Abstract
Effects of different cations on chemical composition and microstructure of pidan white and yolk were investigated. During 3 weeks of pickling and further 3 weeks of ageing, ammonia and ash contents were increased but varied with types of cations used. Lower protein degradation of pidan white was observed in pidan treated with 0.2% PbO2, compared with other treatments. Scanning electron microscopic (SEM) studies indicated that the greater aggregation of egg proteins took place in pidan white treated with PbO2. Yolk of pidan treated with 0.2% PbO2 had more release of free lipid as visualised by confocal laser scanning microscope (CLSM).
INTRODUCTION
Alkaline pickling of eggs has been used for centuries as a method to preserve egg and is widely practiced in China.[Citation1] Pidan is alternatively called as alkali pickled egg which is widely adopted into the local cuisine as an appetizer in Thailand and other South East Asian countries. Pidan is generally produced as a home-based practice, in which the pickling techniques have been passed from one generation to another. Instead of using microorganisms, pidan is made using an alkali-treated pickling.[Citation2] The appearance of pidan differs from fresh eggs in that white becomes a semitransparent tea-brown color, and the yolk is solid or semisolid with a dark-green color. The nutritional value of pidan is slightly decreased compared with fresh eggs, but pidan has an extremely long shelf life and a pleasant fragrant taste that is preferred by most people in Southeast Asian countries.[Citation1]
Salt has been known to have impact on gelation of egg white proteins. Several salts decreased the amount of water bound to the albumen.[Citation3] Egg albumen contained many hydrophobic amino acids and easily formed turbid coagulum even at an alkaline pH when salts are added.[Citation3] Salt at very low concentration aids in protein solubilization prior to heating and provides a cross-link in the network.[Citation4] Nevertheless, further addition of salts simply promotes aggregation. Divalents ions tend to be more effective in aggregation than the monovalent ions, while the influence of polyvalent ions is moderate.[Citation5] Alkaline denaturation and aggregation mainly contributes to gelation as well as characteristic color, aroma and taste of pidan.[Citation1]
Traditionally, pidan pickling mainly relies on lead for its gelation. As a result, the products have high levels of lead residue. Due to the safety concerns, most consumers typically request “lead-free” pidan. Alternatively, zinc is used to produce pidan with no black spots on the eggshell, and the color of the pidan's albumen and yolk was more stable.[Citation6] Thus other divalent cations can be an alternative gel enhancer, especially via salt bridge mechanism. Nevertheless, the basic information related to chemical and microstructural changes of pidan as affected by divalent cations during pickling and aging has not yet been reported. The objective of this study was to monitor the changes in chemical composition and microstructure of pidan white and yolk treated with different cations during pickling and aging.
MATERIALS AND METHODS
Chemicals
Lead dioxide (PbO2), zinc chloride (ZnCl2), calcium chloride (CaCl2), sodium hydroxide, and sodium chloride were purchased from Lab-Scan (Bangkok, Thailand). Nile blue A was obtained from Merck (Darmstadt, Germany). Purity of all salts used was greater than 99%.
Duck Egg Collection
Fresh eggs of duck (Anas platyrhucus) with the weight range of 65–75 g were obtained within one day of being laid from a farm in Rathabhum, Songhkla Province, Thailand. Duck eggs were cleaned and checked for any crack prior to pickling. Eggs were used for pickling within one to two days after collection.
Preparation of Pidan
Clean duck eggs (10 eggs) were soaked in 1 l of pickling solution containing 4.2% NaOH, 5% NaCl and 2% Chinese tea (Chinese tea No. 3, Tesco Lotus, Hat Yai, Thailand) without and with the addition of different cations including PbO2, ZnCl2 and CaCl2. CaCl2 was added in the pickling solution at the concentrations of 0.2% and 0.5% (w/v). For PbO2 and ZnCl2, a level of 0.2% was used. Eggs (10 eggs) were soaked in different pickling solutions (1 l) at room temperature 30 to 32°C for three weeks. Pickled eggs were then removed and coated with white clay paste (Sunrise, Matsuoaka, Japan) using a clay/water ratio of 4:1 (w/v) to obtain a thickness of 2–3 mm. The thickness of clay layer was determined by a micrometer (Mitutoyo, Model ID-C112PM, Serial No. 00320, Mituyoto Corp., Kawasaki-shi, Japan). Coated eggs were left at room temperature (30–32°C) for another three weeks for aging. During pickling (weeks 1 and 3) and aging (week 6), the pidan samples were taken and separated into pidan white and yolk manually. Both pidan white and yolk were subjected to analyses.
