficial in consumption of fruits and vegetables. Previous studies have reported that there is a significant high association between intake of fruits and vegetables with the low rate of heart disease mortality, common cancers and other degenerative diseases.[Citation1,Citation2] These protective effects are considered to be related to the various antioxidants contained in fruits and vegetables.[Citation3,Citation4]
According to FAO,[Citation5] mango is one of the most important tropical fruits in the world and currently ranked fifth in total world production among the major fruit crops. It is a seasonal fruit which normally available in ASEAN market between April to June and September to November.[Citation6] There are about 20% of mango is processed for products such as puree, nectar, leather, pickles, canned slices and chutney.[Citation7] Yamanaka et al.[Citation8] reported that there are 25 Mangifera species found in Peninsular Malaysia and 16 of them are edible. Among edible species, Mangifera caesia (binjai), Mangifera foetida (bacang), and Mangifera odorata (kwini), and Mangifera indica (mangga) are prominent at local markets of Peninsular Malaysia.
Bacang (Mangifera foetida) is an oval shaped underutillized fruit with a smooth yellow-green skin when ripe. It is normally grown wild in dipterocarp forests of Peninsular Malaysia, Peninsular Thailand, Moluccas, Sulawesi, Sumatra, Borneo, and Sulawesi. In Malaysia, it is used to make chutneys as well as pickles.[Citation9,Citation10] According to Tee et al., [Citation11] the edible portion of bacang represents 56% of fruit weight. For every 100 g edible portion of flesh, it contains 78.5 g water, 0.8 g protein, 17.9 g carbohydrates, 16 mg calcium, 19 mg phosphorus, 0.09 mg thiamine, 255 μg carotenes, and 47.4 mg vitamin C. Antioxidant properties of commercial fruits are widely studied.[Citation3,Citation12,Citation13] Study by Gorinstein et al.[Citation14] found that commercial mango had higher antioxidant properties compared to other fruits. Ribeiro et al.[Citation15] reported that commercial mango (Mangifera indica cv Ub′a) pulp had 209 mg phenolic content and 78 mg ascorbic acid per 100 g edible portion. Although many studies have been focused on antioxidant properties of commercial mango (Mangifera indica), no research has been published on Mangifera foetida (bacang). Therefore, there is a need to explore the health promoting properties of this underutilized fruit.
Nowadays, there is an increasing demand for functional foods and nutraceutical products in the world market. Since the fresh bacang is less popular among consumers to consume due to fibrous flesh and strong smell, alternative products from bacang would be warranted. Bacang can be produced into different food ingredients and products such as powder and fiber. In these products, their antioxidant properties should be investigated in order to maintain the nutritional quality. Therefore, this study was aimed to investigate antioxidant properties (antioxidant components and capacity) of fresh and its (powder and fiber) products prepared from bacang fruit.
MATERIALS AND METHODS
Materials
All chemicals used were of analytical grade from Merck (Darmstadt, Germany) unless stated otherwise. Maltodextrin, β-carotene, linoleic acid, Tween 20, butylated hydroxytoluene (BHT), 2,4,6-tripyridyl-1,3,5-s-triazine, Trolox, ABTS, potassium persulfate, sodium carbonate (Na2CO3), galic acid, sodium nitrite (NaNO2), aluminum chloride hexahydrate (AlCl3·6H2O), sodium hydroxide (NaOH), (+)- Catechin, metaphosphoric acid, L-ascorbic acid, sodium salt of 2,6-dichlorophenol-indophenol (DCPIP) were purchased from Sigma Chemical Co (St. Louis, MO, USA). EDTA was from Fisher Scientific (Loughborough, UK).
Methods
Preparation of fresh mango
Bacang fruits were purchased from a fruit farm located at Kedah, Malaysia and convenience sampling was used in the selection the fruits. Upon arrival to our laboratory, the fruits were cleaned and washed under running tap water. The skins were carefully peeled with a sharp knife and the seeds were removed from the flesh. Then, the flesh was cut into small pieces longitudinally and kept frozen at −80°C in an airtight container prior to analysis.
