Abstract
The effect of semi-purified trypsin on the physicochemical properties of durum and bread wheat flour and gluten protein fractions was evaluated. Trypsin was partially isolated from sierra (Scomberomorus sierra) guts extracts by affinity chromatography. Treatment with enzyme caused hydrolysis of 1.16–1.40% in both durum and bread wheat gluten and gluten fractions. The effect of hydrolysis on the isoelectric point was more evident in durum that bread wheat gluten. Addition of the trypsin-like enzyme at three different concentrations induced a decrease in the gluten index. The dough consistency fell after 1.5 h of incubation. It was possible to modify wheat flour proteins by varying S. sierra trypsin-like enzyme concentration and incubation time, depending on the wheat species.
Keywords:
INTRODUCTION
Durum wheat, Triticum durum, is hard wheat that is not ideal for use in the bread making industry due to its gluten characteristics; in contrast, the flour made from the T. aestivum wheat exhibits all the characteristics and properties required for making bread.[Citation1] The main applications of durum wheat, thus, are restricted to pastas and couscous.[Citation2,Citation3] The production of T. durum is important in some regions, mainly in those with water problems, such as Sonora, México, maybe because of semiarid conditions are favorable for production of this cultivar higher in gluten and milling yield.[Citation4] Therefore, durum wheat producers are often seeking alternative applications for durum wheat.
An alternative for the management of wheat flour is the modification of the gluten protein by different agents.[Citation5] The hardness of wheat-flour dough can be decreased upon treatment with oxy-reducer agents, which induce breaking of intermolecular bonds.[Citation6,Citation7] These agents, however, are synthetic and the toxicity of the compounds have yet to be determined.[Citation8] Another method of treatment is the hydrolyzation of peptidic bonds by enzymes; this induces disintegration of the gluten polymeric protein, resulting in more extensible dough.[Citation9]
Marine digestive enzymes have been reported to be capable to hydrolyze vegetable proteins,[Citation10] and sierra fish (Scomberomorus sierra) by-products, such as fish viscera, could be a potential source for proteolytic enzymes.[Citation11] In addition, marine proteolytic enzymes may have some advantage in the limited hydrolysis of durum wheat gluten, like specific breaking protein bonds. Until now, no studies have addressed the effect of marine enzymes on wheat gluten viscoelastic or physicochemical properties. The aim of this study was to investigate the effect of the enzymatic hydrolysis of protease with activity type trypsin extracted from sierra fish viscera, on the change in the degree of hydrolysis and of potential zeta of the gluten and his fractions of two species of wheat. Also it was examined, the change in some physicochemical properties of the dough to predict the field of application.
MATERIALS AND METHODS
Wheat Milling, and Gluten, Gliadin and Glutenin Extraction
T. durum (durum wheat) var. Júpare and T. aestivum (bread wheat) var. Rayón were used in this study. Grains were tempered according to AACC Method 26–95:[Citation12] 17% (v/w) water content for durum wheat and 15% (v/w) for bread wheat. The wheat was milled in a Quadrumat Senior machine (Brabender GmbH and Co, Düsseldorf, Germany) to generate flour. The extraction of gluten from both durum and bread wheat flour was carried out according to AACC Method 38–10.[Citation12] Gliadins and glutenins were extracted from freeze-dried wheat gluten on the basis of their extractability in ethanol.[Citation13] Protein was fractionated by sequential extraction with 70% (v/v) ethanol/water and 50% (v/v) ethanol/water and 0.05 N acetic acid followed by centrifugation to yield gliadin rich fraction and glutenin rich fraction. The fractions were concentrated by solvent evaporation, and freeze-dried.
Extraction of Trypsin-Like Enzyme
Extraction of trypsin from Scomberomorus sierra gut was carried out according to the method described previously,[Citation11] with some modifications. After sulfate ammonium precipitation, the extract was partially purified using SBTI-Sepharose 4B affinity chromatography column.[Citation14,Citation15] The affinity column was prepared according to the manufacturer's specifications (Pharmacia Fine Chemicals, Uppsala, Sweden). During purification, the protein concentration was measured at 280 nm, and the trypsin-like activity was assayed with benzoil-dl-arginin-p-nitroanilide (BAPNA) as a substrate.[Citation16] The fractions with BAPNA activity were pooled and used for the study as described in this paper.
