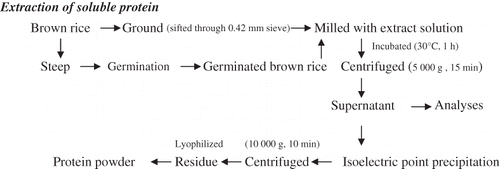
Abstract
Effects of germination methods and extraction conditions on soluble protein yield in brown rice were investigated. Extraction temperature and solid to solvent ratio were significant factors. Highest soluble protein yields were obtained from germinated brown rice at 41.5°C for 2 h using a solid to solvent ratio of 1:10 at pH 8.5. This was about 20% higher than that from non-germinated brown rice. Characteristics of the extracted protein from germinated rice indicated an improved solubility, a lower isoeletric point, a reduction in high molecular weight components and an increase in small peptides compared to non-germinated rice.
INTRODUCTION
Brown rice (BR) is a modality between paddy and white rice, obtained by husking paddy and preserving the embryo, the bran layer, and the aleurone layer. It contains many nutritional components that are almost completely removed from white rice by polishing or milling.[Citation1] BR retains its capacity to germinate in appropriate conditions. During germination, a number of significant biochemical and physiological changes occur, including increases in respiration, nutrient consumption, enzyme activation, and degradation of high molecular weight reserve substances to low molecular weight nutrients. [Citation2–5] At the same time, functional components (such as γ-aminobutyric acid) are increased and anti-nutrients factors (such as phytic acid) are minimized.[Citation2] For this reason, the germination process has been suggested as a technological procedure for improving the nutritional qualities of cereals,[Citation6] and the germinating cereal grain would be a valuable material for production of functional foods being its rich nutrient content, such as the vitamins (E, B1, B2, and B3), the minerals (P, K, Mg, Ca, Zn, S), fiber, ferulic acid, phytic acid, glutathione, and phytosterols et al.[Citation7]
Brown rice protein includes both rice protein and rice bran protein, the nutrition quality of which is just slightly inferior to that of oats, while it surpasses that of wheat and corn.[Citation8] It also has a better amino acid profile than either casein or soy protein.[Citation9] Rice proteins have been recognized as being highly nutritious, hypoallergenic, and particularly healthful for human consumption.
Cereal germination is essentially a process of endogenous enzyme activation and changes in protein are very complex and important during this process. The functional properties of rice and rice bran proteins have been researched well, but the extraction techniques and characteristics of protein from germinated brown rice (GBR) have been little explored. The purpose of this work was, therefore, to optimize extraction techniques via response surface methodology (RSM), examining the effects of extraction temperature, extraction time, solid to solvent ratio and pH on the yield of soluble protein extracted from GBR. Changes in the nature of extractable protein under optimal conditions, including isoelectric points, solubility, and molecular-weight distribution after germination were also characterized.
MATERIALS AND METHODS
Materials
Paddy rice (Oryza sativa L wuyunjing NO.2.) was purchased from Jintan city in Jiangsu Province, P. R. China. The cultivar was harvested in October 2007, and it was stored in a refrigerator at 4°C. It was milled to brown rice with moisture content 10.16%, crude protein 9.06%, crude fat 2.42%, starch 72.44%, and ash 1.24%. Proximate composite was analyzed according to the standard AOAC methods.[Citation10]
Brown Rice Germination
Mature brown rice was steeped in tap water for 6 h, surface-sterilized with 1.0% (v/v) sodium hypochlorite for 30 min at room temperature (25 ± 2°C) and rinsed three times with deionized water. Seed germination was tested in a Petri dish (90 mm in diameter) for soaking culture and in a 100 mL glass beaker (160 mL in volume) for steeping culture. In each Petri dish, 10 g of brown rice seed was randomly placed onto moistened filter paper and 10 mL of deionized water was added. In each beaker, 20 g of brown rice seed, and 100 mL of deionized water were added. Airflow of 1.0 L/min was bubbled into the beaker. Germination was carried out at a constant temperature of 32 ± 1°C in the dark for 12 ∼ 72 h. After germination, the rice was frozen for 20 min in liquid nitrogen and stored at −20°C until further analysis. BR steeped for 6 h was used as a Control. Each treatment was performed in triplicate.
Analysis of Sample
Moisture, ash, and starch content were analyzed according to the standard AOAC methods. The protein content was determined using the Kjeldahl procedure and the obtained nitrogen content was expressed as a protein concentration (N × 5.95). Crude fat was determined by Soxhlet extraction using petroleum ether as the solvent. Soluble protein was determined with the Bradford procedure using bovine serum albumin as a standard.