Determination of Ash Content of Pidan White and Yolk
Pidan white and yolk with different treatments were determined for the ash according to the method of AOAC[Citation7] with the analytical No. of. 920.153.
Determination of Ammonia Content of Pidan White and Yolk
Ammonia content of pidan white and yolk obtained from different treatments was determined by distillation method as described by Parris and Foglia[Citation8] with a slight modification. Fifty ml of 10-fold diluted samples were placed in a Kjeldahl flask containing 100 ml of distilled water and 3 g of MgO. The mixture was distilled and the distillate was collected in 50 ml of 4% boric acid before titration with 0.05 M H2SO4 using methyl red-bromocresol green as an indicator. Ammonia content was calculated and expressed as mg/g sample.
Determination of TCA-soluble Peptide Content of Pidan White
TCA soluble peptide content of pidan white samples treated with 0.2% PbO2, 0.2% ZnCl2 or 0.2% CaCl2 was determined. To 2 g of finely ground pidan white samples, 18 ml of 5% TCA were added and the mixture was homogenized using an IKA Labortechnik homogenizer (Selangor, Malaysia) at a speed of 11,000 rpm for 2 min. The homogenate was allowed to stand at 4°C for 1 h and centrifuged at 8000 × g for 5 min at 25°C using a Mikro 20 centrifuge (Hettich Zentrifugen, Tuttlingen, Germany). TCA-soluble peptide content in the supernatant was measured according to the Lowry method[Citation9] and expressed as μ mol tyrosine/g sample.
Scanning Electron Microscopy (SEM) of Pidan White
Pidan white treated without and with 0.2% PbO2, 0.2% ZnCl2 or 0.2% CaCl2 at week 3 was cut into a piece of 0.5 × 0.5 cm and fixed at room temperature in 2.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 2 h. Fixed samples were rinsed with distilled water three times to remove salt. The samples were dehydrated in graded series of ethanol (50, 70, 80, 90, and 100%) and then were mounted on SEM stubs using a double backed cellophane tape. The samples were coated with gold and examined using a scanning electron microscope (JEOL JSM-5800LV, Tokyo, Japan).
Confocal Laser Scanning Microscopy (CLSM) of Pidan Yolk
Microstructures of pidan yolk treated with 0.2% PbO2, 0.2% ZnCl2 or 0.2% CaCl2 at weeks 3 and 6 of pickling and aging were examined with a confocal laser scanning microscopy (Olympus, FV300, Tokyo, Japan) following the modified method of Mineki and Kobayashi.[Citation10] Yolk of all pidans was suspended in 0.1 % Nile blue A solution at a ratio of 1:10 (w/v) and manually stirred until the uniformity was obtained. Fifty μl of suspension was smeared on the microscopy slide. CLSM was operated in the fluorescence mode at the excitation wavelength of 533 nm and the emission wavelength of 630 nm using a Helium Neon Red laser (HeNe-R) for lipid analysis and at the excitation wavelength of 488 nm and the emission wavelength of 540 nm using a Helium Neon Green laser (HeNe-G) for protein analysis.
