Abstract
Oranges were sanitized, dried, peeled and segmented. Undamaged segments were packed under passive and active MAP with low and high oxygen in PP trays sealed with CPP/OPP film. Overall quality of the segments were monitored for 25 days at 4°C. The results showed that orange segments could be stored under passive and active MAP without significant microbial growth. There were slight changes in acidity and Brix (p ≤ 0.05) with no significant changes in pH and sugar. The hardness increased and color (L, a, b) decreased during storage. Orange segments remained in a commercially viable condition for 10 days under MAP.
INTRODUCTION
Minimal processing has been defined as the handling preparation, packaging and distribution of agricultural commodities in a fresh like state, and may include processes such as dicing, peeling, trimming, slicing and curing.[Citation1] However, the quality of minimally processed fruits rapidly changes during storage because of biological processes such as respiration, ripening and senescence.[Citation2,Citation3] The continuous respiration and metabolism of minimally processed fruits can cause significant changes in the textural, color and flavor qualities.[Citation3,Citation4] Minimal processing may also increase microbial spoilage of fruit through transfer of skin microflora to fruit flesh where microorganisms can grow rapidly.[Citation5]
Modified atmosphere packaging (MAP) is suggested to extend the storage life of fruits and vegetables by controlling respiration rate, senescence and ripening[Citation6,Citation7] by providing low oxygen and high carbon dioxide levels in food packaging. In modified atmosphere (MA) applications, the O2 and CO2 concentrations are modified initially and changes dynamically depending on the respiration rate of commodities and the permeability of the film surrounding the produce.[Citation8,Citation9] MAP can also decrease the rate of browning reactions due to reduced O2 level and elevated CO2 level in the surrounding atmosphere.[Citation7] Packaging under appropriate atmosphere conditions reduced respiration and decreased ethylene production, inhibited or delayed enzymatic reactions, alleviated physiological disorders and preserved the product from quality losses.[Citation8,Citation10–12]
The application of MAP limited the microbial growth and enhanced the quality of strawberries,[Citation13] fresh cut mangoes and pineapples.[Citation14] Soliva-Fortuny and Martin-Bellaso reported that reduced levels of O2 combined with appropriate permeability of plastic package extended the microbiological shelf life of fresh cut pears for almost 3 weeks under refrigerated storage.[Citation10] Soliva-Fortuny, Elez-Martinez and Martin-Bellaso (2004) also indicated similar results for apples.[Citation15] However, packages with low permeability in combination with low O2 atmospheres can stimulate the growth of anaerobic spoilage or pathogenic microorganisms.[Citation12]
Some researchers suggested the use of super atmospheric O2 concentrations ranging from 30 to 100 kPa. Kader and Ben-Yehoshua claimed that elevated O2 atmospheres to control microbiological growth, inhibit enzymatic discoloration and undesirable moisture and odor losses.[Citation16] However, concentrations above 80 kPa may cause pyhtotoxicity. Some authors reported that super atmospheric concentrations are more effective against microorganisms when used with high CO2 levels of 15–20 kPa. High CO2 atmospheres also inhibited most aerobic microorganisms especially gram-negative bacteria and moulds. However, the use of high CO2 concentrations was reported to induce tissue breakdown and formation of large amounts of exudates for fresh-cut apples.[Citation17] High carbon dioxide may also cause undesirable off flavor formation.
Citrus fruits are important because of their nutritional and antioxidant properties.[Citation18] For this reason, citrus fruits are of interest as “ready to eat” products due to convenience and health benefits. There are only few studies found in the literature on the preservation of orange segments. The changes of flavonoids, vitamin C, and antioxidant capacity in minimally processed citrus segments were reported.[Citation18] Another study was on the effect of modified atmosphere packaging on microbial, physicochemical and sensory parameters of “ready to eat” oranges.[Citation19] However, they only applied passive atmosphere in this study. There is negligible study found in the literature about the effect of low and high oxygen applications on the overall quality attributes of orange segments.
The objective of this study was to investigate sensorial, microbiological, physical and chemical quality parameters of minimally processed “ready to eat” orange segments that packaged under three different modified atmospheres: air as passive; low oxygen (20%) and high oxygen (80%) as active MAP treatments using two different PP trays (mono and multilayer PP) at 4°C for 25 days.
