Abstract
Glass transition of rainbow trout muscle was measured by differential scanning calorimetry (DSC) as −13°C. Sucrose and sorbitol (2, 2), sucrose and mannitol (2, 2), sucrose and gum arabic (2, 0.15), sucrose and carrageenan (2, 0.15), sorbitol and mannitol (2, 2), sorbitol and gum arabic (2, 0.15), sorbitol and carrageenan (2, 0.15), mannitol and gum arabic (2, 0.15) and mannitol and carrageenan (2, 0.15) were blended with ground rainbow trout as g/100 g fish and stored for 6 months separately at −9°C, −13°C and −18°C. Total volatile basic nitrogen (TVB-N) and Thiobarbituric acid-reactive substances (TBARS) were determined at 1st, 3rd and 6th months of storage periods. Biopolymers blends, storage temperature and storage period had a significant effect (p < 0.05) on the TVB-N and TBARS values.
INTRODUCTION
Safety and spoilage of foods are the major concern of food manufacturer, retailers and consumers. Recently, it has been stated that aw alone could not sufficient to determine the stability of foods thus the concept of glass transition temperature was proposed.[Citation1] The concept acknowledged that this transition greatly influences food stability, as the water in the concentrated phase becomes kinetically immobilized and therefore does not support or participate in reactions.[Citation2] Due to this very restricted molecular motion, a food product in a glassy state is assumed that deteriorative reactions could no take place during storage.[Citation3] However, it has been recently identified that glassy state may not be sufficient to limit molecular motions.[Citation3–6]
Researches on glass transition of different fish are limited and with the exception of those reported by Levine and Slade,[Citation7] Brake and Fennema,[Citation8] and Jensen et al.[Citation9] the values are inexplicably low (−44°C to −77°C). For cod, reported values are −77°C,[Citation10] −40°C,[Citation11] −15°C,[Citation7,Citation9] and −12°C.[Citation8] For tuna, reported values are −68°C to −71°C,[Citation12] −74°C,[Citation13] −72°C,[Citation9] −54°C,[Citation14] and −11.5°C to −18°C.[Citation7] For king fish, samples with high water contents reported values are −21.4 to −24.78°C.[Citation15]
Although the primary purpose of food freezing is to preserve and to extend shelf life, however some deterioration continues to occur even at frozen storage temperatures especially at temperatures above −10°C. Changes that may occur during frozen storage are lipid oxidation and protein degradation. Rates of chemical deterioration are greatly reduced by freezing, but reactions such as lipid oxidation continue slowly even in frozen state. Most of the chemical changes could be eliminated by reducing temperatures to −80°C, but such temperatures are not economically feasible in most storage facilities.[Citation16] It should, therefore, be informative to study oxidation of lipids in muscle tissue, giving special attention to changes in the rates of these reactions. It is currently not known whether either of these reactions would be diffusion limited.[Citation17,Citation18]
Addition of biopolymers to food systems could be cause the increase of Tg , and they can therefore be stored at higher temperatures with greater stability and longer storage life with respect to diffusion limited reactions.[Citation19] The physical mechanisms of cryoprotection by biopolymers are not fully understood. Despite the fact that some evidence can be produced to support this, it is still difficult to mention about extensive researches in this particular area. Thus, it is significant and meaningful to determine the effects of an added biopolymer on the temperature dependence of reaction rates. The objectives of this study were: (1) to determine the glass transition of rainbow trout using differential scanning calorimetry (DSC), (2) to determine the temperature and storage period dependence of the rates of oxidation of lipids, and protein degradation in frozen ground fish with and without the addition different biopolymer blends.
MATERIALS AND METHODS
Source of Fish and Chemicals
Fresh water rainbow trout with an average 200 g from a farm located in Research and Extension Center of Fisheries Department in Agricultural Faculty at Atatürk University in Erzurum were used in this research. Fillets were obtained from them by removing their heads and bones by hand. Sucrose, sorbitol, gum arabic were provided by Fluka Chemia, and mannitol was provided by Cargill (Cargill S. r. l., Milano), and κ-carrageenan was provided by Incom (Incom A.Ş. Mersin, Turkey).