Statistical Analysis
Completely randomized design was used throughout the study. The experiments were run in triplicate. Data were presented as mean values with standard deviations. One-way analysis of variance (ANOVA) was carried out and means comparisons were performed by Duncan's multiple range tests.[Citation11] Statistical analyses were carried out with the statistical program (SPSS for windows (Version 10), SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Changes in Chemical Composition of Pidan White during Pickling and Aging
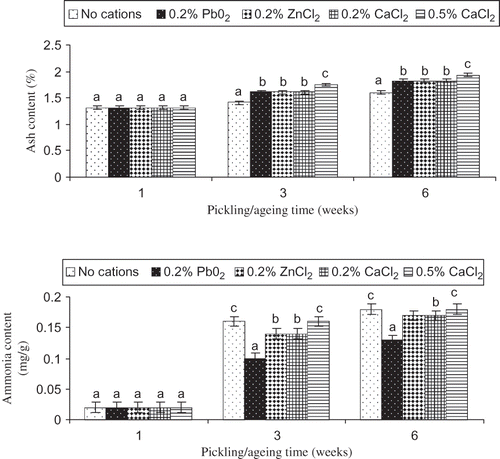
Ash content of pidan white treated with different cations was monitored during pickling and aging (). Continuous increases in ash content were observed in pidan white during pickling and aging (P < 0.05). The increase in ash content indicated the migration of alkali, NaCl and cations from pickling solution. At weeks 3 and 6, the maximum ash content was found in pidan white treated with 0.5% CaCl2 (P < 0.05) and the minimum ash content was obtained in the control sample (without cation) (P < 0.05). However, no differences in ash content were noticeable between pidan white treated with different cations at a level of 0.2% (P > 0.05). Thus, salts in the pickling solution partially contributed to the level of ash in the resulting pidan white. Cations at the higher level most likely penetrated into the egg more effectively as indicated by the greater ash content After aging (week 6), the higher ash content was found in pidan white (P < 0.05), compared with that found after pickling (week 3). This result indicated that the dehydration of pidan white during aging was accompanied with the increases in ash content in pidan white.[Citation12] After three weeks of pickling, eggs were removed from pickling solution containing cations. Therefore, no further migration of cations from pickling solution to egg white took place.
Figure 1 Ash and ammonia contents of pidan white during pickling and aging in the presence or absence of different cations. Bars represent the standard deviations (n = 3). Different letters on the bars within the same pickling/aging time indicate significant differences (P < 0.05).
Ammonia content increased sharply after three weeks of pickling (P < 0.05). The further increase in ammonia content was found after aging (week 6) () Ammonia was formed in the pidan white, possibly by deamidation process.[Citation1] Deamidation of proteins occurs at pH above 8.0, dependent upon the H+ or OH− concentration and adjacent amino acid residue.[Citation13] Lower ammonia content was found in pidan white treated with 0.2% PbO2 after pickling and aging (P < 0.05), suggesting the lower deamidation of pidan white. On the other hand, the deamidation was more pronounced in pidan white treated with 0.5% CaCl2 and the control. In the presence of calcium at a higher level (0.5%), the lesser cross-links might be formed due to the enhanced repulsive force. As a result, the deamidation could occur easily with the looser protein aggregates. For the control, no salt bridges were formed and deamidation occurred more effectively. Ammonia contributes to the unique odor and flavor of pidan. However, different ammonia contents found in pidan white with different treatments had no impact on the pH of pidan white as reported in our previous study.[Citation12] In general, changes in ash and ammonia contents were more pronounced during pickling (weeks 1–3) than during aging (week 6).