MATERIALS AND METHODS
Materials
The Valencia variety oranges (Citrus sinensis) grown in Dortyol, Hatay, the Southern part of Turkey, were provided the day before the processing, and stored at 4°C overnight. Chemicals were supplied by Merck™ (Darmstadt, Germany). PP (Polypropylene) trays (mono and multilayer PP with the dimensions of 144 × 190 × 50 mm) were provided by Huhtamaki Company (Istanbul, Turkey). The lidding material (CPP/OPP with the oxygen and carbon dioxide permeability of 1296 cm3 m−2 day−1 and 3877 cm3 m2 day−1, respectively at 24°C) was provided by A-Pack Company (Istanbul, Turkey).
Experimental Design
Fruits were selected for uniformity based, washed carefully and disinfected with 200 ppm chlorine (NaOCl) for 3 min, dried and peeled manually. Peeled fruits were segmented carefully, and only undamaged segments were weighed as 350 g per PP tray, which was sealed with CPP/OPP film under three different gas compositions. Air composition, low oxygen (20% O2, 10% CO2, 70% N2) and high oxygen (80% O2, 10% CO2, 10% N2) concentrations were used. Modified atmosphere packaging machine (MECA, 501, France) equipped with triple gas mixture (KM60-3, Witt, Germany) was used. The packaged products were stored at 4°C for 25 days. Physical, chemical, microbial and sensory analyses were performed during 0, 2, 5, 10, 15, 20, and 25 days during storage. Analysis was performed in duplicate packages at each sampling time.
Analysis
Determination of the Headspace Atmosphere
The concentrations of oxygen and carbon dioxide inside the packages were measured using a gas analyzer (PBI Dansensor, Ringsted, Denmark). Gas analysis was performed by inserting the needle attached to the gas analyzer through an adhesive seal fixed on the lidding material. The measurements were taken at two different sides of each package and the average of 4 measurements was calculated. The results were represented as O2 and CO2%.
Microbial Analysis
Microbiological growth was determined by total plate count on plate count agar (PCA, Merck), total psychotropic bacteria on plate count agar (PCA, Merck) and by yeast and mould counts on potato dextrose agar (PDA, Merck) acidified with tartaric acid. Two packages were opened under hygienic conditions, and 10 g sample was placed into a sterile stomacher bag with 90 ml of peptone water. Samples were homogenized for 3 min and serial dilutions were made in peptone water. Appropriate dilutions were plated onto duplicate plates of PCA and PDA medium. Plates were incubated at 37°C for 2 days for total mesophilic aerobic bacteria, 7°C for 10 days for total psychotropic bacteria and at 22°C for 5 days for yeasts and moulds. The results were presented as log CFU/g.
Sensory Analysis
Orange segments packaged with different atmospheres were evaluated for visual appearance, aroma, texture, acidity, sweetness, and product acceptability using a five-point scale during 15 days of storage. The sensory attributes were defined based on the important characteristics of orange.[Citation18] Only active MAP samples were evaluated. The score 3 was considered the limit of acceptance. The tasting panel was assessed defined attributes and the scale during the trial period. The testing was done in a clean, quiet, air-conditioned and odor free room where each panelist used separate tables during judgments. Specific attributes and acceptability were evaluated by the six trained panelists on the basis of acceptability, using a scale ranging from 1 to 5 as follows[Citation18]:
| Visual appearance : | = |
5: excellent/fresh; 4: good; 3: acceptable; 2: poor; 1: very poor |
| Aroma: | = |
5: very good/natural; 4: good; 3: acceptable; 2: light strange aroma; 1: strong strange aroma |
| Texture: | = |
5: excellent firmness/juiciness; 4: firm/juicy; 3: acceptable; 2: hard/dry; 1: very hard/dry |
| Acidity: | = |
5: very good; 4: good; 3: acceptable; 2: acidic; 1: very acidic |
| Sweetness: | = |
5: very sweet; 4: sweet; 3: acceptable sweetness; 2: poor in sweetness; 1: not sweet |
| Product acceptability : | = |
5: excellent; 4: good; 3: acceptable; 2: bad; 1: very bad |
The samples were coded with a random 3 digit number and served to selected panelists using completely randomized design.
