Abstract
Prosopis alba is an arboreal legume that occurs naturally in Argentine. Its fruits (algarroba or Prosopis pods) are dried or roasted, ground and then used in foods and feeds. The influence of roasting process on the water sorption isotherms of Prosopis pod flour at three storage temperatures was studied. The equilibrium data for each sorption isotherm were determined by the standard static gravimetric method. Experimental values were fitted to the BET and GAB sorption models. Type II isotherms were obtained according to Brunauer classification. The calculated isosteric heats of sorption (Qs) near the monolayer moisture content were 7.30 and 7.68 kJ/mol for raw and roasted flour, respectively. The results showed that roasting did not significantly change the behavior of the products with regard to water adsorption, although a slight reduction of the tendency to humectation was observed, this being somewhat less spontaneous. In this aspect, the stability of Prosopis flour is similar in the raw and roasted states.
INTRODUCTION
All Prosopis species are tree or bush legumes. The fruit has high protein and carbohydrate contents and varies in size, color and chemical characteristics according to species.[Citation1] Forty-four species of Prosopis genus are known worldwide, in America, south-west Asia and Africa. Twenty-eight species can be found in Argentine, including Prosopis alba Griseb. The fruit is in the form of arched, annular or linear pods, from 12 to 25 cm long, 1.2 to 1.8 cm wide and 0.5 to 0.6 cm thick, with an average weight of 10 g, indehiscent, straw-colored when ripe, very compressed, with parallel edges.[Citation2,Citation3,Citation4] Prosopis pods have been used as a source of food in almost all places where these trees or bushes grow.[Citation5] Burkart[Citation6] describes Prosopis in Santiago del Estero (Argentine) and says that the fruit is a staple in the diet of the local population, who make “patay” (sweet pastry in the form of a dry pancake), “aloja” (fermented alcoholic drink), and “añapa” (sweet non-alcoholic drink). Due to its nutritional value and availability, Prosopis pods can be processed and used as food supplements and ingredients.[Citation5] Recent research in Argentine has focused on the production and characterization of roasted flour made from Prosopis pods as coffee and cocoa substitutes.[Citation2]
Labuza[Citation7] suggested the use of working isotherms based on the actual drying and humidification cycle that the product goes through. If a food is going to be humidified, the adsorption isotherm should be used. This is the case of the roasted flour of Prosopis pod. Characterization of water sorption allows to predict the product behavior and to determine its critical water activity and critical water content that defines the acceptability ranges of the products. The sorption isotherms allow that changes in product water content to be predicted during handling and storage. Sorption data at different temperatures makes thermodynamic analysis of the system possible, providing information about the water-product interactions throughout sorption heat values.[Citation8] The sorption isotherms are useful for theoretical purposes but also have practical uses. Theoretical aspects include both thermodynamic issues (determination of the monolayer moisture content and calculation of the adsorption-desorption enthalpies) and structural aspects (studies of specific surface, pore volume/size distribution ratio and crystallinity). There is little information on sorption isotherms of Prosopis pods available in the scientific literature; the only reference found was about raw flour of Prosopis ruscifolia seeds.[Citation9] The objective of the present work was to study the effects of the roasting process on the sorption isotherms of Prosopis pod flour at the usual storage temperature range (5–45°C).
MATERIALS AND METHODS
Materials and Pre-treatment
The ripe Prosopis pods were handpicked from trees (Prosopis alba Griseb) selected from pure stands in Presidencia Roque Sáenz Peña (Chaco, Argentine). Some of the pods were roasted in a forced air circulation gas oven at 160°C for 60 min. The samples of raw and roasted Prosopis pods were ground in a hammer mill (Retsch Mühle, SK 1, Germany) equipped with a sieve with a 10 mm stainless steel mesh. Sifting was carried out in an electromagnetic lab sifter (Fritsch, Analysette 3, Germany) equipped with stainless steel mesh sieves of 0.85 and 0.25 mm, at 3000 vibrations per minute for 20 min.