Determination of Protein Characteristics
Protein isoelectric point: Determination of protein concentration was carried out according to the Lowry method. BR or GBR powder (1.0 g) was added to 10 mL of deionized water and the pH was adjusted to 1 ∼ 6 using 0.1 mol/L NaOH or 0.1 mol/L HCl. The mixtures were kept on a rotary shaker at 120 r/min and 25°C for 2 h, and then centrifuged at 10,000 g for 10 min. One mL supernatant was then made up to 100 mL and 0.5 mL of Na2CO3 and 0.1 mL of Folin reagent were added. The reaction solution was incubated at 30°C for 15 min.
Protein solubility: BR or GBR powder (1.0 g) were added to 50 mL deionized water at pH 3 ∼ 10. The mixtures were stirred with a magnetic stirrer at 25°C for 1 h, then centrifuged at 4,000 g for 10 min. The supernatants were then assayed for protein content using the following formula:
Protein molecular-weight: Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Anderson et al.[Citation11] using a condensed gel concentration of 5% and a separation gel concentration of 12.5%, was used to determine the molecular weights of the extracted proteins. Electrophoresis was carried out at 60 mA for 1 h, then 110 mA for 3 ∼ 4 h, with 0.025 mol/L tris hydrochloric acid solution (Tris-HCl) as the electrode buffer (pH 8.3). Images of the gels were obtained with the ImageMaster VDS (Pharmacia Biotech) and data were analyzed using the ImageMaster 1D Elite V4.00 software provided.
Experimental Design and Statistical Analysis
First, a single factor experiment was performed to analyze the effects of four factors (temperature, time, solvent-solid ratio, and pH) on the amount of protein extracted from GBR. Second, a three-level three-factor Box-Behnken design was chosen for the optimized extraction technique. Experimental factors and levels are listed in . The optimization of protein extraction parameters (temperature, time, and solvent-solid ratio) were performed using above design and a second-order polynomial model was developed. Third, the characteristics of soluble protein extracted under the optimal conditions were studied.
Table 1 Experimental factors, codes and treatment levels of the independent variables
All trials were carried out in triplicate and the means were calculated. Analysis of variance (ANOVA) and Duncan's multiple range tests were used to determine significant differences in protein yield extracted from GBR under different conditions. A second-order polynomial equation was established based on analysis of Box-Behnken experimental data, and the optimal conditions for extraction were obtained using Design Expert version 7.0 software (Version 7.0 Stat-Ease Inc., Minneapolis, MN, USA).
RESULTS AND DISCUSSION
Effects of Germination Method and Time on the Content of Soluble Protein
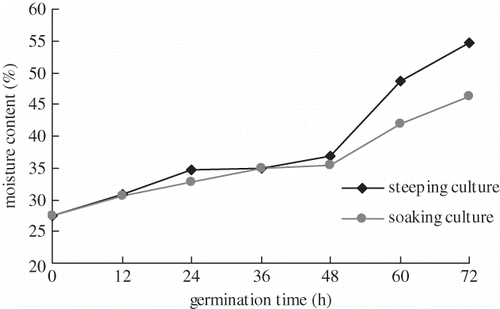
describes relationship between germination time and moisture content using different methods. It revealed that the moisture content increased gradually with a protracting in germination time from 0 to 48 h, the same result was obtained for both steeping and soaking culture. When the germination time exceeds 48 h, the moisture content of brown rice was increased significantly (P < 0.01).
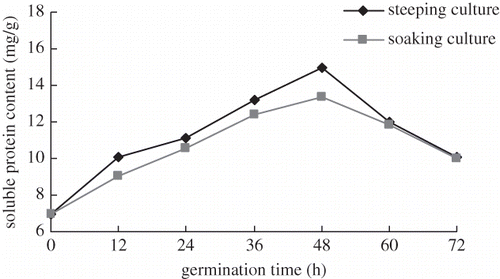
The growth conditions during the germination process have important effects on the composition of secondary metabolites of nutritional importance.[Citation12] The results of soaking culture and steeping culture are shown in . Soluble protein in both methods increased up to the 48th h of germination, after which it started to decrease. The solution protein content of steeping culture was higher by 59.98%, 114.52%, and 45.47% at germination 24, 48, and 72 h, respectively with respect to control sample. Simultaneity, soluble GBR protein obtained by the steeping method was always higher than that obtained by the soaking method. Therefore, GBR steeped for 48 h was used for all further experiments.