Sample Preparation
Fillets were ground once through a 3-mm plate, and then mixed separately with biopolymer blends [(sucrose and sorbitol (2, 2), sucrose and mannitol (2, 2), sucrose and gum arabic (2, 0.15), sucrose and carrageenan (2, 0.15), sorbitol and mannitol (2, 2), sorbitol and gum arabic (2, 0.15), sorbitol and carrageenan (2, 0.15), mannitol and gum arabic (2, 0.15) and mannitol and carrageenan (2, 0.15) g/100 g fish)] for 2 min using a Hobart chopper. Each ground fish samples (75 g) with and without the addition different biopolymer blends were vacuum packaged and stored for 24 h at 4°C to allow biopolymer diffusion and then frozen at −40°C. The frozen fish samples were stored at −9°C, −13°C and −18°C for the periods of 1, 3, and 6 months. At different temperatures and storage periods, the stored samples were taken for analysis.
Measurement of Tg by DSC
The glass transition value was determined by DSC (DSC-50, Shimadzu Corporation, Kyoto, Japan) equipped with a low temperature cooling unit (LTC-50, Shimadzu Corporation, Kyoto, Japan). The DSC was calibrated using mercury and indium standards, with onset temperatures of −38.9°C and 156.6°C, respectively. Approximately 10 mg fish samples were weighed into aluminum DSC pans, hermetically sealed, and then loaded onto the DSC instrument at room temperature, using an empty pan as a reference. Nitrogen at a flow rate of 30 ml/min was used as a carrier gas. Samples were then cooled at 5°C/min to −80°C, held for 15 min, warmed up to the annealing temperature (−15°C), held for 1 h, re-cooled to −80°C at 5°C/min, held for 15 min and then scanned at 5°C/min to 20°C. Measurements were recorded with three replicates. Each thermogram was analyzed for the onset, mid, and end of glass transition. Glass transition is reported as the mid point of the step.
TVB-N Determination
TVB-N was determined using the procedure of Anonymous.[Citation20] The method is based on the extraction of TVB-N using alkaline solution and the titration of the recovered ammonia as follows: ten grams of fish were homogenized with an Ultra-Turrax (Type T-25 basic, Germany) for 60 s in 90 ml of 0.6 N perchloric acid. The homogenate were filtered with filter paper. 50 ml of fish extract and few drops of phenolphthalein indicator were placed in the distillation flask. Then 6.5 ml of 20% sodium hydroxide solution was added, the apparatus immediately sealed and the end of the steam distillate collected in a flask containing 100 ml of 0.3% boric acid and few drops of Tashiro indicator (methyl red/methylene blue 2:1). The steam distillation procedure was continued until 250 ml of distillate had been collected. The obtained basic solution was titrated with 0.01 N hydrochloric acid by color change. The TVB-N content was determined after blanc correction that has been determined by the steam distillation of 0.6 perchloric acid sample. The results were expressed as mg TVB-N/100g fish mixture.
TBARS Determination
TBARS values for lipid oxidation was determined according to Lemon.[Citation21] This is based on an aqueous acid extraction of sample (with trichloroacetic acid containing Eyhlenediamin tetraecetic acid (EDTA) and n-propyl gallate) prior to reaction with thiobarbituric acid (TBA). One g fish sample was blended into 6 ml of extraction solution. The samples were homogenized with an Ultra-Turrax (Type T-25 basic, Germany) for 15 s. The homogenate was filtered through Whatman nr 1 filter paper (Maidstone, England). Filtrate (1 ml) was mixed with 1 ml of TBA and vortexed. The mixture was heated at 100°C for 40 min in heating block. After cooling, the sample was centrifuged at 2000 g for 5 min. Absorbance was determined at 532 nm against blank containing 1 ml TCA extraction solution and 1 ml TBA solution. The TBARS values were expressed as mmol TBARS per kg mixture. A standard curve was prepared using tetraethoxypropane.