Isotherm Measurements
The equilibrium data to construct the sorption isotherms of raw and roasted Prosopis pod fraction (flour) retained by the 0.25 mm mesh were determined by the static gravimetric method standardized by the European Cooperation in Scientific and Technological Research Project COST-90.[Citation10,Citation11] This method is based on the determination of the moisture content of the sample after reaching equilibrium in air of known relative humidity. Triplicate samples of approximately 1 g of Prosopis flour were uniformly distributed in small dishes, which were placed on tripods in hermetically closed containers into which saturated salt solutions of known water activities (a w) had previously been poured.[Citation12,Citation13] The volume of solution ranged from 40 ml for low a w solutions, to 20 ml for high a w solutions according to AOAC Official Method 978.18D[Citation14] to keep the internal relative humidity of each container constant at a known level.
The samples of Prosopis raw and roasted flour were kept at constant temperatures of 5°C, 25°C and 45°C until constant weight, equilibrating a w with the relative humidity inside the airtight containers. This was considered when the weight variation between two consecutive sample weightings was less than 1 mg/g.[Citation15] Since this procedure required a 10-day stabilization time, the possible microorganism growth (which can happen in samples equilibrated with a w > 0.65 saturated salts) was prevented by the addition of 1 mg/g of potassium sorbate, which do not affect the sorption process.[Citation16] When equilibrium was reached, the moisture content of the raw and roasted flour samples was calculated (EquationEq. 1), since initial moisture content and initial and final weights were known.
The experimental values of w and a w were fitted by the BET model[Citation18] for a w < 0.5. At higher a w levels, the physical meaning of the model is doubtful[Citation19,Citation20] because the principal phenomena that describe water interaction in the product are not due to sorption but are rather of the solute-solvent type, and water comes out mobilizing solutes in the liquid phase.[Citation21] Parameters w 0 (monolayer moisture content) and C (constant related to the heat implied in the sorption process) were estimated by linear regression (EquationEq. 2). The BET equation in linear form is expressed as:
The specific surfaces (S) of Prosopis flour were obtained from the w 0 values (EquationEq. 3). This is the area available for water adsorption phenomena of the adsorbent substrate and very important for establishing superficial reaction rates.[Citation22]
The experimental data were also fitted by the GAB model (Guggenheim-Anderson-de Boer)[Citation23], which was referred to as the best predictive model for foodstuffs by the COST-90 project for a wide range of a w (0.1 to 0.9).[Citation10,Citation24] Its parameters, w 0 (monolayer moisture content), C (Guggenheim constant related to monolayer sorption heat) and K (correction factor related to multilayer sorption heat), were fitted by non-linear regression throughout the a w interval studied (EquationEq. 4).
Applying Clausius-Clapeyron equation (EquationEq. 5) for liquid-vapor equilibrium permitted the enthalpy associated with the sorption process to be calculated.
The entropy of sorption (ΔS) was calculated by applying equation:
The isosteric heat of sorption (Q s, enthalpic change associated with the sorption process) was calculated as follows:
RESULTS AND DISCUSSION
The initial water content determined in raw and roasted flour of Prosopis pod was w = 7.17 ± 0.05 (g water/100 g sample) and w = 6.86 ± 0.03 (g water/100 g sample), respectively. gives the water sorption isotherms obtained for raw and roasted flour, which show the sigmoid form, typical of most foodstuffs. These curves correspond to type II isotherms according to Brunauer classification.[Citation25] As expected, a decrease in water adsorption capacity was observed when the temperature was raised. This is in agreement with the exothermic character of the adsorption process. Roasting results in larger differences between the isotherms of raw and roasted flour, the latter showing a lower capacity for water adsorption. This can be explained by chemical modifications in the proteins associated with the denaturalization phenomenon and the increase in interactions with other components such as carbohydrates and lipids generated by the roasting process.[Citation26] A rise in protein hydrophobicity, due to the high roasting temperatures, could mean there are fewer sites available for water adsorption in the substrate.