Physicochemical parameters
| Color: | = |
The color measurements were performed with a chromameter (CR 400, Minolta, Osaka, Japan). The colorimeter was calibrated to a standard white tile. The L, a, b were recorded in the middle of the one side of each segment. Twenty segments (10 from each package) were measured for each treatment on each sampling day. |
| Instrumental Texture: | = |
The firmness was measured with a texture analyzer (TA-XT Plus, Stable Micro Systems, Surrey, England) using HDP/BS blade set. A speed of 12 mm/s and penetration distance of 35 mm was used to cut the segments at the center, and the hardness was expressed as maximum cutting force (N). The segments similar in thickness were selected for texture measurement. The data are presented as means of 20 independent measurements. |
| Acidity: | = |
Sample (∼50 g) was blended for 2 min and filtered by using cheesecloth. The juice of the sample was used for chemical analysis.[Citation21] Titratable acidity was determined by potentiometric titration with 0.1 N NaOH up to pH 8.1 using 10 mL of macerate diluted with 50 mL of water. Results were expressed as citric acid %. |
| Soluble solids: | = |
Soluble solids were measured by hand refractometer (Model N-50E; Atago, Tokyo, Japan) and expressed as °Brix at 20°C. |
| pH: | = |
pH was determined by immersing pH electrode directly to orange juice by using pH meter (Model pH-315i; WTW, Weilheim, Germany). |
| Sugar content: | = |
The juice of the sample was centrifuged at 3000 rpm for 10 min, and the supernatant was filtered by using 0.45 μm pore size filter to determine sugar content (fructose, glucose, sucrose, total sugar) of grapefruit segments by using HPLC (Shimadzu, Japan) equipped with refractive index detector (Model RID-10A, Shimadzu, Japan).[Citation20] |
Statistical Analysis
All data were subjected to analysis of variance (ANOVA) and a Duncan multiple comparison test to determine significant differences between treatments using MSTAT statistical package. Significance of differences was represented at 5%.
RESULTS AND DISCUSSION
Gas Composition in the Packages
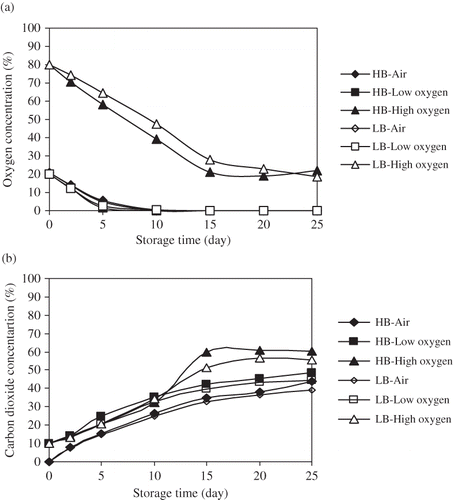
Headspace atmosphere composition in PP trays is shown in and b. Carbon dioxide contents of mono and multilayer packages at all treatments (air, low O2, and high O2) increased with the increase in storage time. However, the oxygen contents dramatically decreased for all treatments with increased storage. Oxygen levels in mono and multilayer PP packages reached to 0% for air and low oxygen; however, it was 39.23% in multilayer and 47.53% monolayer PP at high oxygen treatment on the storage day of 10. The oxygen and carbon dioxide reached equilibrium on the fifteenth day of storage at only high oxygen application. At the end of the storage, the O2 contents of multilayer and monolayer PP were 21.95% and 18.63%, respectively, at high oxygen atmosphere application. These changes in the atmosphere were related to the continuous respiration of minimally processed oranges. It was reported that separation of segments affected the respiratory metabolism and the atmosphere inside package (rich CO2 poor O2) than whole oranges.[Citation19] Permeability of films to respiration gases was also the main factor, which determined package atmosphere. Our study showed that there was no significant difference between mono and multilayer PP packages in terms of atmosphere indicating the permeability occurred through the sealing lid rather than the PP tray walls. Furthermore, the atmospheres of both PP packages underwent anaerobic condition at low oxygen and air treatments after 10 days. This might be dangerous for fruit marketability because anaerobic respiration stimulates the growth of anaerobic spoilage and pathogenic microorganisms.[Citation13,Citation22] Thus, it is very important to consider the atmosphere in the packages in terms of the quality of orange segments as stated by the study.[Citation19] At this point, low oxygen and air applications should be limited to 10 days considering the atmosphere.