Preparation of mango powder
The mango flesh was ground into small pieces using a wet blender (National; model: MX-291N, Matsushita Electric Industrial Co., Osaka, Japan) and immediately filtered in order to obtain fibrous and puree parts. The fibrous part was used to prepare mango fiber whereas the puree part was diluted with distilled water (puree: water, 1:1, w/v). After that, about 12% maltodextrin was added into the mixture, and mixed using homogenizer (National Juicer; model: MJ-C90N, Matsushita Electric Industrial Co., Osaka, Japan). The mixture was then placed in the freeze dryer. After three days, the freeze-dried sample was ground and filtered through a 0.05 μm sieve. The yield was considered as mango powder.
Preparation of mango fiber
The mango fiber was prepared according to the method described by Larrauri et al.[Citation16] Fresh mango flesh was wet milled with water in the ratio of 1 to 1 (w/v). Puree obtained was removed using a presser and the mango pomaces were washed with hot water at 95°C for 5 minutes in the ratio of 1 to 2 and then pressed. The washing and pressing steps were repeated using tap water. The pomaces were then dried using an air-oven at 50°C for overnight. The dried pomaces were then finely ground using ball mill and sieved into 0.25 mm particle size to obtain mango fiber powder. The fiber powder was stored at room temperature in an airtight container prior to analysis.
Preparation of extract
The extract was prepared according to the procedure of Velioglu et al.[Citation17] Fresh, powder and fiber of mango were mixed with 80% aqueous acetone for 2 h at 50°C using an orbital shaker (Unimax 1010, Heidolph Instruments GmbH & Co. KG, Germany). The ratio between the extraction medium to sample was 1 to 20. The extract was filtered through Whatman No. 1 filter paper. The filtrate was considered as mango extract and kept frozen at −20°C prior to analysis. The extract obtained was used for the determination of antioxidant capacity and reducing, flavonoid as well as carotenoid contents.
Determination of antioxidant capacity
The FRAP value was estimated according to the method described by Benzie and Strain[Citation18] with slight modifications. First, fresh FRAP reagent was prepared by mixing 2.5 ml of 10 mM TPTZ solution in 40 mM hydrochloric acid with 2.5 ml of a 20 mM FeCl3 solution and 25 ml of a 0.3 M acetate buffer (pH 3.6). After that, 50 μl of the sample solution, 150 μl of water, and 1.5 ml of FRAP reagent were mixed. The absorbance was read after 4 min at 593 nm using SECOMAM Anthelie Advanced 5 spectrophotometer (Anthelie Junior, 95335 Domont Codex, France). Ferrous sulfate (FeSO4.7H20) with concentration ranged from 0–20 μmol/L was used to plot a standard curve. The results were expressed as mmol Fe (II) per 100 g dry weight of sample.
TEAC assay was carried out using a SECOMAM Anthelie Advanced 5 spectrophotometer by improved ABTS+ method as described by Li et al.[Citation19] with slight modifications. Briefly, ABTS+ radical cation was generated by a reaction between 7 mmol/L ABTS and 2.45 mmol/L potassium persulfate. The reaction mixture was allowed to stand in the dark for 16 hr at room temperature. The ABTS+ solution was diluted with acetone to an absorbance of 0.700 ± 0.050 at 734 nm before use. All samples were diluted appropriately to provide 20–80% inhibition of the blank absorbance. Fifty microliters of the diluted sample were mixed with 1.9 ml of diluted ABTS+ solution. The mixture was allowed to stand for 6 min at room temperature and absorbance was immediately read at 734 nm. Trolox with concentration ranged from 0–20 μmol/L was used as a standard. The results were expressed as μmol Trolox/100 g dry weight of sample.
