Abstract
Chhana, an Indian cottage cheese, is one of the most popular heat-acid coagulated milk products. It forms the base material for a variety of milk-based sweets. Application of high pressure treatment for denaturation of milk protein has successfully been carried out. In the present study, high pressure in the range of 200–400 MPa for 0 to 100 min at coagulation temperature of 30–70°C was applied at constant acidity of milk-acid mixture in order to study the coagulated milk gel properties for preparation of chhana. The experiments were conducted using a central composite rotatable experimental design. Linear and non-linear regression equations, using the coded values of the independent variables were developed to study the effect of process variables on coagulated gel properties viz., lag time, mean coagulation rate and inflexion time. The optimum values of independent variables corresponding to minimum lag time, maximum coagulation rate and minimum inflexion time was obtained were high pressure - 280 MPa; pressurization time - 47 min; and coagulation time - 52°C. At this optimum condition of independent variables the lag time, mean coagulation time and inflexion time were 0.0028 min, 3.87 min, and 5.19 min, respectively.
INTRODUCTION
Chhana, an Indian counter part of soft cottage cheese is a heat-acid coagulated product of milk. It forms the base material for production of a variety of milk products like paneer, rasogolla, sandesh, and gulabjaman in Asian countries. Pattern of milk consumption indicates that about 6% of milk produced in India is coagulated to produce chhana.[Citation1]
Chhana is a rich source of fat and protein. It also contains fat-soluble vitamins A and D. With high protein and low sugar content, chhana is highly recommended for diabetic patients.[Citation2] According to the definition of the Bureau of Indian Standard (BIS), chhana should not contain more than 70% moisture and milk fat should not be less than 50% of the dry matter.[Citation3] According to the Prevention of Food Adulteration Rules, chhana is defined as a milk product obtained by precipitating a part of milk solid by boiling whole milk of cow and or buffalo or a combination thereof by addition of lactic acid, citric acid or any other suitable coagulating agent and subsequent drainage of whey.[Citation4]
For preparation of chhana, whole milk of cow and/or buffalo is heated to its boiling point. The heated milk is allowed to cool to 70°C and a coagulating solution of lactic acid, citric acid or calcium lactate is added to coagulate the milk. Coagulation of milk involves destabilization of casein micelles. Acid affects the stability of casein directly by disturbing the charges carried by particles and indirectly by releasing the calcium ion from colloidal calcium-caseinate-phosphate complex. The destabilization results in formation of large, firm, and cohesive structural aggregates of casein micelles in which milk fat, other colloidal and soluble solids are entrained along with deproteinated whey. The whey is drained out and the dewheyed product is known as chhana.
Quality of chhana is affected by the heat treatment given to milk prior to its acidification, acidity of milk-acid mixture at the time of coagulation and residence time of the coagulum prior to separation of milk solids, besides the type of milk and its initial composition.[Citation5] The heat treatment of milk before acidification is characterized by the temperature to which milk is heated, the rate of heating, the temperature to which the milk is cooled and the rate of cooling.[Citation6] Heating causes denaturation of whey proteins and they get associated with casein micelles. The degree of denatured whey proteins depends on the time-temperature combination given to milk and is mainly determined by the maximum temperature to which milk is heated.[Citation7–11]
High pressure processing is one of the most promising non-thermal processes for food processing and of great concern in food industry because of its potential to achieve interesting functional, structural, chemical, and sensory attributes in the final product. High pressure processing have been found to improve the cheese making properties such as coagulation time, cheese yield, gel strength and effect on syneresis.[Citation12–17] Some authors reported milk curdling by renneting under pressure.[Citation12,Citation18] Many authors have reported that the coagulation properties of milk improved after pressure treatment,[Citation15,Citation18,Citation19] however, there is little information available in the literature characterizing the influence of pressure processing conditions (pressure, pressurization time, and coagulation temperature) on acidified milk gel properties.
The specific objective of the present research was to study the effect of high pressure treatments viz., applied pressure and pressurization time, and coagulation temperature on acidified gel kinetics viz., lag time, mean coagulation rate, and inflexion time of milk, and to optimize the applied pressure, and pressurization time, and coagulation temperature based on minimum lag time, minimum coagulation time and minimum inflexion time for preparation of chhana.