Trypsin-Like Activity
Trypsin amidase activity of the extract was assayed using BAPNA as a substrate.[Citation16] BAPNA was dissolved in 1 ml DMSO to make a 1 mM solution of the substrate and then made to 100 ml with 50 mM TRIS-HCl, 20 mM CaCl2 and pH 7.8 buffer. For 1.25 ml of fresh substrate solution, 0.025 ml of the enzyme preparation was added. After 15 min, 0.25 ml of 30% acetic acid was added and the absorbance at 410 nm was measured against a blank of water. One BAPNA unit of activity was defined as [(ΔAbs410nm/min × 1000 × 1.5)/8800 × mg protein in the assay], where 8800 is the extinction coefficient of p-nitroaniline at 410 nm and 1.5 the volume of the reaction mixture.[Citation16] Protein concentrations of the samples were determined by the protein-dye binding method.[Citation17] Bovine serum albumin was used as the standard (Sigma Chemicals, St. Louis, MO, USA).
SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Separation of proteins contained in the extract preparation was done by electrophoresis using 12% acrylamide.[Citation18] Extract preparations were diluted 1:1 in sample buffer (0.5 M Tris-HCl, pH 6.8, glycerol, 10% SDS, 0.05% bromophenol blue) and an aliquot of 25 μL was loaded into the gel and subjected to electrophoresis at a constant current of 15 mA per gel using a Mini-Protean II Cell Apparatus (Bio-Rad, Mississauga, ON, Canada). Molecular weight standards were included on each plate.
Inhibition Assay
In order to confirm the trypsin activity of the affinity fraction, an inhibition assay was developed. The enzyme solution was incubated with an equal volume of the inhibitor solution. The final concentrations of the inhibition solution are specified in . The mixture was left to stand at 30°C for 30 min. Thereafter, the remaining activity was determined using the BAPNA method, and percent inhibition was calculated.[Citation19]
Table 2 Effect of various inhibitors on the trypsin activity of sierra gut enzyme extract
Degree of Hydrolysis
Degree of hydrolysis (DH) was determined using the o-phthaldialdehyde spectrophotometric assay. This assay is based on the reaction of o-phthaldialdehyde (OPA Sigma-Aldrich St Louis, MO, U.S.A.) and 2-mercaptoethanol with amino groups released during proteolysis of a protein substrate, and is specific for primary amino acids, peptides, and proteins. Color development was read at Abs340nm.[Citation20,Citation21] Briefly, 10 mg N of crude protein from wheat gluten was homogenized in 10 ml of 50 mM Tricine, pH 8.0. Three samples with enzyme and three without enzyme were subjected to analysis of the amino groups formed during hydrolysis. Samples were homogenized for 1 h, and pH was adjusted to 8.0 with NaOH 0.1 N. One ml of the sierra fish trypsin-like protein (0.28 U) was added to gluten and gluten fractions, and samples were incubated for 1 h at 37°C. The volume was adjusted to 25 ml with deionized water, and 2.5 ml were removed for further analysis. Ten ml of 1% SDS (sodium dodecyl sulfate) were added to these samples. Vessels were covered with a watch glass, and were incubated at 75°C for 45 min. The volume was adjusted to 25 ml with 1% SDS. Samples (0.125 ml) were transferred to a test tube containing 2 ml OPA solution (160 mg o-phthaldialdehyde in 4 ml ethanol) and incubated for 2 min at 25°C. Color development was read at an absorbance of 340 nm in a spectrophotometer. A standard curve was obtained from absorbance at 340 nm against leucine concentration (0, 0.075, 0.1125 y 0.185 μmoles), and total meq per sample were calculated. DH was then estimated as follows:
Zeta Potential Determination
Aqueous colloidal dispersions of wheat gluten, native and hydrolyzed freeze-dried gluten were prepared as follows for zeta potential determinations. Ten mg of gluten were added to 100 mL of an aqueous solution of 1.0 mM NaCl, which was used to fix the ionic strength of the solution, and dispersed under magnetic agitation. The pH was then adjusted to the desired final value with dilute solutions of nitric acid or sodium hydroxide, and the sample was stirred for 15 min. The zeta potential (ζ) of gluten was determined at different pH values from electrokinetic experiments using a Zeta Meter 3.0+ unit (Zeta-Meter, Inc., New York). This apparatus includes a microprocessor that first measures the electrophoretic mobility of colloidal particles dispersed in aqueous solutions, and then automatically calculates the zeta potential (in mV) using the Smoluchowski model, which is the most elementary expression for zeta potential calculations from electrophoretic mobility (U) measurements. This model can be written as:
Gluten Index Determination
Dough was formed with 10 g flour and 4.8 ml partially purified trypsin solution. Three levels of the trypsin-like enzyme (0.0, 4.6 and 10.0 mU) were tested (three replicates) to compare the reaction times of the different dosages. Gluten with and without enzyme was incubated at 37°C.[Citation22] Samples were taken at 0.0, 0.5, 1.0, 2.0, and 3.0 h. Wet gluten, dried gluten, and GI were established according to AACC Method 38-12A,[Citation12] using a glutomatic (Perten, Huddinge, Sweden).