Statistical Analysis
Statistical analysis was conducted according to completely random block design with two replicates. A one-way analysis of variance (ANOVA) was performed to test the significance among treatments. Data were analyzed with the SPSS.[Citation22] Comparisons of mean values were made using the Duncan test.
RESULTS AND DISCUSSION
Tg Value of Fish Sample
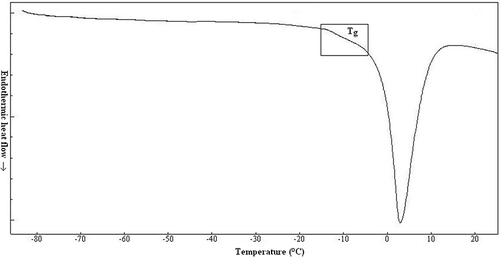
A typical thermal curve of fish heated from −80°C to 20°C with annealing is shown in . The DSC results revealed two characteristic thermal events: the maximal-freeze concentration conditions (Tm′ and Tg′′′) and ice melting. In the literature, it was mentioned that annealing needs to achieve maximal-freeze-concentration condition (that is, real Tm′, and Tg′′′). Tm′ is the characteristic transition (end-freezing point) for the maximum possible formation of ice. The maximum formation of ice could be reached during DSC measurement by controlling the cooling rate and annealing condition. In this research, according to Rahman[Citation23] and Rahman et al.,[Citation24] glass transition (Tg′′′) was defined as the glass transition of the solids matrix (mixture of solids and unfreezable water) in the frozen sample determined from DSC thermogram. The glass transitions were calculated as the initial, mid and end transitions temperatures, according to Rahman et al.,[Citation24] and determined as −14.16 ± 0.14, −13.00 ± 0.31, and −10.95 ± 0.28°C, respectively. Tg′′′ value as the mid point of transition of rainbow trout was determined as −13°C from DSC curves. This value is close to the values reported by Levine and Slade,[Citation7] Brake and Fennema,[Citation8] Jensen et al.[Citation9]
TVB-N Values During Storage
Addition of biopolymer blends caused a decrease in the TVB-N value compared to control (). These results showed that the best biopolymer blends for rainbow trout are sucrose and sorbitol and sucrose and mannitol during the frozen storage. By adding biopolymers to food systems, their Tg can be increased and they can, therefore, be stored at higher temperatures with greater stability and longer storage life.[Citation19] The stabilizing effects associated with stabilizers have been widely explained by preferential interaction and glass transition. The preferential interaction involves preferential exclusion of solute molecules from the hydration shell of proteins. This exclusion prevents proteins from unfolding and thus, its native conformation is stabilized.[Citation25,Citation26] A higher protection for sucrose and sorbitol was associated with a lower molecular weight of sorbitol resulting in a higher effect on the surface tension of water. Also, addition of sucrose resulted in an unfavorable free-energy change which led to the stabilizing solute being excluded from the surface and the protein being preferentially hydrated. Both sugars and polyols stabilize proteins by the mechanism of solute exclusion from the hydration ratio (water molecules on the vicinity) of proteins.[Citation27,Citation28] This means that solutes are excluded from the surface of the proteins and do not react with them.
Table 1 Effect of type of biopolymer blends, storage temperature and storage period on TVB-N values
The effect of storage temperatures on TVB-N values are shown in . As can be seen in , TVB-N values of samples at −9°C were higher than −13°C and −18°C for all treatments. Significant differences were found for general effect of temperature (). Compared to −9°C, samples stored at −13° and 18°C had lower TVB-N values. These results are ascribed to the glass transition. Rahman[Citation2] reported that the food is stable at and below its Tg whereas its deterioration or reaction rates are higher above Tg . In addition, the Tg means the temperature at which the viscosity of amorphous materials becomes high, and the molecular mobility is the lower.[Citation29,Citation30]
Table 2 Effect of type of biopolymer blends, storage temperature and storage period on TBARS' values
The effect of storage period on TVB-N values was shown in . TVB-N values increased with increasing frozen storage periods. TVB-N values increased with storage period and the highest values were determined at the end of the 6th month. This result might be attributed to protein degradation during the storage period. Considered to all treatments, the lowest TVB-N values were obtained for the samples with sucrose and sorbitol at the end of storage period. According to these results possible to say that the best biopolymer blends are sucrose and sorbitol.