MATERIALS AND METHODS
Milk Sample Preparation
Cow milk was used to study the acid coagulation of milk. The raw milk obtained from a particular cow from the nearby cow yard at the University of Reading, UK was dried into powder using a buchi spray dryer (Model–456/R, USA). The milk was dried in order to maintain the composition of its constituents same, which was further reconstituted immediate after drying. This spray dried milk powder was reconstituted by adding distilled water. The fat content in the reconstitute milk was balanced at 4% making a mass balance over fat and solid-not-fat content. The reconstituted milk samples were stirred at the ambient temperature for at least 10 h before further treatment to ensure complete equilibration.
Moisture content of reconstituted milk and chhana was determined as per IS: 5162.[Citation20] Fat content of the milk was determined by Gerber method.[Citation21] Lactose content (% actic acid) and titratable acidity (% lactic acid) of the milk were determined by using Lane-Eynon method as described in BIS.[Citation22] Ash content of milk was determined according to ISI: 5162.[Citation20] Protein content was determined by Kjeldahl method using 6.38 as nitrogen to protein conversion factor.[Citation22] The proximate compositions of milk, as obtained from the experiments were: moisture 0.861 ± 0.015, protein 0.047 ± 0.008, fat 0.041 ± 0.007, lactose 0.0361 ± 0.002, and ash 0.011 ± 0.001 kg per kg milk. The average titratable acidity of milk was 0.168 ± 0.005% equivalent lactic acid.
High Pressure Treatment
A high pressure unit (Model S-FL-085-9-W, Stansted Fluid Power, UK) was used in this study to subject milk to various pressure conditions. The high pressure unit consists of a pressure chamber, with an internal diameter of 10 cm and height of 55 cm with an enclosed metal jacket. The metal jacket allows temperature control in the pressure chamber by circulating hot/cold water at the desired coagulation temperature. Water containing 2% water-soluble oil (part no 5019, ABB Autoclave Engineers, UK) was used as the hydrostatic fluid in the pressure chamber. The milk filled in test pouches were submerged in water in the pressure chamber and subjected to various pressure and time combinations as mentioned in . Adiabatic heating results in temperature rise in test samples and pressure medium (approx. 3°C/100 MPa). In order to achieve and maintain an approximate average coagulation temperature during test run, milk samples were equilibrated to lower initial temperatures and the temperature of circulating water was kept slightly below the coagulation temperature. Average temperature indicated during the run was within ±2°C of the set point. Following treatment, test pouches were removed from the chamber and processed for analysis.
Table 1 Experimental design of independent variables
A central composite rotatable design (CCRD) was employed to carry out the experiments at various combinations of independent variables, e.g., applied pressure, pressurization time and coagulation temperature. The levels chosen for pressure X 1, time X 2, and coagulation temperature X 3 were; X 1: 200, 270, 300, 330, and 400 MPa; X 2: 0, 14.5, 50, 85.5, and 100 min; and X 3: 30, 40, 50, 60, and 70°C. These values were considered based on literature and preliminary trails. In the design (), the real values of independent variables viz., pressure, pressurization time and coagulation temperature were coded as x1 , x2, and x3 . The coded values were set to lie in the range +1.682 and −1.682.[Citation23–25] The relationship between the real and coded variables is given by the following equations:
To find the effect of independent variables x (viz., applied pressure, pressurization time and coagulation temperature) on the dependent variables y (viz., lag time (LT), mean coagulation time (MCT) and inflexion time (IT)), following linear and non-linear regression forms of equations were fitted.
Milk Coagulation and Rheological Parameters Measurement
Citric acid solution was used as the coagulating agent for coagulation of milk. Strength of the acid solution required for the coagulation was calculated based on acidity and density of milk, acidity of milk-acid mixture, and ratio of milk and acid solution at the time of coagulation.[Citation26] The ratio of weight of milk to citric acid solution was fixed at 5:1.[Citation6] The acidity of milk-acid mixture at the time of coagulation was fixed at 0.52% lactic acid.[Citation6] Using the procedure, the strength of the citric acid solution used was 1.62 gm per 100 gm of citric acid solution.
The reconstituted milk was treated with high pressure as per the experimental design given in . The citric acid solution, previously heated to different coagulation temperatures as mentioned in was immediately added to the high pressure treated samples. Immediately after addition of citric acid solution to milk samples, 1 ml sample of acid treated mass were poured into three disposable 10 mm polythene micro-cuvettes and transferred to a Beckman DU 65 spectrophotometer (Beckman Instrument, Inc., Fullerton, CA) equipped with a Beckman auto 6-sampler accessory, a six-sample cell holder. Three of the six cells were used to simultaneously follow coagulation in three replicate samples. The coagulation temperature was maintained with circulation hot/cold water. Optical density (OD) was recorded at 700 mm at 12 s intervals for 50 min by using a Beckman DU Data Leader software.