Microbial Quality of Orange Segments
There was no growth of mesophilic aerobic bacteria, psychotropic bacteria and yeasts and molds observed at all treatments including both types of packages. Yeasts and molds are the natural microflora of fruits because yeasts and molds can grow at lower pH comparing to bacteria. However, effective washing and sanitation practices of the fruit could retard the spoilage especially by yeasts. Sanitation with 200-ppm chlorine before peeling, low pH (3.5) of the product, and modified atmosphere combined with low storage temperature provided good microbial quality. High carbon dioxide was indicated to be effective in inhibiting aerobic microorganisms and yeasts.[Citation11] It was reported that there was slight or no microbial growth during 11 days storage of minimally processed orange segments under passive modification.[Citation19] It was also stated that low number of colonies observed at Torocco orange slices packaged with 3 different films.[Citation23]
Sensory Quality of Orange Segments
Sensory attributes and product acceptance of orange segments were determined for low and high O2 treatments at both packages during 15 days of storage as presented in . In terms of visual appearance, all treatments except high O2 (in multilayer PP) were acceptable (>3) for 15 days storage. Aroma, acidity, sweetness, and product acceptability scores decreased by increased storage and the attributes were in acceptability limit (3.0) at all treatments including both packages till 10 days of storage. Although orange segments under high oxygen was acceptable in terms of texture on the day 15, overall there was no difference was observed between low and high oxygen applications in terms of product acceptance. The product acceptability was limited to 10 days at all applications, which was considered “commercially viable.” Orange segments under low oxygen atmosphere were limited to 10 days due to both oxygen concentration and sensory quality, however, the segments under high oxygen were limited to 10 days due to only sensory quality. This is probably the negative effect of high carbon dioxide on the sensory attributes and the product acceptance.
Table 1 Sensory quality of orange segments stored at 4°C
Physical and Chemical Qualities of Orange Segments
Fruit color is an important attribute in purchase decisions especially if the product is packaged and cannot be touched or smelled. The L value of orange segments is presented in (Data not shown for a and b values). There were no significant effects of MAP treatment and packaging material on the L, a and b values (p > 0.05). However, the effect of storage time on the L and a values was found significant (p ≤ 0.05). L value tended to be constant for the first 10 days of storage; however, started to decrease especially after the 15 days of storage at all treatments. The a value started to decrease after the 2nd day of storage at all MAP treatments applied. Value b, indication of yellowness, did not change much till the storage day of 20. However, it was significantly dropped after the storage day of 20 probably due to the prolonged storage.
Table 2 Physical parameter (Color, L* value) of orange segments stored at 4°C
As well as color properties, the textural properties were important for fresh-cut fruits. Hardness (max cutting force, N) of the orange segments is presented in . The deterioration of the textural properties often results in soft or hard texture and thus, decreases in consumer acceptance. During the increased storage, the cutting force (N) also increased at all treatments as a result of drying surfaces of the segments related to water loss. This might be related to decrease in L value. There were no significant effects of packaging material and MAP treatment on the hardness (p > 0.05). It was stated that water loss of minimally processed fruits was one of the important problem and this might be overcome using packaging materials with low water vapor transmission rate and low storage temperature.[Citation12]
Table 3 Physical parameter (Hardness, max cutting force, N) of orange segments stored at 4°C
Chemical attributes (titratable acidity, pH, soluble solid and sugar content) of orange segments packaged under modified atmosphere in two PP trays is shown in . There was no significant difference between two PP packages including active and passive MAP treatments (p > 0.05) in terms of titratable acidity (TA). However, TA significantly decreased especially on storage day 2 and remained unchanged for the rest of the storage time at all treatments including both PP packages. This decrease might be due to increased respiration following the minimal processing as indicated by Kim, Smith, and Lee.[Citation24] Acids are used quickly during respiration compared the other compounds. For citrus fruits (grapefruit, orange, mandarin), citric acid is a major organic acid followed by malic acid and quinic acid.[Citation25] It was stated that organic acids content might decrease due to fruit maturation.[Citation26] In addition, it was mentioned that loss in total acid content of fruits during storage could be minimized by using low oxygen and high carbon dioxide atmosphere applications.[Citation6,Citation27] A study reported that there was no significant change in total acidity of orange segments packaged under passive MAP with two films in different permeabilities during 11 days of storage.[Citation19]
Table 4 Chemical parameters (titratable acidity, pH, soluble solids, sugars) of orange segments stored at 4°C
A slight increase in pH value was observed on the day 2 of storage and remained almost the same for the rest of the storage. This change was parallel with the acidity. It was also reported that there was no significant change in pH of orange segments during 11 days of storage.[Citation19]
Initial sugar content of orange segments was 2.49% for fructose, 2.51% for glucose, and 5.65% for sucrose. The sugar content (fructose, glucose, sucrose and total sugar) of orange segments did not change significantly during the storage (p > 0.05). There were no significant effects of packaging material and MAP application on the fructose, glucose, sucrose and total sugar content of orange segments (p > 0.05). Studies in fresh cut pears[Citation28] and kiwifruit[Citation29] reported that the sugar content did not significantly change under refrigerated storage. It was also indicated that MAP does not result in significant change in sugar content of minimally processed fruits.[Citation11]
The effect of storage time was found significant on the soluble solids (p ≤ 0.05). Although the decrease was found statistically significant, soluble solids tended to show slight decrease during storage at all applications. Since the sugar content remained unchanged during storage, the decrease in soluble solids might be due to the use of organic acids.