TBARS
TBARS values were increased with addition of biopolymer blends compared to control, except for sucrose and sorbitol, sorbitol and gum arabic and sorbitol and carrageenan (). The increase TBARS values caused by biopolymer blends were probably due to physicochemical changes. In glassy system with high water content, the system is mostly made up of glass, and freezing of water can also occur. Jittinandana et al.[Citation31] stated that added biopolymer to system may create certain aqueous microenvironments in frozen system where (1) prooxidants may become more concentrated, or (2) improved hydration of biopolymer could aid in diffusion of water-soluble pro-oxidants to the lipid–water interface, thereby facilitating lipid oxidation. Compared to other cryoprotective agents the inhibitory effect of sorbitol and gum arabic on the lipid oxidation is probably due to a surface active agent of gum arabic and with a lower molecular weight of sorbitol. Gum arabic has a protein moiety which is covalent association to polysaccharide subunits and responsible for the surface activity. The protein-rich high molecular weight fraction of gum arabic is preferentially adsorbed onto the hydrophobic surface while the carbohydrate portion inhibits flocculation and coalescence by electrostatic repulsions and steric forces.[Citation32,Citation33]
Table 3 General effect of storage temperature on TVB-N and TBARS' values
TBARS values of samples stored at −9°C, −13°C and −18°C are shown in . As can be seen in , TBARS values of samples at −9°C were higher than −13°C and −18°C. Significant differences were found for general effect of temperature (). Compared to −9°C, samples stored at −13° and −18°C had lower TBARS values. Rates of diffusion limited reactions are affected by temperature. The reaction may occur also at temperature Tg and below Tg , probably due to the small size of the diffusing oxygen molecules.[Citation29,Citation34,Citation35]
The effect of storage period on TBARS values were shown in . TBARS values increased with increasing frozen storage periods. These results suggest that increased storage periods during frozen storage result in excessive damage for these more sensitive products. Lee et al.[Citation36] reported that good quality meat products exhibited TBARS values less than 0.46 mg/kg and spoiled meat products exhibited TBARS values more than 1.2 mg/kg. Frozen rainbow trout samples stored at any temperature in this research did not rich over 1.2 mg/kg of TBARS after 6th month storage period.
CONCLUSION
In conclusion, the result of the study showed that the biopolymer blends containing sucrose and sorbitol and sorbitol and gum arabic were effective on TVB-N and TBARS values under frozen storage conditions for six months. There were no statistical differences between TVB-N and TBARS values in the samples stored at Tg and −18°C. The relationship between deterioration processes important for preservation of quality and glass transition temperatures still needs to be evaluated to optimize frozen storage conditions. At this point, further research is necessary for determining about the relationship between significant deterioration processes and observed transition temperatures.
REFERENCES
- Sablani , S.S. , Kasapis , S. and Rahman , M.S. 2007 . Evaluating water activity and glass transition concepts for food stability . Journal of Food Engineering , 78 : 266 – 271 .
- Rahman , M.S. 2006 . State diagram of foods: Its potential use in food processing and product stability . Trends in Food Science and Technology , 17 : 129 – 141 .
- Orlien , V. , Andersen , M.L. , Jouhtimaki , S. , Risbo , J. and Skibsted , L.H. 2004 . Effect of temperature and glassy states on the molecular mobility of solutes in frozen tuna muscle as studied by electron spin resonance spectroscopy with spin probe detection . Journal of Agricultural and Food Chemistry , 52 : 2269 – 2276 .