Farinograph Assays
Flour samples (50 g) were placed in a farinograph (Brabender GmbH and Co, Düsseldorf, Germany) and the percentage of water absorption was determined for each flour type according to AACC method 54–21.[Citation12] This water quantity containing the semipurified trypsin at 3 levels (0.0, 23.0 and 46.0 mU of activity) was added to the flours and mixed for 20 min (three replicates). Dough was incubated for 1.5 h (in bowl of farinograph) followed by 20 min of mixing to register consistency (expressed in Brabender units, BU) after enzyme action. The net consistency falling (NCF) was calculated as the difference between consistency falling with and without enzyme (BU after incubation and second mixing time).
Statistical Analysis
Mean of DH and GI values were compared by the Tukey test using NCSS, version 2001.
RESULTS AND DISCUSSION
Trypsin-Like Active Extract from Sierra Fish Viscera
The specific trypsin-like activity of the protease after affinity chromatography was approximately 52-fold greater than the crude extract from gut (). In others studies, the use of affinity chromatography on SBTI-Sepharose 4B was highly effective in concentrating trypsin activity.[Citation23–25] The specific activity of 2.22 U/mg was used to hydrolyze gluten proteins. The affinity fraction from the trypsin-like active extract separated into three bands (), one with an estimated molecular weight of 27 kDa. Different molecular weights have been reported for purified trypsin from fish digestive organs, depending on the fish species, in most instances is with the range of 24–29 kDa.[Citation14,Citation15,Citation23–26] The trypsin was strongly inhibited by a serine proteinase inhibitor, such as soybean trypsin inhibitor and TLCK (97–86%) (). These results for molecular weight and effects of inhibitors, confirmed that the extract from sierra fish viscera was serine proteinase, most likely trypsin.
Figure 1 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of protease extracted from S. sierra viscera after affinity chromatography. Lane A: molecular weight standards; Lane B: S. sierra trypsin-like enzyme extract.
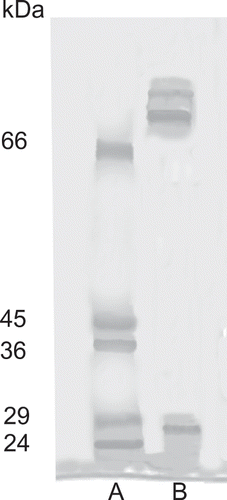
Table 1 Protein concentration, specific activity and purification level during extraction of trypsin from sierra (Scomberomorus sierra ) gut
Degree of Hydrolysis
shows the degree of hydrolysis (DH) of the gluten from both durum and bread wheat. The mean DH of bread wheat gluten was 1.40%, while the mean of durum wheat was 1.27% (with significant difference p < 0.05). This was similar for efficiency of gluten hydrolysis published for other food-grade proteases;[Citation27] but less competent than trypsin from porcine pancreas.[Citation28] The glutenin fraction of both durum and bread wheat flours showed the highest DH. Glutenin is more sensitive to enzymatic proteolysis.[Citation27,Citation29] Despite the low concentration of lysine and arginine content in gluten, the proportion of these amino acids is higher in glutenin than in gliadin.[Citation30] This is consistent with the observed effect of trypsin, which has high affinity to substrates with lysine and arginine residues,[Citation31] causing higher DH in glutenins.