β-carotene bleaching activity was determined according to the method described by Amin and Tan.[Citation20] One milliliter of β-carotene solution (0.2 mg/ml chloroform) was pipetted into a 50 ml round bottom flask containing 0.02 ml of linoleic acid and 0.2 ml of 100% Tween 20. After evaporation at 40°C for 10 minutes using rotary evaporator to remove chloroform, the mixture was immediately diluted with 100 ml of distilled water. The distilled water was added slowly to the mixture with vigorous agitation to form an emulsion. Five ml aliquots of the emulsion were transferred into different test tubes containing 0.2 ml of samples in 80% acetone at 1 mg/ml. The tubes were then gently mixed and placed at 45°C in a water bath for 2 h. Absorbance of the samples was measured at 470 nm using a SECOMAM Anthelie Advanced 5 spectrophotometer at initial time (t = 0) against a blank, consisting of an emulsion without β-carotene. Standards at same concentration with samples were used as comparison. 80% acetone (0.2 ml) was used as control. The measurement was carried out at 20 min intervals. The antioxidant activity (AA) was measured in terms of successful bleaching of β-carotene according to EquationEq. (1):
Determination of Antioxidant Components
Total reducing content was determined using the method described by Velioglu et al.[Citation17] with slight modifications. First 0.2 ml of sample extract was transferred into a test tube. Then, 1.5 ml of Folin-Ciocalteau reagent was added into the extract, and the mixture was allowed to stand at room temperature. After 5 min, 1.5 ml of sodium carbonate solution (0.566 M) was added into the mixture. Absorbance was measured using a SECOMAM Anthelie Advanced 5 spectrophotometer at 725 nm after standing 90 min at room temperature. Galic acid with concentrations ranged from 0.02 to 0.10 mg/ml were used as the standard. Results were expressed as gallic acid equivalents (GAE) in milligrams per 100 grams of a dry weight basis.
The total flavonoid content was estimated according to the method of Shin et al.[Citation13] with slight modifications. An aliquot of sample extract (1 ml) was added to 4 ml of distilled water. After that, 0.3 ml of sodium nitrite solution (5% NaNO2) was added to the mixture, which was allowed to stand for 5 min at room temperature. Then, 0.3 ml of aluminum chloride hexahydrate solution (10% AlCl3·6H2O) was added into the mixture and allowed to stand for another 6 min at room temperature. Then, 2 ml of sodium hydroxide (NaOH) (1 M) was added, and the total was made up to 10 ml with distilled water. The absorbance was read immediately using a SECOMAM Anthelie Advanced 5 spectrophotometer at 510 nm. (+)-Catechin with different concentrations ranged from 0.2 to 1.0 mg/ml was used to plot a standard curve. The results were expressed as gram of (+)- catechin equivalents per 100 gram of the dry weight basis.
Total carotenoid content was determined by a spectrophotometric method described by Choi et al.[Citation21] with some modifications. Approximate 5 ml of sample extract was mixed with 5 ml of distilled water and 1 ml of a mixture of hexane/acetone/methanol (50/25/25, v/v) solution. The mixture was then homogenized using a magnetic stirrer (Stirring Hotplate HS070N2, FAVORIT, Japan) and centrifuged at 3000 rpm for 10 min. The absorbance of the upper layer was measured at 450 nm. Total carotenoid of the samples was calculated as μg β-carotene per 100 g dry weight of sample using the following equation[Citation22]:
The ascorbic acid content was determined according to the method of AOAC.[Citation23] About 2 g of sample was mixed with 2 ml of 0.750 M metaphosphoric acid. Then, the mixture was diluted to 100 ml with 0.375 M metaphosphoric acid. The diluted suspension was filtered, and 10 ml aliquot of the filtrate was pipetted into a small conical flask. The filtrate was titrated immediately with the standardized solution of 2,6-dichlorophenol-indophenol to a faint pink.