Changes in Chemical Composition of Pidan Yolk during Pickling and Aging
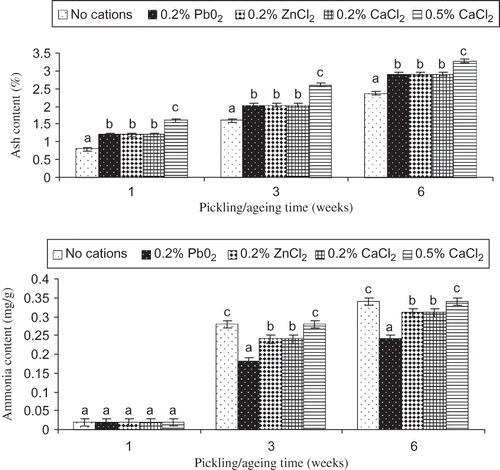
Changes in ash and ammonia contents of pidan yolk treated with different cations were monitored during pickling and aging (). Continuous increases in ash and ammonia contents in pidan yolk were observed during pickling and aging, regardless of type of cations used (P < 0.05). At the same pickling time (weeks 1 and 3) and aging time (week 6), ash and ammonia contents of pidan yolk varied with the treatments. The increase in ash content in pidan yolk indicated the migration of alkali, NaCl and cations from pickling solution through pidan white The continuous increase in the salt content of pidan yolk was reported previously.[Citation14] It was noted that the highest ash content was obtained in pidan yolk treated with 0.5% CaCl2. Yolk from pidan treated without cations showed the lowest ash content (P < 0.05). At the same level of cations used, similar ash content was found in all samples, regardless of type of cations (P > 0.05). After aging (week 6), slight increase in ash content was obtained in pidan yolk (P < 0.05), irrespective of the treatments. This result indicated that the migration of inorganic matter from egg albumin to yolk still occurred during aging. Ammonia content increased sharply after three weeks of pickling (P < 0.05). Only slight increase was noticeable after aging (week 6). Similar result was observed in comparison with that of pidan white, in which the lowest ammonia content was found in pidan with 0.2% PbO2 treatment. Pidan yolk treated with 0.5% CaCl2 or without cation contained the highest ammonia content (P < 0.05). No difference in ammonia content was observed between pidan yolks treated with 0.2% ZnCl2 and 0.2% CaCl2 (P < 0.05). The result suggested that deamidation also occurred in pidan yolk at different degrees, depending on the treatments. The deamidation of pidan yolk gradually proceeded with increasing pickling and aging time. Different cations exhibited the different impact on deamidation. Association of cations to proteins, particularly via amino groups, more likely impeded the deamidation process. Therefore, the chemical compositions of yolk were governed by cations to some degree. This might be associated with different characteristics of resulting pidan yolk.
Degradation of Pidan White Proteins during Pickling and Aging
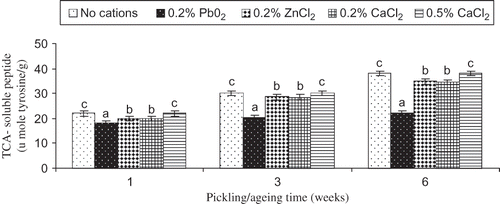
An increase in TCA-soluble peptide content was observed in pidan white during pickling and aging, irrespective of treatments (). Degradation of ovoalbumin and other egg white proteins resulted in the increases in free amino acid and small peptides, which partially contributed to development of flavor and aroma of pidan. Among all samples, pidan white treated with 0.2% PbO2 showed the lowest TCA-soluble peptide content during pickling and aging (P < 0.05). This revealed that Pb cation induced the formation of cross-links in pidan white, which were more resistant to degradation at alkaline pH when compared to the control (without cations) and other samples. TCA-soluble peptide contents of pidan white treated with 0.2% ZnCl2 or 0.2% CaCl2 were lower than that found in pidan treated with 0.5% CaCl2 (P < 0.05). The excess amount of calcium might result in the increased repulsion between egg white proteins, leading to the ease of hydrolysis under alkaline condition. Compounds such as peptides and amino acids may contribute to the taste in a complex manner, exceeding the taste properties of the pure compounds due to synergistic interactions.[Citation15] This chemical process causes an “inorganic version” of fermentation, which breaks down some of the complex flavorless proteins into simpler flavorful counterparts.[Citation16] Free amino acids released from hydrolysis react with carbonyl group of sugar which may also contributes to volatile Mailliard flavor compounds[Citation17] as Maillard reaction indicators[Citation18] and different sulfur flavor compounds may arise from methionine or cysteine under alkaline condition[Citation19] which contribute to the cysteine flavor to pidan. The degree of degradation of egg white protein was associated with the hardness of pidan white. PbO2 treated pidan with the lowest degradation exhibited the higher hardness during pickling and aging.[Citation14] Thus, the type of played a role in protein aggregation of pidan white, determining the textural property and characteristics of resulting pidan.