- Champion , D. , Le Meste , M. and Simatos , D. 2000 . Towards an improved understanding of glass transition and relaxations in foods: molecular mobility in the glass transition range . Trends in Food Science and Technology , 11 : 41 – 55 .
- Le Meste , M. , Champion , D. , Roudaut , G. , Blond , G. and Simatos , D. 2002 . Glass transition and food technology: A critical appraisal . Journal of Food Science , 67 : 2444 – 2458 .
- Sablani , S.S. , Al-Belushi , K. , Al-Marhubi , I. and Al-Belushi , R. 2007 . Evaluating stability of vitamin C in fortified formula using water activity and glass transition . International Journal of Food Properties , 10 : 61 – 71 .
- Levine , H. and Slade , L. 1989 . Response to the letter by Simatos, Blond, and Le Meste on the relation between glass transition and stability of a frozen product . Cryo-Letters , 10 : 347 – 370 .
- Brake , N.C. and Fennema , O.R. 1999 . Glass transition values of muscle tissue . Journal of Food Science , 64 : 10 – 15 .
- Jensen , K.N. , Jorgensen , B.M. and Nielsen , J. 2003 . Low-temperature transitions in cod and tuna determined by differential scanning calorimetry . Lebensm.-Wiss. U.-Technol. , 36 : 369 – 374 .
- Nesvabda , P. 1993 . “ Glass transitions in aqueous solutions and foodstuffs ” . In The glassy state in foods , Edited by: Blanshard , J.M.V. and Lillford , P.J. 523 – 526 . Nottingham : Nottingham University Press .
- Simatos , D. and Blond , G. 1993 . “ Some aspects of the glass transition in frozen foods systems ” . In The glassy state in foods , Edited by: Blanshard , J.M.V. and Lillford , P.J. 395 – 415 . Nottingham : Nottingham University Press .
- Inoue , C. and Ishikawa , M. 1997 . Glass transition of tuna flesh at low temperature and effects of salt and moisture . Journal of Food Science , 62 : 496 – 499 .
- Orlien , V. , Risbo , J. , Anderson , M.L. and Skibsted , L.H. 2003 . The question of high-or low-temperature glass transition in frozen fish. Construction of the supplamented state diagram for tuna muscle by differential scanning calorimetry . Journal of Agricultural and Food Chemistry , 51 : 211 – 217 .
- Rahman , M.S. , Kasapis , S. , Guizani , N. and Amri , O.S. 2003 . State diagram of tuna meat: freezing curve and glass transition . Journal of Food Engineering , 57 : 321 – 326 .
- Sablani , S.S , Rahman , M.S. , Al-Busadi , S. , Guizani , N. , Al-Habsi , N. , Al-Belushi , R. and Soussi , B. 2007 . Thermal transitions of king fish whole muscle, fat and fat-free muscle by differential scanning calorimetry . Thermochimica Acta , 462 : 56 – 63 .
- Aberle , E.D. , Forrest , J.C. , Gerrard , D.E. , Mills , E.W. , Hedrick , H.B. , Judge , M.D. and Merkel , R.A. 2001 . Principles of Meat Science , 9 – 197 . Iowa : Kandall/Hunt Publishing Company .
- Brake , N.C. and Fennema , O.R. 1999 . Lipolysis and lipid oxidation in frozen minced mackerel as related to Tg, molecular diffusion, and presence of gelatin . Journal of Food Science , 64 : 25 – 32 .
- Rahman , M.S. , Al-Belushi , R.S. , Guizani , N. , Al-Saidi , G.S. and Soussi , B. 2009 . Fat oxidation in freeze-dried grouper during storage at different temperatures and moisture contents . Food Chemistry , 114 : 1257 – 1264 .
- Herrera , J.J. , Pastoriza , L. , Sampedro , G. and Cabo , M.L. 1999 . Effect of various cryostabilizers on the production and reactivity of formaldehyde in frozen-stored minced blue whiting muscle . Journal of Agricultural and Food Chemistry , 47 : 2386 – 2397 .