Statistical Analysis
Results were analyzed using the Statistical Package for the Social Sciences (SPSS version 15). Data were expressed as means ± standard deviation (SD) of triplicate determinations. ANOVA and Tukey tests were used to determine the mean differences. Pearson correlation test was used to determine the correlation between the antioxidant capacity and antioxidant components. The value of P < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
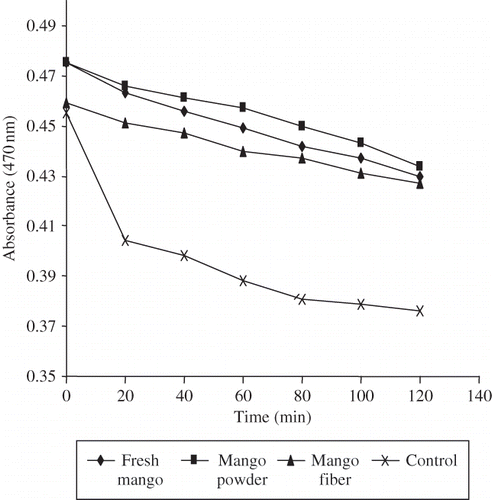
The β-carotene bleaching rates of the samples are shown in . There was a decrease in absorbance value of β-carotene in the absence of samples due to the oxidation of β-carotene and linoleic acid. Bacang fiber had the lowest antioxidant activity compared to fresh and powder samples (). Previous studies showed different antioxidant compounds may act through different mechanisms; therefore, no single method can fully evaluate the total antioxidant capacity of foods.[Citation24] Due to this reason, at least three antioxidant methods with different principles should be carried out to determine the antioxidant capacity of the samples. In this present study, three antioxidant assays were carried out in order to determine the antioxidant capacity of mango extracts, namely FRAP, TEAC, and β-carotene bleaching assays. These assays, based on different chemical mechanisms, were selected to take into account the wide variety and range of action of antioxidant compounds present in mango extracts.
The antioxidant capacity of mango sample as assessed by FRAP, TEAC, and β-carotene bleaching assays are shown in . Fresh bacang exhibited the highest antioxidant capacity among the samples based on the FRAP and TEAC assays followed by fiber and powder. The TEAC value of fresh bacang was comparable to the result of commercial mango (Mangifera indica L.) reported by Soong and Barlow.[Citation25] The present results revealed that antioxidants found in fresh bacang exhibited the highest reducing properties and ability to scavenge the ABTS radical cation compared with its powder and fiber. The results clearly show a decrease in antioxidant capacity of fresh bacang after powder and fiber preparations. Fresh bacang showed a higher reduction in antioxidant capacity after preparation of powder than fiber ().
Table 1 Antioxidant capacity of fresh, powder and fiber of bacang.
Soong and Barlow[Citation25] found a higher antioxidant capacity in freeze-dried mango flesh compared to fresh ones. This could be due to differences in sample preparation. The antioxidant compounds in the sample are preserved after freeze-drying. However, in the present study, preparation of fiber product involved heat treatment, and most of antioxidant components were lost. For preparation of bacang powder, the addition of maltodextrin could influence the antioxidant properties. A study by Quek et al.[Citation26] showed that maltodextrin influenced the physicochemical properties of watermelon powders.
On the other hand, the increment of antioxidant activity in powder and fiber was observed after preparing these products from fresh ones (). Bacang fiber possessed the highest antioxidant activity followed by powder and fresh mango. It could be due to different stability of antioxidant compounds present in mango fiber. A study by Larrauri et al.[Citation27] showed that antioxidant compounds associated with dietary fibers may responsible to antioxidant activity of mango peel fibers. Our study found that bacang (Mangifera feotida) had higher total dietary fiber (6 g/100 g) than Mangifera indica L (2 g/100 g) as reported by Ramulu and Roa.[Citation28]
There were four types of antioxidant components being analyzed in this study, which included phenolic compounds, flavonoid, carotenoid, and ascorbic acid. In this present study, the total phenolic content of sample was determined using the Folin-Ciocalteu method. However, it is important to mention that the Folin-Ciocalteu assay might overestimate the content of phenolics substances, since the reducing agents such as ascorbic acid, sugars and others present in the food can interfere in this assay.[Citation29]
shows the total reducing (considered as phenolic), flavonoid, carotenoid and ascorbic acid contents in bacang samples. Fresh bacang exhibited the highest content of antioxidant compared to its powder and fiber products. There are several studies showed that mango flesh rich in carotenoids, vitamin C and polyphenols.[Citation30,Citation31,Citation32] The results indicate the lost of antioxidant content in fresh bacang was in the range of 67–92% and 53–74% after powder and fiber preparations, respectively ().
Table 2 Total reducing, flavonoid, carotenoid and ascorbic acid contents of fresh, powder and fiber of bacang.