Changes in Microstructure of Pidan White during Pickling and Aging
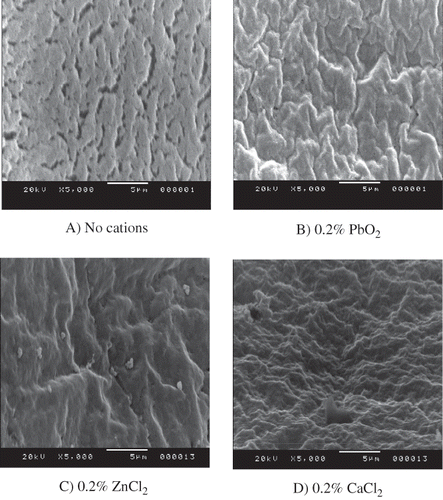
Microstructures of pidan white treated with 0.2% PbO2, 0.2% CaCl2 or 0.2% ZnCl2 and the control (without cations) obtained after three weeks of pickling, visualised by SEM, are shown in . Pidan white samples from most treatments including the control, 0.2% ZnCl2, 0.2% CaCl2 and 0.5% CaCl2 were partially liquified after aging (week 6). Thus, pidan white samples obtained after 3 weeks of pickling were used for microstructure study. Heterogeneous aggregates with the cracks were observed in pidan white of the control. Pidan gels had a more compact structure—without the gap or void in the network when treated with 0.2% PbO2, 0.2% ZnCl2 or 0.2% CaCl2. Smoother surface of pidan was observed in pidan treated with 0.2% PbO2 or 0.2% ZnCl2 whereas different surface was found in pidan white treated with 0.2% CaCl2. Some researchers[Citation20] reported that egg white gel examined with SEM was very coarse with large irregularly shaped voids. Nevertheless, ovalbumin gels showed the homogeneous microstructure.[Citation21] Alkaline conditions are known to unfold protein molecules.[Citation22] Those unfolded proteins could be cross-linked to form protein networks, particularly in the presence of cation via salt bridge mechanism. Thus, the appropriate cations were most likely involved in ion-induced gelation of pidan white.
Changes in Microstructure of Pidan Yolk during Pickling and Aging
The confocal laser scanning microscope (CLSM) micrographs of pidan yolk after pickling (week 3) and aging (week 6) using a two-channel technique, in which both protein and lipid were stained, are illustrated in and , respectively. Pidan yolk samples from some treatments including the control and 0.5% CaCl2 treated sample were very sticky and adhesive with pidan white after pickling and aging (weeks 3 and 6). This characteristic was not desirable for consumers. Thus, pidan yolk samples obtained from treatment of 0.2% PbO2, 0.2% ZnCl2 or 0.2% CaCl2 were used for microstructure study. A combined image is also presented. Proteins in yolk were organized into micellar and granular structures together with polar and non-polar lipid molecules[Citation23] In general, the lipids in egg yolk are organized in lipoproteins and are dispersed in aqueous phase.[Citation24] After three weeks of pickling, alkali more likely penetrated into yolk and pidan yolks became hardened. The dehydration together with the release of lipids in pidan yolk might be associated with such a change. Irregular shapes of both lipid and protein were found in pidan yolk, irrespective of treatments. Nevertheless, the release of lipids and alteration of lipid shape were more pronounced in pidan yolk with 0.2% PbO2 treatment. In general, the distribution of both protein and lipid of yolk treated with 0.2% ZnCl2 or 0.2% CaCl2 was similar. Viscous continuous phase of yolk treated with 0.2% ZnCl2 or 0.2% CaCl2 might promote the stability of emulsions because it impeded the movement and coalescence of the dispersed lipids.
Figure 5 Confocal laser scanning microscope (CLSM) micrographs of exterior yolk for (3A)protein distribution, (3B) lipid distribution, and (3C) and combined image of protein and lipid of pidan treated with different cations after pickling (week 3). Pb: 0.2% PbO2; Zn: 0.2% ZnCl2; Ca: 0.2% CaCl2. Magnification: 200X (zoom X2.5).