Properties of High Pressure Treated Acid Coagulated Milk Gel
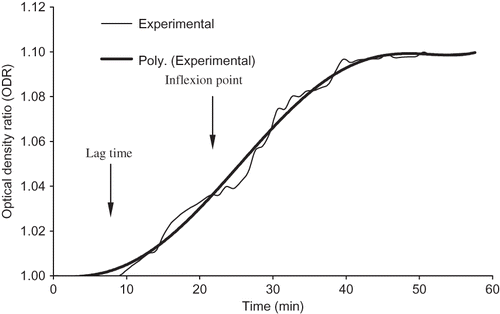
Optical density ratio was used as an index to quantify the gel quality. The optical density ratio (ODR) was calculated by dividing the measured optical density value by the average of initial optical density values for the first 5 data points (1 min) after acidification. Since the optical density increased with time, the ODR vs. time represented a normalized transient optical density curve (), the coagulation process can be modeled as a sigmoid equation[Citation27–28] as shown in EquationEq. (6):
RESULTS AND DISCUSSION
shows the different parameters of the coagulation curve at various levels of coagulation variables. The coagulation curves thus obtained are appeared to be good fit for the ODR and time data. The model fitted had R 2 values in the range of 0.929–0.997. As shown in the , the coagulation started at approximately 5 min after acid addition and reached the peak value at the inflexion point approximately 18 min. An average coagulation rate was computed from observed rates around the inflexion point to be 0.0038 min−1, while the peak value was 0.0043 min−1. The inflexion times for various levels of the treatments were obtained from the first derivative of the .
Table 2 Estimated values of quality of acid gel
Effect of Independent Variables on High-Pressure Treated Acid Coagulated Gel Properties
The values of the coagulation process responses obtained for different combinations of pressure treatment and coagulation temperature are mentioned in . Linear regression EquationEqs. (10), Equation(11), and Equation(12), using the coded values (x 1, x 2, and x 3) of the independent variables (X 1, X 2, and X 3) have been developed to find out the effect of high pressure (x 1), pressurization time (x 2) and coagulation temperature (x 3) on the acidified milk gel properties (viz., lag time (LT), mean coagulation rate (MCT), and inflexion time (IT).
Table 3 Estimated values of quality parameters of liquid honey powder obtained from the optimized combinations of the ingredients
The relationship between the real (X) and the coded (x) values are:
By comparing the coefficients and sign of the coefficients of EquationEqs. (10), Equation(11), and Equation(12), and the surface plots drawn with coded values of independent variables for various responses, we may arrive at the following conclusions.
EquationEquation (10)
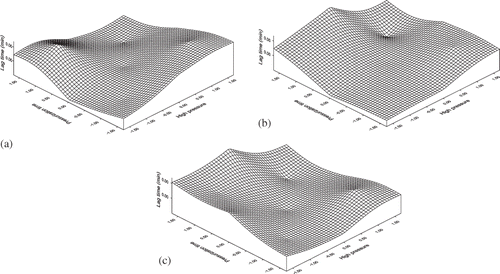
It is observed that lag time will increase with increase in pressurization time, applied pressure, and coagulation temperature. The high pressure has the significant effect on lag time compared to coagulation temperature. Surface plots demonstrating the effect of coded values of different process variables on lag time are shown in . The lag time increased with an increase in applied pressure and pressurization time (). Moreover, the effect of coagulation temperature was more significant followed by pressurization time and applied high pressure. Both increased value of pressure and coagulation time increased the lag time equally as observed from . The effect of pressurization time and coagulation temperature () on lag time increased, but the effect of coagulation temperature was more prominent than the pressurization time.