It was found that FRAP, TEAC values, total phenolic, flavonoid, carotenoid and ascorbic acid contents were in the order of fresh > fiber > powder, whereas for β-carotene bleaching assay, the order was bacang fiber > powder > fresh. The ranking order obtained using β-carotene bleaching assay was different from those obtained using FRAP and TEAC assays. The differences could be due to characteristics of antioxidant components present in the studied samples. Phenolic and flavonoid compounds may act as chain breakers and radical scavengers, which contribute to the antioxidant properties.[Citation33,Citation34] In addition, principles of the assays used could also be factors influence the results as FRAP assay measures chain breaking potential and TEAC measures the ability to scavenge the ABTS radical cation, while β-carotene bleaching assay measures the ability to neutralize the free radicals.[Citation24,Citation35]
One-way ANOVA revealed a significant difference (P < 0.05) in the FRAP, TEAC values, total phenolic, flavonoid, and ascorbic acid contents. Further analysis using Tukey test revealed that fresh bacang and bacang powder had comparable antioxidant capacity as determined by β-carotene bleaching assay. Moreover, results were also indicated that bacang powder and bacang fiber had comparable in carotenoid content. The FRAP, TEAC values, phenolic, flavonoid, carotenoid, and ascorbic acid contents in fresh bacang were found to be significantly higher (P < 0.05) compared to fiber and powder form. In addition, the FRAP, TEAC values, phenolic, flavonoid, carotenoid, and ascorbic acid contents in bacang fiber was also found to be significantly higher (P < 0.05) than those contained in bacang powder.
There were very high positive correlations between the means of antioxidant capacity assessed by FRAP and TEAC (r = 0.997), FRAP and total reducing content (r = 0.998), FRAP and total flavonoid content (r =0.99), FRAP and total carotenoid (r = 0.99) and FRAP and ascorbic acid content (r = 0.99) (). In addition, a positive and high significant correlations were also observed between TEAC and total phenolic content (r = 0.99), TEAC and total flavonoid (r = 0.98), TEAC and total carotenoid (r = 0.99) as well as TEAC and ascorbic acid content (r = 0.99). The positive correlations were in agreement with previous studies.[Citation36, Citation37, Citation38] There was, however, no significant correlation was found between β- carotene bleaching activity and antioxidant components.
Table 3 Correlations between antioxidant capacity and antioxidant components
CONCLUSION
Antioxidant capacity assessed by FRAP and TEAC assays exhibited that fresh bacang had the highest antioxidant properties followed by its powder and fiber products. Processing applied during preparation of bacang powder and fiber decreased the antioxidant properties and components. Results indicated that antioxidant capacity exhibited positive and high correlations with antioxidant components studied. Antioxidant components such as phenolic compounds, flavonoid, carotenoid, and ascorbic acid could play major contributors to antioxidant capacity of this fruit. Although the highest antioxidant properties and components in fresh bacang, there is still a need to prepare alternative products such as powder and fiber that could serve as source of nutraceuticals and functional foods.
ACKNOWLEDGEMENTS
The work was financially supported by Ministry of Science, Technology, and Innovation of Malaysia (Project No. 05-01-04-SF0048).
REFERENCES
- Sujatha , R. 2003 . The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis . America Journal Clinical Nutrition , 78 ( Suppl ) : 552S – 558S .
- Knekt , P. , Kumpulainen , J. , Järvinen , R. , Rissanen , H. , Heliövaara , M. , Reunanen , H. T. and Aromaa , A. 2002 . Flavonoid intake and risk of chronic diseases . America Journal Clinical Nutrition , 76 : 560 – 568 .
- Leong , L.P. and Shui , G. 2002 . An investigation of antioxidant capacity of fruits in Singapore markets . Food Chemistry , 76 : 69 – 75 .
- Zin , Z.M. , Hamid , A.A. , Osman , A. , Saari , N. and Misran , A. 2007 . Isolation and Identification of Antioxidative Compound from Fruit of Mengkudu (Morinda citrifolia L.) . International Journal of Food Properties , 10 : 363 – 373 .