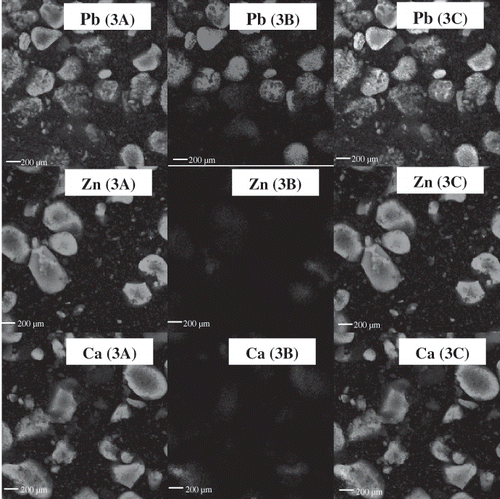
Figure 6 Confocal laser scanning microscope (CLSM) micrographs of exterior yolk for protein distribution (6A), lipid distribution (6B) and combined image of protein and lipid (6C) of pidan with different treatments after aging (week 6). Pb: 0.2% PbO2; Zn: 0.2% ZnCl2; Ca: 0.2% CaCl2. Magnification: 200X (zoom X2.5).
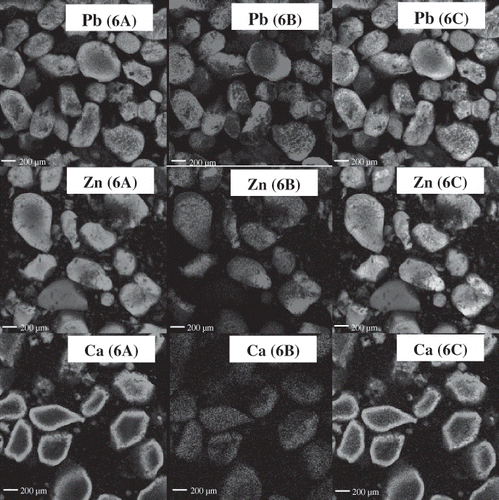
During aging, the greater dehydration with the release of lipid in pidan yolk might reduce emulsion capacity of protein portion, causing the change in the structure of lipid and protein. However, the amount of lipid released was greater in yolk treated with 0.2% PbO2. The greater release of free lipid from lipoprotein of pidan yolk was obtained when 0.2% PbO2 was used as indicated by the denser lipid granules appeared in the combined image (). In the presence of alkali, free lipids underwent saponification. Saponified lipid was postulated to trap the protein, leading to the formation of shielding surface. As a result, the dehydration was lowered and the soft yolk pidan was obtained in pidan yolk treated with 0.2% PbO2. Yolk treated with 0.2% PbO2 was softer in texture than those from pidan yolk treated with other cations. For yolk of pidan treated with 0.2% CaCl2 or 0.2% ZnCl2, less release of free lipids might be associated with hard pidan yolk. Yolk lipid molecules are somehow involved in gel structure formation.[Citation23] CLSM from pidan treated with 0.2% CaCl2 or 0.2% ZnCl2 confirmed that binding of lipid to protein resulted in the hard aggregated yolk, whereas 0.2% PbO2 treatment yielded soft yolk containing more released lipids with less association with proteins.
CONCLUSIONS
During pickling and aging, degradation and aggregation of proteins took place and were governed by divalent cations introduced. Treatment with 0.2% ZnCl2 or 0.2% CaCl2 yielded pidan white with higher protein degradation and ammonia formation. With the treatment of 0.2% PbO2, the lower degradation and ammonia content were observed in pidan white and the softer yolk with more released lipids was obtained. Thus, the type and level of cations used for pidan production had the influence on chemical composition and microstructure of both pidan white and yolk.
ACKNOWLEDGMENTS
The authors would like to express their sincere thanks to Graduate School of Prince of Songkla University and National Research Council of Thailand for the financial support.
REFERENCES
- Hou , H.C. 1981 . “ Egg preservation in China ” . In Food and Nutrition Bulletin‘ , 17 – 20 . Tokyo : The United Nations University Press .
- Wang , J. and Fung , D.Y.C. 1996 . Alkaline fermented foods: a review with emphasis on pidan fermentation . Critical Review of Microbiology , 22 : 101 – 138 .