EquationEquation (11)
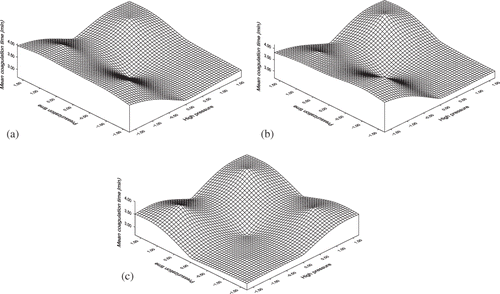
The mean coagulation time will decrease with increase in applied high pressure, but it increases with increases in pressurization time and coagulation temperature. Pressurization time has the significant effect on the mean coagulation time compared to applied pressure. The effect of various coded values of process variables on the time at inflexion point is shown in . With coagulation temperature fixed, a decrease in applied pressure and increases in pressurization time resulted in an increase in the inflexion time (). Longer inflexion times were apparent at longer pressures and pressurization time. There was a reduction in the inflexion time as the pressure levels and coagulation temperature increased. The treatment effect on inflexion time appears to be more sensitive at the higher coagulation temperature (EquationEq. 11).
EquationEquation (12)
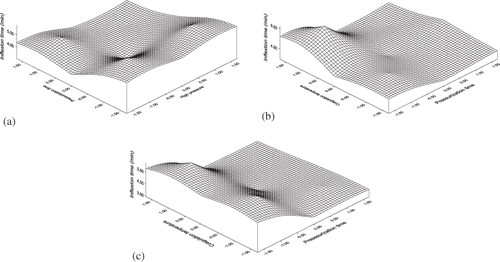
The equation shows that inflexion time increases with increase in applied pressure and pressurization time, but it decreases with coagulation temperature. Pressurization time has the most significant effect on inflexion time than applied pressure. shows the response surface plots of the combined effect of coded values of different processing variables on inflexion time.
Optimum Coagulation Conditions
In order to find out the level of applied pressure (X 1), pressurization time (X 2), and coagulation temperature (X 3) required to attain the minimum lag time, maximum coagulation rate and minimum inflexion time, second order regression equations were fitted with coded values of X 1, X 2, and X 3 by using the experimental values of the response given in .
The range of optimum values of x 1, x 2, and x 3 at the desired values, i.e., minimum lag time, minimum coagulation time and minimum inflexion time were found out by solving the above equations using Micro Soft Excel Solver. The values of x 1, x 2, and x 3 thus obtained are: applied pressure (x 1): 0.8; pressurization time (x 2): 0.627; and coagulation temperature (x 3): 0.867. The real value (viz., X 1, X 2, and X 3) of these process variables was obtained by using EquationEqs. (17)—Equation(18) and they are: applied high pressure (X 1): 280 MPa, pressurization time (X 2): 47 min and coagulation temperature (X 3): 52°C. Using optimum ranges of the independent variables, x 1, x 2 and x 3 as obtained above, the values of lag time, mean coagulation time and inflexion time of high pressure treated coagulated acidified milk gel were estimated using EquationEqs. (16), Equation(17), and Equation(18). The estimated values of the above parameters are given in .
CONCLUSIONS
High pressure processing significantly affected the coagulation properties of acid cogulated milk. The coefficients obtained from ODR vs. time curve showed that higher-pressure levels and higher temperatures significantly decreased the lag, inflexion time, and coagulation time. A multiple correlation model was developed for each coagulation parameter to relate them to the process variables viz. pressure, pressurization time and coagulation temperature. The optimum values of independent variables corresponding to minimum lag time, maximum coagulation rate and minimum inflexion time was obtained were: high pressure - 280 MPa; pressurization time - 47 min; and coagulation time - 52°C. At this optimum condition of independent variables the lag time, mean coagulation time and inflexion time were 0.0028 min, 3.87 min, and 5.19 min, respectively.
ACKNOWLEDGEMENT
The author is thankful to the Society of Chemical Industry (SCI), UK, for felicitating as Seligman APV Fellow for the year 2007, and for providing financial support to get in hand experience of high pressure machine at The University of Reading, UK.
REFERENCES
- Aneja , R.P. , Mathur , B.N. , Chandan , R.C. and Banerjee , A.K. 2002 . Technology of Indian Milk Products , 6 – 8 . New Delhi : A Dairy India Publication .
- De , S. 1980 . Outline of dairy technology , 128 New Delhi : Oxford University Press .
- Bureau of Indian Standards.BIS. IS-5162 . 1996 . Specification of chhana , New Delhi : Bureau of Indian Standards .
- PFA . 1976 . The Prevention of Food Adulteration Act, 1954 , 105 New Delhi : Eastern Book Company .
- Jagtap , G.E. and Shukla , P.C. 1973 . A note on the factors affecting the yield and quality of chhana . Journal of Food Science and Technology , 10 ( 2 ) : 73 – 75 .