Figure 1 Water sorption isotherms of raw and roasted flour of Prosopis pod at 5 °C (▴), 25 °C (•) and 45 °C (▪). Experimental data (open symbols, raw flour; closed symbols, roasted flour).
shows the values obtained for each parameter of the BET model and also the specific adsorption surface estimated from the w 0 parameter. For the adsorption of water vapor, the monolayer moisture content was defined as the water content for which many foodstuffs present the greatest stability in relation to chemical reactions.[Citation21] A w 0 value of 4% (dry basis) at 25°C for roasted flour indicates that, at higher water content, it will present instability against many deterioration processes. The stickiness of flour together with the crystallization of its sugars, may seriously affect its quality and stability. The shape of the isotherm is related to values of the C parameter. EquationEq. (2) corresponds to the difference between two hyperbolas (each quotient represents a hyperbola). This difference depends on the value of C, since it only appears in the second quotient. It can be demonstrated that only after a certain C value there is an inflection point reached in the curve, the isotherm then being type II. The inflection point only appears for values of C > 2.[Citation21] C values are related to sorption heat, and low parameter values, such as those found in this product, indicating low sorption energy. A decrease in the parameter values can be observed due to the effects of roasting. Both monolayer moisture content and the C parameter decrease, indicating the product will have affinity for water. This could be related to the degradation-oxidation of many hydrophilic compounds during roasting of Prosopis pod.
Table 1 Parameter values obtained from fitting the BET model to isotherms of raw and roasted flour of Prosopis pod and specific surface of adsorption (S)
The values obtained for each of the parameters of the GAB model, all of which show to be similarly affected by roasting, are given in . Monolayer moisture content is very similar to that obtained with the BET model. The advantage of GAB model over the BET model is that it is possible to predict the water content-a w relationship in the broadest a w interval.
Table 2 Parameter values obtained from fitting the GAB model to the isotherms of raw and roasted flour of Prosopi s pod
Clausius-Clapeyron analysis of sorption data at different temperatures (5, 25, and 45°C)[Citation23] allowed to obtain values for enthalpy (ΔH), Gibbs free energy (ΔG) and entropy (ΔS) in function of the moisture content of raw and roasted flour. to show typical ΔH, ΔG and ΔS values, at different water content levels (w e). The water content at which ΔH and ΔS are at a maximum roughly coincides with the BET monolayer value, which represents the amount of water covering the primary polar sites by one layer. When most of the accessible points are saturated, the water vapor will be adsorbed at the primary points of less accessible zones with a greater density of polymeric segments, so that the associated sorption heat will be at a maximum just before the monolayer is complete. The spontaneity of the sorption process in the Prosopis flour is described by the negative ΔG values. The drop in ΔG when water content increases, approaching zero at high moisture levels, shows that adsorption process loses energy as humidity increases.
Figure 2 Enthalpy of sorption (ΔH) as a function of equilibrium moisture (w e) content for raw (○) and roasted (•) flour of Prosopis pod.
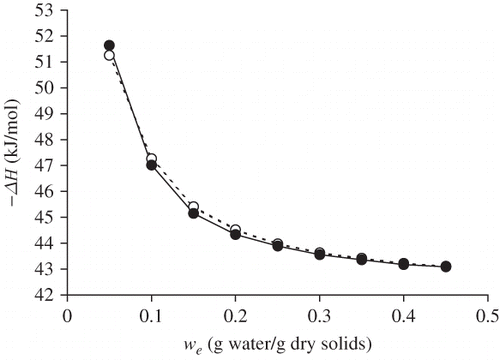
Figure 3 Gibbs free energy of sorption (ΔG) at 25°C as a function of equilibrium moisture content (w e) for raw (○) and roasted (•) flour of Prosopis pod.