- Food and Agriculture Organization. FAOSTAT database collections, agricultural data, food and agriculture organization of the United Nations. 2004 http://faostat.fao.org/faostat/collections?subset=agriculture (http://faostat.fao.org/faostat/collections?subset=agriculture) (Accessed: 16 August 2007 ).
- FAMA. Menuju Ke Arah Kualiti Malaysia's Best. FAMA, Kuala Lumpur. 2006 http://www.famaxchange.org/ (http://www.famaxchange.org/) (Accessed: 15 February 2008 ).
- Abdalla , A.E.M. , Darwish , S.M. , Ayad , E.H.E. and El-Hamahmy , R.M. 2007 . Egyptian mango by-product 2: Antioxidant and antimicrobial activities of extract and oil from mango seed kernel . Food Chemistry , 103 ( 4 ) : 1141 – 1152 .
- Yamanaka , N. , Hasran , M. , Xu , D.H. , Tsunematsu1 , H. , Idris , S. and Ban , T. 2006 . Genetic relationship and diversity of four Mangifera species revealed through AFLP analysis . Genetic Resources and Crop Evolution , 53 : 949 – 954 .
- Boer, E. Mangifera L. In Plant Resources of South-East Asia. No. 5(2): Timber tree: Minor commercial timber; Lemmens, R.H.M.J.; Soerianegara, I.; Wong, W.C.; Eds.; Prosea Foundation, Bogor, Indonesia, 1995 http://www.worldagroforestry.org/sea/Products/AFDbases/af/asp/SpeciesInfo.asp?SpID=18087 (http://www.worldagroforestry.org/sea/Products/AFDbases/af/asp/SpeciesInfo.asp?SpID=18087) (Accessed: 10 January 2010 ).
- Bompard, J.M. Mangifera foetida Lour. and Mangifera pajang Kostermans. In Plant Resources of South-East Asia. No. 2: Edible fruits and nuts; Coronel, R.E.; Verheij, E.W.M.; Eds.; Prosea Foundation, Bogor, Indonesia, 19928 http://www.worldagroforestry.org/sea/Products/AFDbases/af/asp/SpeciesInfo.asp?SpID=18090 (http://www.worldagroforestry.org/sea/Products/AFDbases/af/asp/SpeciesInfo.asp?SpID=18090) (Accessed: 10 January 2008 ).
- Tee , E.S. , Mohd Ismail , N. , Mohd Nasir , A. and Khatijah , I. 1997 . Nutrient composition of Malaysian foods , 72 – 73 . Kuala Lumpur : Institute Medical for Research .
- Lim , Y.Y. , Lim , T.T. and Tee , J.J. 2007 . Antioxidant properties of several tropical fruits: A comparative study . Food Chemistry , 103 : 1003 – 1008 .
- Shin , Y.J. , Liu , R.H. , Nock , J.F. , Holliday , D. and Watkins , C.B. 2007 . Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry . Postharvest Biology and Technology , 45 : 349 – 357 .
- Gorinstein , S. , Zemser , M. , Haruenkit , R. , Chuthakorn , R. , Grauer , F. , Martin-Belloso , O. and Trakhtenberg , S. 1999 . Comparative content of total polyphenols and dietary fiber in tropical fruits and persimmon . Journal of Nutritional Biochemistry , 10 : 367 – 371 .
- Ribeiro , S. M. R. , deQueiroz , J. H. , Lopez , M. E. , deQueiroz , R. , Campos , F. M. and Santana , H. M. P. 2007 . Antioxidant in mango (Mangifera indica) pulp . Plant Foods for Human Nutrition , 62 : 13 – 17 .
- Larrauri , J.A. , Goni , I. , Martin-Carron , N. , Ruperez , P. and Saura-Calixto , F. 1996 . Measurement of health-promoting properties in fruit dietary fibers: antioxidant capacity, fermentability and glucose retardation index . Journal of the Science of Food and Agriculture , 71 : 515 – 519 .
- Velioglu , Y.S. , Mazza , G. , Gao , L. and Oomah , B.D. 1998 . Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products . Journal of Agricultural and Food Chemistry , 46 : 4113 – 4117 .