- Choudhary , R.L. , Berg , V.D. , Singh , M.D. and Das , H. 1998 . Effect of heat treatment on recovery of solids in chhana produced from cow and buffalo milks . Journal of Food Science and Technology , 35 ( 1 ) : 30 – 34 .
- Walstra , P. and Jennes , R. 1983 . Dairy Physics and Chemistry , 446 New York : John Wiley and Sons .
- Singh , H. and Creamer , L.K. 1992 . “ Heat stability of milk ” . In Advanced Dairy Chemistry: Protein , Edited by: Fox , P.F. 621 – 656 . London : Elsevier Science Publishers Ltd .
- Singh , H. 1995 . Heat induced changes in casein, including interactions with whey proteins . International Dairy Federation Bulletin , 238 : 86 – 104 .
- O'Connell , J.E. and Fox , P.F. 2003 . “ Heat-induced coagulation of milk ” . In Advanced Dairy Chemistry: Proteins , 3rd , Edited by: Fox , P.F. and McSweeney , P.L.H. Vol. 1 , 879 – 945 . New York : Kluwer Academic Publishers .
- Singh , H. 2004 . Heat stability of milk . International Journal of Dairy Technology , 57 : 111 – 120 .
- Pandey , R.K. , Ramaswamy , H.S. and St-Gelais , D. 2003 . Effect of high pressure processing on rennet coagulation properties of milk . Innovative Food Science and Emerging Technologies , 4 : 245 – 256 .
- Desobry-Banon , S. , Richard , F. and Hardy , J. 1994 . Study of acid and rennet coagulation of high pressurized milk . Journal of Dairy Science , 77 : 3267 – 3274 .
- Johnston , D.E. , Austin , B.A. and Murphy , R.J. 1993 . Properties of acid-set gels prepared from high pressure treated skim milk . Milchwissenschaft , 48 : 206 – 209 .
- Lopez-Fandino , R. , Carrascosa , A.V. and Olano , A. 1996 . The effect of high pressure on whey protein denaturation and cheese-making properties of raw milk . Journal of Dairy Science , 79 : 929 – 936 .
- Patterson , M.F. , Qinn , M. , Simpson , R. and Gilmore , A. 1995 . Sensitivity of pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods . Journal of Food Protection , 58 : 524 – 529 .
- Pandey , P.K. , Ramaswamy , H.S. and St-Gelais , D. 2000 . Water holding capacity and gel strength of rennet curd as affected by high-pressure treatment of milk . Food Research International , 33 : 655 – 663 .
- Ohmiya , K. , Fukami , K. , Shimizu , S. and Gekko , K. 1987 . Milk curdling by rennet under high pressure . Journal of Food Science , 52 : 84
- Buffa , M. , Trujillo , A.J. and Guamis , B. 2001 . Rennet coagulation properties of raw, pasteurised and high pressure-treated goat milk . Milchwissenschaft-Milk Science International , 56 ( 5 ) : 243 – 246 .
- Bureau of Indian Standards. IS. 1224 . 1977 . Determination of Fat by Gerber Method, Part I: Milk , New Delhi : Bureau of Indian Standards .
- Bureau of Indian Standards. IS. 5162 . 1980 . Specification for Chhana, Bureau of Indian Standards New Delhi, , India
- Bureau of Indian Standards. BIS SP: 18 . 1981 . Handbook of Food Analysis, Part XI: Dairy Products , 98 New Delhi, , India : Bureau of Indian Standards .
- Das , H. 2005 . Food Processing Operations Analysis , 1st , 432 – 439 . New Delhi : Asian Books Private Limited .
- Myers , P.H. 1971 . Response surface methodology , Boston : Allyn and Bacon .
- Jaya , S. , Das , H. and Mani , S. 2006 . Optimization of Maltodextrin and Tricalcium Phosphate for Producing Vacuum Dried Mango Powder . International Journal of Food Properties , 9 ( 1 ) : 13 – 24 .
- Jonkman , M.J. and Das , H. 1993 . Optimization of process parameters for production of chhana from low fat cow milk . Journal of Food Science and Technology , 30 : 417 – 421 .
- Caron , A. , St-Gelais , D. and Pouliot , Y. 1997 . Coagulation of milk enriched with ultrafiltred or diafiltered microfiltered milk retentate powders . International Dairy Journal , 7 : 445 – 451 .
- St-Gelais , D. and Savoie , L. 1993 . Coagulation of milk enriched with low mineral retenate powders . Milchwissenschaft , 48 ( 11 ) : 603 – 606 .