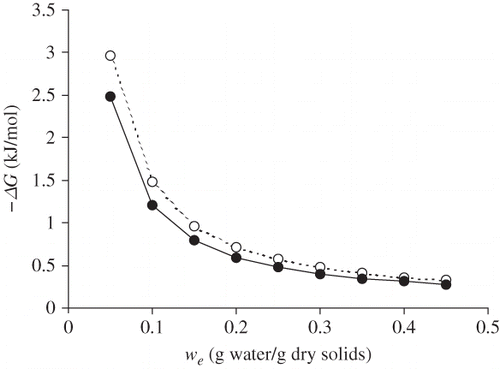
Figure 4 Entropy of sorption (ΔS) at 25°C as a function of equilibrium moisture (w e) content for raw (○) and roasted (•) flour of Prosopis pod.
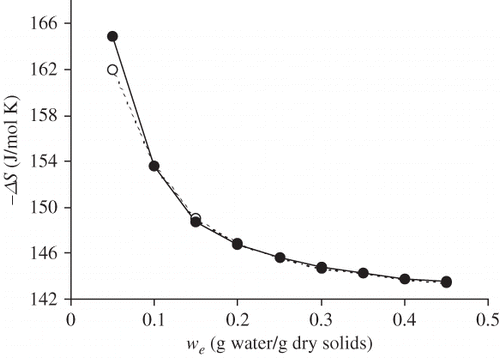
The ΔH values were similar for roasted and raw flour, which indicates that the roasting process did not cause differences in the binding forces involved in the sorption mechanism. The ΔG values of the process are lower in the case of roasted flour, which reflects the lesser tendency of the roasted product to humectation. The entropy values (ΔS) were similar for raw and roasted flour, which indicates a similar degree of the ordering of water molecules in both products.
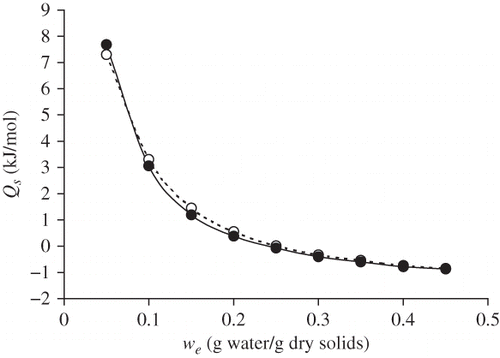
shows how isosteric heat of sorption (Q s) quickly decreases when moisture rises, for both raw and roasted flour. Q s was lower in the roasted flour at almost all moisture values, except near the monolayer moisture content. The high dependence of Q s on moisture content has generally been associated with the high polar activity of sites on the surface of foodstuffs with low water content.[Citation27] Q s has negative values at high moisture values for both raw and roasted flour. Such negative Q s values at high moisture have been reported for foodstuffs containing sugar, such as raisins.[Citation28] The negative values represent the situation where the isosteric heat of sorption is lower than the heat of vaporization of pure water, then water molecules react preferably with other water molecules.[Citation29] The dissolution of sugars and other macromolecules, together with capillary condensation, are the predominant phenomena at high water contents.[Citation27,Citation29–31]
CONCLUSIONS
Roasting does not modify Prosopis pod flour behavior regarding water adsorption to any great extent. The study of the sorption isotherms shows that the stability of the product, and therefore its recommended storage conditions, are not modified by roasting. However, the roasting process provides a product with other characteristics of color, aroma, and taste, which make it more attractive to consumers and thus the treatment becomes recommendable for Prosopis pods.
ACKNOWLEDGMENTS
The authors acknowledge the Secretaria General de Ciencia y Tecnica of the Universidad Nacional del Nordeste (Argentine) for the financial support given to this investigation and the R&D+i Linguistic Assistance Office at the Universidad Politecnica de Valencia (Spain) for their help in translating this paper.