It is known that during germination a sequence of metabolic changes such as an increase of moisture content and a loss of dry matter. The activation of the respiratory process and protein synthesis are triggered. Hydrolytic enzymes generate the motion of the stored substances.[Citation13] The synthesis of enzymes during germination might be responsible for the apparent increase in the content of the soluble protein. Furthermore, the endogenous proteases were capable of extensively hydrolyzing the storage protein of BR to become soluble nitrogenous substances and supply the embryo with nitrogen and amino acids.[Citation14] Some researchers had proved that soluble protein was the major nitrogen fraction in the grain germinated in the dark for 6 days.[Citation15]
Relationship between Extraction Conditions and Soluble Protein yield
Effect of extraction temperature on content of soluble protein
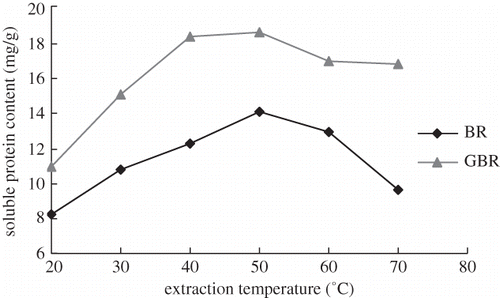
The content of soluble protein increased rapidly with an increase in temperature from 20 to 50°C, due to improvement in protein solubility and swelling of the starch granules.[Citation16] Appropriate heat treatments might partially break down hydrogen and disulfide bonds, resulting in an improvement in protein dissolution rate. However, when temperatures exceeded 50°C, no further improvements in protein extraction were noted. Above 60°C, the starch in solution turned to a viscous paste, preventing further protein recovery. High temperature also promoted aggregation and cross-linking of partially hydrolyzed proteins, thus decreasing their solubility.[Citation17] As seen in , the most effective extraction temperature for GBR protein was lower than that for BR protein. The reasons might be that BR was in rich phytate and insoluble cellulose, which may have combined with protein, leading to poorer solubility. After germination, the phytic and insoluble cellulose were hydrolyzed to soluble substances; therefore, more protein was released. It is therefore, inferred that the for protein extraction temperature should not exceed 50°C for GBR.
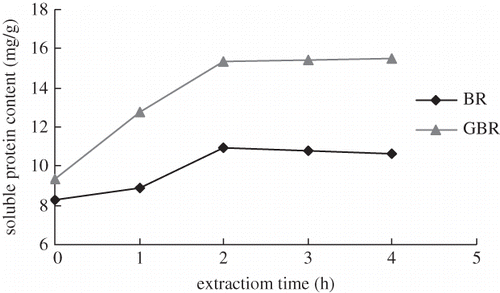
Effect of extraction time on content of soluble protein
shows that protein content increased with the time, increasing from 0 to 2 h, but there were no further increased after 2 h, suggesting that extraction was completed by this time.
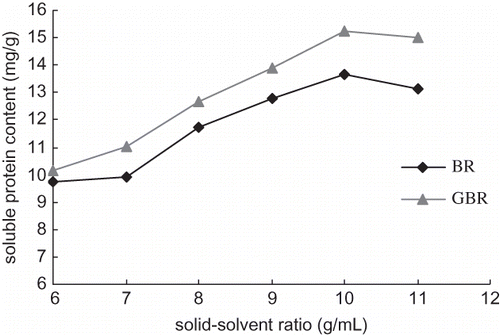
Effect of solid-solvent ratio on content of soluble protein
When the solid to solvent ratio was lower than 1:10, more extract solution was required to complete the protein extraction, as shown in . However, when the ratio was higher than 10, the effect of added solvent was reduced because of the excess extract solution. This suggested that a solid to solvent ratio of 1:10 was the optimal ration for soluble protein extraction.