- Benzie , I.F.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma as a measure of antioxidant power, the FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Li , H.B. , Wong , C.C. , Cheng , K.W. and Chen , F. 2008 . Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants . LWT- Food Science and Technology , 41 : 385 – 390 .
- Amin , I. and Tan , S.H. 2002 . Antioxidant activity of selected seaweeds . Malaysian Journal of Nutrition , 8 : 167 – 177 .
- Choi , Y.M. , Jeong , H.S. and Lee , J.S. 2007 . Antioxidant activity of methanolic extracts from some grains consumed in Korea . Food Chemistry , 103 : 130 – 138 .
- De Ritter , E. and Purcell , A.E. 1981 . “ Carotenoid analytical methods ” . In Carotenoid as colorants and vitamin A precuisorsed , Edited by: Bauernfeind , J.C. 815 – 923 . New York : Academic Press .
- AOAC . 1990 . Official Methods of Analysis , 16th , 16 – 17 . Gaithersburg, MD : AOAC International .
- Pellegrini , N. , Serafini , M. , Colombi , B. , Del Rio , D. , Salvatore , S. , Bianchi , M. and Brighenti , F. 2003 . Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays . Journal of Nutrition , 133 : 2812 – 2819 .
- Soong , Y.Y. and Barlow , P.J. 2004 . Antioxidant activity and phenolic content of selected fruit seeds . Food Chemistry , 83 : 411 – 417 .
- Quek , S.Y. , Chok , N.K and Peter , S. 2007 . The physicochemical properties of spray-dried watermelon powders . Chemical Engineering and Processing , 46 : 386 – 392 .
- Larrauri , J.A. , Rupérez , P. and Saura-Calixto , F. 1997 . Mango peel fibres with antioxidant activity . Z Lebensm Unters Forsch , 205 : 39 – 42 .
- Ramulu , P. and Rao , P.U. 2003 . Total insoluble and soluble dietary fibre contents of Indian fruits . Journal of Food Composition and Analysis , 16 : 677 – 685 .
- Deepa , N. , Kaur , C. , Singh , B. and Kapoor , H.C. 2006 . Antioxidant activity in some red sweet pepper cultivars . Journal of Food Composition and Analysis , 19 : 572 – 578 .
- Godoy , H.T. and Rodriquez-Amaya , D.B. 1987 . Changes in individual carotenoids on processing storage of mango (Mangifera indica) slices and puree . International Journal of Food Science and Technology , 22 : 451 – 460 .
- Shivashankara , K.S. , Isobe , S. , Al-Haq , M.M. , Takenaka , M. and Shiina , T. 2004 . Fruit antioxidant activity, ascorbic acid, total phenol, quercetin, and carotene of Irwin mango fruits stored at low temperature after high electric field pretreatment . Journal of Agricultural and Food Chemistry , 52 : 1281 – 1286 .
- Schieber , A. , Ullrich , W. and Carle , R. 2000 . Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection . Innovative Food Science and Emerging Technologies , 1 : 161 – 166 .
- Burda , S. and Oleszek , W. 2001 . Antioxidant and antiradical activities of flavonoids . Journal of Agricultural and Food Chemistry , 49 : 2774 – 2779 .
- Khokhar , S. and Apenten , R.K.O. 2003 . Iron binding characteristics of phenolic compounds: some tentative structure-activity relations . Food Chemistry , 81 : 133 – 140 .
- Antonio , C. and Marino B , A. 2005 . Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties . International Journal of Food Properties , 8 : 521 – 528 .
- Benzie , I.F. and Stezo , Y.T. 1999 . Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 47 : 633 – 636 .
- Sun , T. and Ho , C.T. 2005 . Antioxidant activities of buckwheat extracts . Food Chemistry , 90 : 743 – 749 .
- Proteggente , A.R. , Pannala , A.S. , Paganga , G. , Buren , L.V. , Wagner , E. , Wiseman , S. , Van De Put , F. , Dacombe , C. and Rice-Evans , C.A. 2002 . The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition . Free Radicals Research , 36 : 217 – 233 .