REFERENCES
- Fagg , C. and Stewart , J. 1994 . The value of Acacia and Prosopis in arid and semi-arid environments . Journal of Arid Environments , 27 : 3 – 25 .
- Prokopiuk , D. , Cruz , G. , Grados , N. and Chiralt , A. 2001 . “ Estudio comparativo entre algarroba argentina (Prosopis alba Griseb) y algarroba peruana (Prosopis pallida) ” . In Series de Ciencia e Ingenieria de Alimentos, Investigacion del Postgrado del Instituto de Alimentos para el Desarrollo, Departamento de Tecnologia de Alimentos , Edited by: Fito , P. , Chiralt , A. , Andres , A. and Martinez-Navarrete , N. Vol. 2 , 545 – 554 . Spain : Editorial de la Universidad Politecnica de Valencia .
- Roig , F. 1993 . “ Informe nacional para la seleccion de germoplasma en especies de Prosopis de la Republica Argentina ” . In Conservacion y Mejoramiento de Especies del Genero Prosopis. Quinta Reunion Regional para America Latina y el Caribe de la Red de Forestacion del Centro Internacional de Investigaciones para el Desarrollo , 1 – 36 . Mendoza, Argentine : Instituto Argentino de Investigaciones de las Zonas Aridas .
- Burkart , A. 1976 . A monograph of the genus Prosopis . Journal of the Arnold Arboretum , 57 ( 3 ) : 217 – 249 . (4), 450–525
- Cruz , G. 1999 . Production and characterization of Prosopis seed galactomannan , 113 Zurich : PhD Thesis, Swiss Federal Institute of Technology .
- Burkart , A. 1952 . Las leguminosas argentinas, silvestres y cultivadas , 126 – 143 . Buenos Aires, Argentine : Ediciones Acme Agency .
- Labuza , T. 1984 . Moisture sorption: practical aspects of isotherm, measurement and use , 149 Saint Paul, MN : American Association of Cereal Chemists .
- Martinez-Navarrete , N. and Chiralt , A. 1996 . Influence of roasting on the water sorption isotherms of nuts . Food Science and Technology International , 2 ( 6 ) : 399 – 404 .
- Freyre , M. , Baigorria , C. , Rozycki , V. , Bernardi , C. , Martinez-Navarrete , N. and Camacho , M. 2001 . “ Composición y propiedades de semillas de vinal (Prosopis ruscifolia L.) ” . In Series de Ciencia e Ingenieria de Alimentos, Investigacion del Postgrado del Instituto de Alimentos para el Desarrollo, Departamento de Tecnologia de Alimentos , Edited by: Fito , P. , Chiralt , A. , Andres , A. and Martinez-Navarrete , N. Vol. 2 , 229 – 240 . Spain : Editorial de la Universidad Politecnica de Valencia .
- Wolf , W. , Spiess , W. and Joung , G. 1985 . “ Standardization of isotherm measurements (COST Project 90 and 91 bis) ” . In Properties of water in foods , Edited by: Simatos , D. and Multon , J. 661 Dordrecht, , Netherlands : Martinus Nijhoff Publishers .
- Spiess , W. and Wolf , W. 1983 . “ The results of the Cost 90 Project on water activity ” . In Physical properties of food , Edited by: Jowitt , R. , Escher , F. , Hallström , B. , Meffert , H. , Spiess , W. and Vos , G. 65 – 91 . London and New York : Applied Science Publishers .
- Kanade , P. and Pai , J. 1988 . Moisture sorption method for hygroscopic samples using a modified proximity equilibration cell . Journal of Food Science , 53 ( 4 ) : 1218
- Greenspan , L. 1977 . Humidity fixed points of binary saturated aqueous solutions . Journal of Research of the National Bureau of Standards - A. Physics and Chemistry , 81a ( 1 ) : 89 – 96 .