Effect of extraction solution pH on content of soluble protein
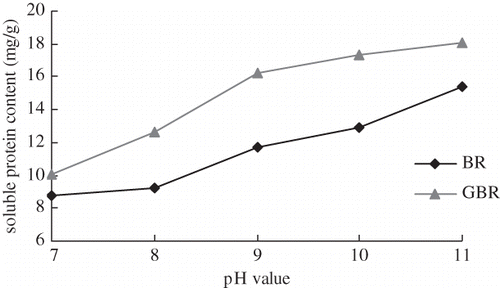
shows that soluble protein in both BR and GBR were increased as the pH of the extraction solution increased from pH 7 to 11. Generally, the majority of rice protein was glutenin, which dissolved readily in an alkaline extraction medium. On the contrary, highly alkaline solutions destroyed the structure of protein, starch, and phytate, and thus, the extraction content was significantly increased. A further concern is that exposure of protein to extreme alkaline conditions may adversely change its nutritional characteristics, for example through conversion of cysteine and serine residues to toxic lysinoalanine.[Citation18] Wang[Citation9] found that alkaline solutions could accelerate protein denaturation and hydrolysis, enhance Maillard reactions, cause protein discoloration, and lower its commercial quality. Hamada[Citation18] advocated that the pH value of an extraction solution for soluble protein should not be higher than 9.0. Thus, pH 8.5 was selected in latter optimization experiments.
Figure 6 Relationship between extraction solvent pH value and protein content. Extraction conditions: temperature 30°C; time 2 h; and solid:solvent ratio 1:9.
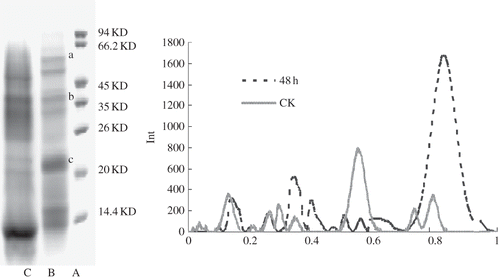
The results from the above experiments indicated that extraction efficiency from GBR was always higher than that from BR. The former was, on average, 1.27 times higher than that of the latter. In germinated rice grains, an increase in oxygen uptake preceded the increase in ATP level, which coincided with the period of rapid increase in activities of hydrolases and the degradation of endosperm reserves. The increase in content of soluble protein during germination must be from the degradation of glutelin by protease, in addition to the synthesis of new soluble proteins (enzymes) from the liberated free amino acids.[Citation15] Okada[Citation19] reported that the increase in soluble protein was 13.8% after germinating 3 days.
Optimization for the Conditions for Soluble Protein Extraction
Extraction temperature, time, and solid to solvent ratio were selected as independent variables. Three levels of the independent variables were chosen according to the results of previous experiments, and the amounts of soluble protein extractable from GBR were taken as response values. Experimental result was listed in . Second-order polynomial equation was established on the basis of analysis of the Box-Behnken experimental data as shown in EquationEq. (2):
Table 2 Observed value of soluble protein content of BR and GBR obtained by RSM experiments
By applying analysis of variance (ANOVA) to the model, which was found to be very significant (P < 0.01), the optimal condition for GBR extraction was found to be an extraction temperature of 41.5°C, an extraction time of 2.0 h and a solid to solvent ratio of 1:10, giving a final soluble protein yield of 22.02 mg/g. Under the same extraction conditions, the protein recovery from BR was 18.28 mg/g. These optimum points were further verified with additional experiments, which indicated that this model is feasible and reliable for predicting the amount of protein extractable from GBR.
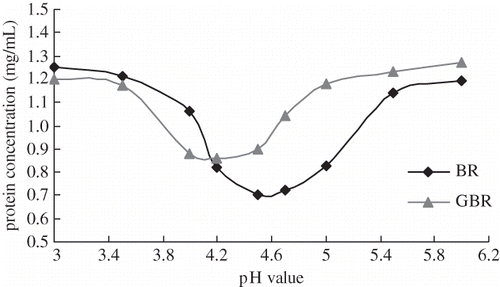
Determination of Isoelectric Points of Soluble Protein in GBR
At isoelectric point, the protein solubility is the lowest. The relationship between pH and the protein extractability from GBR is presented in . Protein extractions from BR and GBR were the lowest at pH range of 4.5 ∼ 4.7 and 4.0 ∼ 4.2, respectively. When the pH was below 4.2, protein extracted from GBR was lower than that of extractable from BR. However, when the pH was above 4.2, the results were found to be reversed. After germination for 48 h, insoluble glutelin in BR has been degraded, and new proteins have been synthesized to enhance protein concentration at an appropriate range of pH. Thus, the isoelectric point of extractable proteins changed from 4.5 ∼ 4.7 to 4.0 ∼ 4.2 in GBR.