CONCLUSION
Orange segments modified atmosphere packaged and stored at low temperature were in commercially viable condition for 10 days. Although the orange segments were stable in terms of chemical and microbiological qualities for 25 days of storage, the sensory panel did not find the product acceptable after 10 days. There was no significant difference observed between mono and multilayer PP with the same lid. This indicated that the permeability occurred through the lid instead of PP tray walls as expected. There would have been differences for products stored longer. The atmosphere also changed from aerobic to anaerobic after 10 days, which could stimulate growth of anaerobic spoilage and pathogenic microorganisms. Although the package atmosphere was aerobic during 25 days at high oxygen applications, the segments were also not acceptable by the sensory panel after 10 days possibly due to negative effects of high amount of carbon dioxide on flavor. Overall, the shelf life of orange segments was suggested as 10 days under low and high oxygen modified atmosphere packaging using PP based packaging material.
ACKNOWLEDGMENTS
This paper is produced from the part of master thesis of the first author completed at Mustafa Kemal University. The authors acknowledge project funding provided by Commission of Scientific Research Projects of Mustafa Kemal University (Project No. 06 M 1501) and Prime Ministry State Planning Organization in Turkey (Project No. 03 K 120860). We are grateful to Nafiz Celiktas for statistical analysis and Elif Erturk for her help in sugar analysis. We also thank A-Pack (Istanbul, Turkey) for providing packaging film, Huhtamaki (Istanbul, Turkey) for providing PP trays, and Citexco (Hatay, Turkey) for providing oranges. The authors are thankful to Gulbahar Yetis, Huseyin Senyurt, Suleyman Uzan, and Hamit Artar for their help in the process and laboratory analysis.
REFERENCES
- Shewfelt , R.L. 1987 . Quality of minimally processed fruits and vegetables . Journal of Food Quality , 10 : 143 – 156 .
- Labuza , T.P. and Brenee , W.M. 1989 . Applications of ‘active packaging’ for improvement of shelf life and nutritional quality of fresh and extended shelf-life foods . Journal of Food Processing and Preservation , 13 : 1 – 69 .
- Liu , C-L. , Hsu , C-K. and Hsu , M-M. 2007 . Improving the quality of fresh-cut pineapples with ascorbic acid/sucrose pretreatment and modified atmosphere packaging . Packaging Technology and Science , 20 ( 5 ) : 337 – 343 .
- O'Connor-Show , R.E. , Roberts , R. , Ford , A.L. and Nottingham , S.M. 1994 . Shelf life of minimally processed honeydew, kiwifruit, papaya, pineapple and cantaloupe . Journal of Food Science , 59 ( 6 ) : 1202 – 1206 . 1215
- Bracket , R.E. 1992 . Shelf stability and safety of fresh produce as influenced by sanitation and disinfection . Journal of Food Protection , 55 : 808 – 814 .
- Zagory , D. and Kader , A.A. 1998 . Modified atmosphere packaging of fresh produce . Food Technology , 42 ( 9 ) : 70 – 77 .
- Gunes , G. and Lee , C.Y. 1997 . Color of minimally processed potatoes as affected by modified Atmosphere packaging and antibrowning agents . Journal of Food Science , 62 ( 3 ) : 572 – 582 .
- Erkan , M. and Wang , C.Y. 2006 . Modified and controlled storage of subtropical crops . Postharvest Biology and Technology , 5 ( 4 ) : 1 – 8 .
- Chauhan , O.P. , Raju , P.S. , Dasgupta , D.K. and Bawa , A.S. 2006 . Instrumental textural changes in banana (var. Pachbale) during ripening under active and passive modified atmosphere . International Journal of Food Properties , 9 ( 2 ) : 237 – 253 .
- Soliva-Fortuny , R.C. and Martin-Bellaso , O. 2003 . Microbiological and biochemical changes in minimally processed fresh cut Conference pears . European Food Research and Technology , 217 : 4 – 9 .