- AOAC Official Method 978.18D . 2000 . “ Water activity of canned vegetables. Preparation of reference salt slushes ” . In Official methods of analysis of AOAC international , 17th , Edited by: Horwitz , H. Vol. II (42) , 2 Gaithersburg, MD : AOAC International .
- Labuza , T. , Kaaname , A and Chen , J. 1985 . Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods . Journal of Food Science , 50 : 385 – 391 .
- Pilosof , A. 2000 . “ Propiedades de hidratacion ” . In Caracterizacion funcional y estructural de proteinas , Edited by: Pilosof , A. and Bartholomai , G. 17 – 29 . Argentine : Editorial Universitaria de Buenos Aires .
- AOAC Official Method 925.10. Solids (total) and moisture in flour . 2000 . Official methods of analysis of AOAC international , 17th , Edited by: Horwitz , H. Vol. II (32) , 1 Gaithersburg, MD : AOAC International .
- Brunauer , S. , Emmett , P. and Teller , E. 1938 . The adsorption of gases in multimolecular layers . Journal of American Chemical Society , 60 : 309 – 320 .
- Iglesias , H. and Chirife , J. 1976 . Prediction of effect of temperature on water sorption isotherms of food material . Journal of Food Technology , 11 : 109 – 118 .
- Van den Berg , C. 1985 . “ Development of BET - like models for water of food. Theory and relevance ” . In Properties of water in foods , Edited by: Simatos , D. and Multon , J. 119 Dordrecht, , Netherlands : Martinus Nijhoff Publishers .
- Martinez-Navarrete , N. , Andres , A. , Chiralt , A. and Fito , P. 1998 . Termodinamica y cinetica de sistemas alimento entorno , 137 – 221 . Spain : Editorial de la Universidad Politecnica de Valencia .
- Labuza , T. 1980 . The effect of water activity on reaction kinetics of food deterioration . Food Technology , 34 ( 4 ) : 36 – 51 . 59
- Van den Berg , C. and Bruin , S. 1981 . “ Water activity and its estimation in food systems: theoretical aspects ” . In Water activity: Influences on food quality , Edited by: Rockland , L. and Stewart , G. 1 – 55 . London and New York : Academic Press .
- Schär , W. and Rüegg , M. 1985 . The evaluation of GAB constants from water vapor sorption data . Lebensmittel Wissenschaft und Technologie , 18 : 225 – 229 .
- Leung , H. 1986 . “ Water activity and other colligative properties of foods ” . In Physical and chemical properties of foods , Edited by: Okos , M. 138 – 185 . St. Joseph, MI : American Society of Agricultural Engineers .
- Perez , I. 1995 . Estudio de algunos cambios quimicos y microestructurales producidos en el proceso de elaboracion del turron de Jijona , 100 Spain : Doctoral Thesis, Universidad Politecnica de Valencia .
- Kapsalis , J. 1987 . “ Influences of hysteresis and temperature on moisture sorption isotherms ” . In Water activity: theory and applications to food , Edited by: Rockland , L. and Beuchat , L. 173 – 213 . New York : Marcel Dekker .
- Saravacos , G. , Tsiourvas , D. and Tsami , E. 1986 . Effect of temperature on the water adsorption isotherms of sultana raisins . Journal of Food Science , 51 ( 2 ) : 381 – 383 . 387
- Okos , M. , Narsimhan , G. , Singh , R. and Weitnauer , A. 1992 . “ Food dehydration ” . In Handbook of food engineering , Edited by: Heldman , D. and Lund , D. 437 – 536 . New York : Marcel Dekker .
- Haque , A. , Shimizu , N. , Kimura , T. and Bala , B. 2007 . Net isosteric heats of adsorption and desorption for different forms of hybrid rice . International Journal of Food Properties , 10 ( 1 ) : 25 – 37 .
- Tsami , E. 1991 . Net isosteric heat of sorption in dried fruits . Journal of Food Engineering , 14 : 327 – 335 .