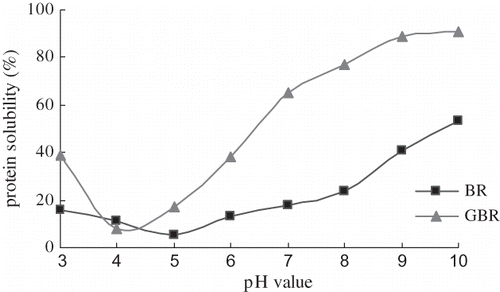
The Change in Solubility of Soluble Protein in GBR
illustrated that the amounts of soluble protein were elevated after germination. BR protein is difficult to dissolve due to extensive disulfide bonding and aggregation. Phytase activity increases earlier than that of other enzymes. Its activity increased within the first day of soaking, before soluble protein increased in the germinating grain.[Citation15] Phytate was decomposed to relax protein aggregation. Ilankovan et al.[Citation17] revealed that enzymatic hydrolysis of rice endosperm protein improved its solubility and emulsification properties. Seed germination involved activation of endogenous proteases. Through this process, protein stored in the endosperm was degraded and the soluble nonprotein components of GBR increased. This might be the reason why solubility of GBR protein was increased.[Citation20] Hamada et al.[Citation21] found that small levels of deamidation (6–16%) increased soy protein solubility under mildly acidic conditions (pH 4–6). also showed that the solubility of BR protein was low in the pH range from 5 to 8, and it increased slowly as the pH increased. Rice grains contain mostly glutelin (about 80%) which is a high molecular weight protein composed of subunits bound by disulfide linkages and which is soluble only in dilute acid or alkali.[Citation17] Germination greatly increased the solubility of the protein, particularly at acidic (pH < 4) and alkaline (pH > 7) conditions. The lowest solubility of BR and GBR protein was at pH of 5.0 and 4.0, respectively. These results were consistent with the isoelectic point test.
Molecular Weight Distribution of Soluble Protein in GBR
SDS-PAGE was used to determine the molecular weights of polypeptides and the overall protein composition of GBR and BR extracts, as shown in . The molecular weights of polypeptides ranged from 14 to 90 KD where detected in BR. Compared with ungerminated BR controls (Lane B), germinated rice seeds (Lane C) showed a reduction in the high molecular weight proteins prevalent in the ungerminated seeds and their replacement with enhanced levels of low molecular weight polypeptides. The predominant bands in GBR were changed to proteins of MW < 40 KD. Peptides under 14KD were also more abundant in GBR than that in BR. These results confirmed that proteins were effectively degraded to small peptides and amino acids by endogenous enzymatic proteolysis. Hari[Citation22] separated the glutelin protein fraction of rice seeds by SDS-PAGE, and found a molecular size range of three glutelin protein subunits, 51KD, 34 KD to 37 KD, and 21 KD to 22 KD. In , the molecular weight of bands of a, b and c in lane B were 50.9 KD, 36.3 KD and 22.3 KD, and they might be that fraction of glutelin protein. After germination, bands a, b, and c in lane C were very faint or had disappeared, indicating that soluble protein increases were mostly due to the glutelin hydrolysis in GBR.
CONCLUSION
The soluble protein content in GBR germinated by steeping culture for 48 h was about 11.84% greater than that obtained by soaking culture. Soluble protein content reached a peak in GBR at 48 h. Extraction condition (temperature, time, and solid to solvent ratio) was optimized and a polynomial regression model was established by RSM. When extraction temperature was 41.5°C, time was 2 h, and solid to solvent ratio was 1:10 at pH 8.5, the soluble protein yield of GBR was 22.02 mg/g, which was 1.2-fold than that of BR extracted under the same conditions. The experiments that verified these optimum conditions indicated that this model could be used to predict the soluble protein yields in GBR. Compared with BR, the GBR protein solubility improved, the isoeletric point declined, the high molecular weight composition decreased, and content of small peptides increased.
ACKNOWLEDGMENTS
This research was financially supported by Jiangsu Science & Technology Department of China (BE2008309).
Notes
REFERENCES
- Ohtsubo , K. , Suzuki , K. , Yasui , Y. and Kasumi , T. 2005 . Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder . Journal of Food Composition and Analysis , 18 : 303 – 316 .
- Chavan , J.K. and Kadam , S. S. 1989 . Nutritional improvement of cereals by sprouting . Critical Reviews in Food Science and Nutrition , 28 ( 5 ) : 401 – 437 .