- Soliva-Fortuny , R.C. and Martin-Bellaso , O. 2003 . New advances in extending the shelf life of fresh-cut fruits: A Review . Trends in Food Science and Technology , 14 : 341 – 353 .
- Martin-Bellaso , O. and Soliva-Fortuny , R.J. 2006 . Effect of modified atmosphere packaging on the quality of fresh cut fruits . Postharvest Biology and Technology , 1 ( 3 ) : 1 – 8 .
- Garcia , J.M. , Media , R.J. and Olias , J.M. 1998 . Ouality of strawberries automatically packed in different plastic films . Journal of Food Science , 63 ( 6 ) : 1037 – 1041 .
- Martinez-Ferrer , M. , Harper , C. , Perez-Munaz , F. and Chaparro , M. 2002 . Modified atmosphere packaging of minimally processed mango and pineapple fruits . Journal of Food Science , 67 ( 9 ) : 3365 – 3371 .
- Soliva-Fortuny , R..C. , Elez-Martinez , P. and Martin-Bellaso , O. 2004 . Microbiological and biochemical stability of fresh-cut apples preserved by modified atmosphere packaging . Innovative Food Science Emerging Technologies , 5 : 215 – 224 .
- Kader , A.A. and Ben-Yehoshua , S. 2000 . Effects of super atmospheric oxygen levels on postharvest physiology and quality of fresh fruits and vegetables . Postharvest Biology Technology , 20 : 1 – 13 .
- Soliva-Fortuny , R.C. , Oms-Oliu , G. and Martin-Bellaso , O. 2002 . Effects of ripeness stages on the storage atmosphere, color and textural properties of minimally processed apple slices . Journal of Food Science , 67 : 1958 – 1963 .
- Del Caro , A. , Piga , A. , Vacca , A. and Agabbio , M. 2004 . Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage . Food Chemistry , 84 : 99 – 105 .
- Pretel , M.T. , Fernandez , P.S. , Romajoro , F. and Martinez , A. 1998 . The effect of modified atmosphere packaging on “Ready to Eat” oranges . Lebensmittel-Wissenschaft and Technologie , 31 : 322 – 328 .
- Escolana , V.H. , Aguayo , E. and Artes , F. 2005 . Overall quality throughout shelf life of minimally processed fennel . Journal of Food Science , 70 ( 1 ) : S13 – S17 .
- AOAC . 1990 . Official methods of analysis , Edited by: Horwitz , W. Washington, DC : AOAC International .
- Kader , A.A. , Zagory , D. and Kerbel , E.L. 1989 . Modified atmosphere packaging of fruits and vegetables . Critical Reviews in Food Science and Nutrition , 28 ( 1 ) : 1 – 30 .
- Rapisarda , P. , Caggia , C. , Lanza , C.M , Bellamo , S.E. , Pannuzzo , P. and Restuccia , C. 2006 . Physiochemical, microbiological and sensory evaluation of minimally processed Tarocco clone oranges packaged with 3 different permeability films . Journal of Food Science , 71 ( 3 ) : S299 – S306 .
- Kim , D.M. , Smith , N.L. and Lee , C.Y. 1993 . Quality of minimally processed apple slices from selected cultivars . Journal of Food Science , 58 : 1115 – 1117 .
- Cemeroglu , B. , Yemenicioglu , A. and Ozkan , M. 2001 . Meyve ve sebzelerin bilesimi ve sogukta depolanmalari , Ankara : Gida Teknolojisi Dergisi Yayinlari .
- Weichmann , J. 1986 . The Effect of Controlled Atmosphere Packaging on the Sensory and Nutritional Quality of Fruits and Vegetables . Horticultural Reviews , 8 : 101 – 127 .
- Damarlı , E. 2005 . “ Yerli kiraz ve kayısının modifiye atmosferde paketlenmesi ” . Istanbul, , Turkey : Ph.D. Thesis. Istanbul Technical University .
- Senesi , E. , Galvis , A. and Fumagalli , G. 1999 . Quality indexes and internal atmosphere packaged fresh-cut pears (Abete fetel and Kaisert varieties) . Italian Journal of Food Science , 2 : 111 – 120 .
- Agar , I.T. , Massantini , R. , Hess-Pierce , B. and Kader , A.A. 1999 . Postharvest CO2 and ethylene production and quality maintenance of fresh-cut kiwifruit slices . Journal of Food Science , 64 : 433 – 440 .