- Anonymous . 1988 . Unterschung von Lebensmitteln Bestimmung des Gehaltes von flqchtigen stickstoffhaltigen Basen (TVB-N) in Fischen und Fischerzeugnissen Referenzerfahren , 80 Amtliche Sammlung von Untersuchunsverfahren nach 35 LMBG .
- Lemon , D. W. 1975 . An improved TBA test for rancidity . New Series Circular, Halifax Laboratory, Halifax, Nova Scotia , 51 : 1 – 14 .
- SPSS . 2004 . SPSS for Windows , Chicago, IL : SPSS Inc. .
- Rahman , M.S. , Sablani , S.S , Al-Habsi , N. , Al-Maskri , S. and Al-Belushi , R. 2005 . State diagram of freeze-dried garlic powder by differential scanning calorimetry and cooling curve methods . Journal of Food Science , 70 : E135 – E141 .
- Rahman , M.S. 2004 . State diagram of date flesh using differential scanning calorimetry (DSC) . International Journal of Food Properties , 7 : 407 – 428 .
- MacDonald , G.A. and Lanier , T. 1991 . Carbohydrates as cryoprotectants for meats and surimi . Food Technology , March : 150 – 159 .
- Ohkuma , C. , Kawai , K. , Viriyarattanasak , C. , Mahawanich , T. , Tantratian , S. , Takai , R. and Suzuki , T. 2008 . Glass transition properties of frozen and freeze-dried surimi products: Effects of sugar and moisture on the glass transition temperature . Food Hydrocolloids , 22 : 255 – 262 .
- Sultanbawa , Y. and Li-Chan , E.C.Y. 1998 . Cryoprotective effects of sugar and polyol blends in ling cod surimi during frozen storage . Food Research International , 31 : 87 – 98 .
- Uresti , R.M. , Velazquez , G. , Vazquez , M. , Ramirez , J.A. and Torresc , J.A. 2005 . Effect of sugars and polyols on the functional and mechanical properties of pressure-treated arrowtooth flounder (Atheresthes stomias) proteins . Food Hydrocolloids , 19 : 964 – 973 .
- Lievonen , S.M. and Roos , Y.H. 2002 . Water sorption of food models for studies of glass transition and reaction kinetics . Journal of Food Science , 67 : 1758 – 1766 .
- Roudaut , G. , Simatos , D. , Champion , D. , Contreras-Lopez , E. and Le Meste , M. 2004 . Molecular mobility around the glass transition temperature: a mini review . Innovative Food Science and Engineering Technologies , 5 : 127 – 134 .
- Jittinandana , S. , Kenney , P.B. and Slider , S.D. 2005 . Cryoprotectants preserve quality of restructured trout products following freeze–thaw cycling . Journal of Muscle Foods , 16 : 354 – 378 .
- Phillips , G.O. and Williams , P.A. 2000 . Handbook of hydrocolloids , Edited by: Phillips , G.O. and Williams , P.A. 132 – 145 . New York : CRC Press . (Gum Arabic)
- Izydorczyk , M. , Cui , S.W. and Wang , Q. 2005 . “ Food Carbohydrates: Chemistry, Physical Properties and Applications ” . In Polysaccharide Gums: Structures, Functional Properties, and Applications , Edited by: Cui , S.W. 261 – 305 . New York : CRC Press .
- Bhandari , B.R. and Howes , T. 1999 . Implication of glass transition for the drying and stability of dried foods . Journal of Food Engineering , 40 : 71 – 79 .
- Hansen , E. , Lauridsen , L. , Skibsted , L.H. , Moawad , R.K. and Andersen , M.L. 2004 . Oxidative stability of frozen pork patties: effect of fluctuating temperature on lipid oxidation . Meat Science , 68 : 185 – 191 .
- Lee , Y.C. , Yang , H.S. and Kim , D.H. 2002 . Shelf-life determination of precooked frozen pork meat patties at various temperatures . Journal of Food Processing and Preservation , 26 : 165 – 177 .