- Ahmed , F.A. , Abdel-Rahim , E.A. , Abdel-Fatah , O.M. , Erdmann , V.A. and Lippmann , C. 1995 . The changes of protein patterns during one week of germination of some legume seeds and roots . Food Chemistry , 52 ( 4 ) : 433 – 437 .
- Callis , J. 1995 . Regulation of Protein Degradation . The Plant Cell , 7 ( 7 ) : 845 – 857 .
- Morita , N. , Maeda , T. , Watanabe , M. and Yano , S. 2007 . Pre-Germinated Brown Rice Substituted Bread: Dough Characteristics and Bread Structure . International Journal of Food Properties , 10 ( 4 ) : 779 – 789 .
- Mbithi-Mwikya , S. , Van , C.J. , Yiru , Y. and Huyghebaert , A. 2000 . Nutrient and antinutrient changes in finger millet (Eleusine coracan) during sprouting . Food Science and Technology-Lebensmittel-Wissenschaft and Technologie , 33 ( 1 ) : 9 – 14 .
- Jiwan , S.S. , Yearul , K. and Fatma , G.H. 2007 . Functional Foods from Cereal Grains. International . Journal of Food Properties , 10 ( 2 ) : 231 – 244 .
- Shih , F.F. , Champagne , E.T. , Daigle , K. and Zarins , Z. 1999 . Use of Enzymes in the Processing of Protein Products from Rice Bran and Rice Flour . Nahrung/Food , 43 : 14 – 18 .
- Wang , M. , Hetliarachchy , N. S. , Qi , M. , Burjs , W. and Siebenmorgen , T. 1999 . Preparation and Functional Properties of Rice Bran Protein Isolate . Journal of Agriculture and Food Chemistry , 47 ( 2 ) : 411 – 416 .
- Association of Official Analytical Chemists (AOAC) . 1990 . Official methods of analysis , 15th , 51 – 53 . Washington, DC : AOAC .
- Anderson , A. , Hettiarachchy , N. and Ju , Z. Y. 2001 . Physicochemical Properties of Pronase-Treated Rice Glutelin . JAOCS , 78 ( 1 ) : 1 – 6 .
- Yu , H. K. , Pascale , R. , Fernand , L. , Juana , F. and Concepcion , V.V. 2004 . Effects of different germination conditions on the contents of free protein and non-protein amino acids of commercial legumes . Food Chemistry , 86 ( 4 ) : 537 – 545 .
- Wanasundara , P.K.J.P.D. , Shahidi , F. and Brosnan , M.E. 1999 . Changes in flax (Linum usitatissmum) seed nitrogenous compounds during germination Food Chemistry . 65 : 289 – 295 .
- Georg , U. , Peter , K. and Herbert , W. 2006 . Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals . Journal of Cereal Science , 44 ( 3 ) : 368 – 371 .
- Evelyn , P.P. and Bienvenido , O.J. 1972 . Biochemical Changes in the Rice Grain during Germination . Plant Physiol. , 49 : 751 – 756 .
- Sano , Y. 1984 . Differential regulation of waxy gene expression in rice endosperm . Theoretical and Applied Genetics , 68 ( 5 ) : 467 – 473 .
- Ilankovan , P. , Hettiarachchy , N.S. , Christian , S. and Markus , I.B. 2007 . Hydrophobicity, Solubility, and Emulsifying Properties of Enzyme-Modified Rice Endosperm Protein . Cereal Chemistry , 84 ( 4 ) : 343 – 349 .
- Hamada , J.S. 1997 . Characterization of Protein Fractions of Rice Bran to Devise Effective Methods of Protein Solubilization . Cereal Chemistry , 74 ( 5 ) : 662 – 668 .
- Okada , T. 2001 . Physiological Function of Rice Germ Enriched with GABA . Food Industry , 36 ( 6 ) : 7 – 8 . (Japanese)
- Ilankovan , P. , Hettiarachchy , N.S. and Christian , S. 2008 . Preparation of Rice Endosperm Protein Isolate by Alkali Extraction . Cereal Chemistry , 85 ( 1 ) : 76 – 81 .
- Hamada , J.S. and Marshal , W.E. 1989 . Preparation and Functional Properties of Enzymatically Deamidated Soy Proteins . Journal of Food Science , 54 ( 3 ) : 598 – 601 .
- Hari , B. K. and Thomas , W.O. 1986 . Structural Relationship among the Rice Glutelin Polypeptides . Plant Physiol. , 81 : 748 – 753 .