- Bull , H.B and Breeze , K. 1970 . Water and soluble binding proteins of egg . Archives of Biochemistry and Biophysics , 137 : 299 – 303 .
- Mullvihill , D.M. and Kinsella , J.E. 1988 . Gelation characteristics of whey proteins and β-lactoglobulin . Journal of Food Science , 53 : 31 – 35 .
- Nakamura , T. , Utsumi , S. and Mori , T. 1984 . Network structure formation in thermally induced gelation of glycinin . Journal of Agricultural and Food Chemistry , 32 : 349 – 352 .
- Chen , J.W. and Su , H.P. 2004 . A new process for preparing spots-free pidan . Journal of Chinese Animal Science , 33 : 79 – 88 .
- AOAC International . 2000 . Official methods of analysis , 399 – 406 . Washington, DC : AOAC .
- Parris , N. and Foglia , T.A. 1983 . Simplified alcoholic extraction for ammonia in meat tissue . Journal of Agricultural and Food Chemistry , 31 : 887 – 889 .
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.L. and Randall , R.J. 1951 . Protein measurement with Folin phenol reagent . Journal of Biological Chemistry , 193 : 256 – 275 .
- Mineki , M. and Kobayashi , M. 1997 . Microstructure of yolk from fresh eggs by improved method . Journal of Food Science , 62 : 757 – 61 .
- Steel , R.G.D. and Torrie , J.H. 1980 . Principle and procedure of statistics , 2nd , 454 New York : McGraw-Hill .
- Palanivel , G. ; Soottawat , B. Chemical composition, physical properties and microstructure of pidan white as affected by different divalent and monovalent cation . Journal of the Science of Food and Agriculture (In review).
- Riha , W.E.I II. , Izzo , H.V. , Zhang , J. and Ho , C.T. 1996 . Nonenzymatic deamidation of food proteins . Critical Reviews in Food Science and Nutrition , 36 : 225 – 255 .
- Palanivel , G. ; Soottawat , B. Chemical composition, physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cation . LWT- Food Science and Technology 2010 , 43 , 77 – 85 .
- Solms , J. 1969 . The taste of amino acids, peptides, and proteins . Journal of Agricultural and Food Chemistry , 17 : 686 – 688 .
- Harold , Mc.G. 2004 . On Food and Cooking: The Science and Lore of the Kitchen , 896 New York : Scribner .
- Pripis-Nicolau , L. , De Revel , G. , Bertrand , A. and Maujean , A. 2000 . Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions . Journal of Agricultural and Food Chemistry , 48 : 3761 – 3769 .
- Herrera-Jimenez , M. , Escalona-Buendia , H. , Ponce-Alquicira , E. , Verde-Calvo , R. and Guerrero-Legarreta , I. 2007 . Release of five indicator volatiles from a model meat emulsion to study phase contribution to meat aroma . International Journal of Food Properties , 10 : 807 – 818 .
- Zhang , Y. and Ho , C.T. 1991 . Comparison of the volatile compounds formed from the thermal reaction of glucose with cysteine and glutathione . Journal of Agricultural and Food Chemistry , 39 : 760 – 763 .
- Woodward , S.A and Cotterill , O.J. 1987 . Texture and microstructure of cooked whole egg and heat-formed gels of stirred egg yolk . Journal of Food Science , 52 : 63 – 67 .
- Heertje , I. and van Kleef , F.S.M. 1986 . Observations on the microstructure and rheology of ovalbumin gels . Food Microstructure , 5 : 91 – 98 .
- Creighton , T.E. 1993 . “ Proteins in solution and in membranes ” . In Proteins , 2nd , Edited by: Creighton , T.E. 507 New York : W.H. Freeman and Company .
- Kiosseoglou , V. 2003 . Egg yolk protein gels and emulsions . Current Opinion on Colloidal Interface Science , 8 : 356 – 370 .
- Schreiner , M. 2006 . Optimization of solvent extraction and direct transmethylation methods for the analysis of egg yolk lipids . International Journal of Food Properties , 9 : 573 – 581 .