Table 3 DH of different protein fractions from gluten of both durum and bread wheat hydrolyzed by semipurified trypsin
Electrokinetic Potential (Zeta Potential)
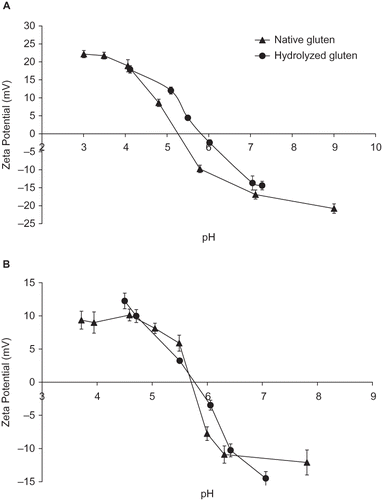
The zeta potentials of native and hydrolyzed gluten are presented in . The sign and magnitude of the zeta potential is pH-dependent, and the variation in this parameter () indicates that the particles of durum wheat gluten contained ionizable surface groups which were exposed due to the enzymatic hydrolysis. The zero zeta potential from untreated durum wheat gluten was at pH 5.1, whereas the zero zeta potential after enzyme incubation was at pH 5.8. Isoelectric point of hydrolyzed gluten moved to a less acid pH () suggesting that amino groups in the protein, (maybe provided by glutamine), were exposed after hydrolysis. The most abundant (about 35%) amino acid in gluten proteins is glutamic acid, but occurs mainly as glutamine,[Citation30,Citation32] and is therefore, positively charged at pH values acidic. Thus, the treatment with the enzyme barely modified the zero zeta potential of the bread wheat gluten (). In other studies,[Citation33] the concentration of glutamine in the gluten modified by other enzyme has been reported is nearly identical to the original gluten. Therefore, addition of fish trypsin was more effective on the modification of isoelectric point of durum gluten.
Table 4 Variation of isoelectric point (zero zeta potential) between native and hydrolyzed gluten particles
Gluten Index
The variations in the gluten index during hydrolysis in both durum and bread wheat flour are shown in . The overall trend of a decrease in GI in response to hydrolysis was more drastic in bread wheat than in durum wheat. After 3 h of treatment with the highest concentration of enzyme, the GI of the two gluten types was nearly 55. Significant differences (p > 0.05) were detected between the GI readings of both flours at every 0.5 h of incubation with semipurified trypsin. These results indicate that gluten index in durum wheat gluten was decreasing in response to increasing enzyme concentration and incubation time due to the disaggregation of native proteins by enzymatic hydrolysis. These results agree with previous findings,[Citation22] who reported the progressive decrease of gluten index during incubation of wheat gluten infested with heteropterous proteases.
Figure 3 Gluten index in response to various enzyme doses at different incubation times. (a) Bread wheat flour and (b) durum wheat flour.
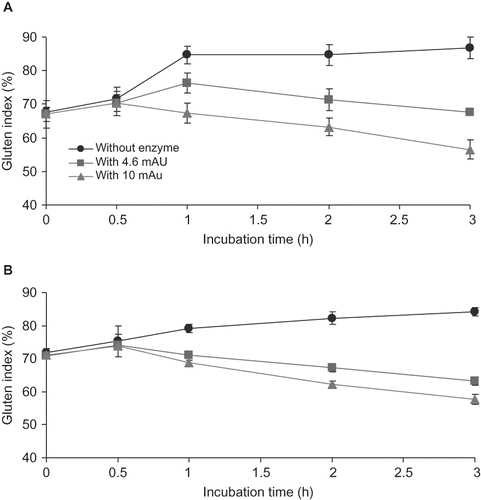
GI is a parameter related to wheat gluten quality, or strength.[Citation34] At the molecular level, the high molecular weight glutenin subunits form a gluten network support;[Citation35] these proteins are hydrolyzed by proteases,[Citation29] and consequently the gluten network becomes weak, resulting in the decrease of GI. The GI of bread wheat flour in the absence of enzyme incubation (0 h) was 67.6, and similar values were observed with the durum wheat flour after 2 h of incubation with 0.0046 AU (GI = 67.2), and after 1 h with 0.01 AU (GI = 68.7). Thus, by varying the enzyme concentration and incubation time, the GI of durum wheat became similar to that of bread wheat.
Farinograph Results
Enzyme incubation assays were carried out at constant consistency of 500 BU. The flour without enzymatic treatment, 63% and 65% of water absorption was detected in bread and durum wheat, respectively. During the first 20 min of mixing, most of parameters were relatively unaffected (). The main variation observed was in the consistency falling after 1.5 h of incubation. Durum and bread wheat dough lose 90 and 180 BU, respectively, in the absence of enzyme. Increasing the amount of semipurified trypsin resulted in an increase of consistency falling (loss of consistency) in both durum and bread wheat dough (). While the highest consistency falling was detected with bread wheat dough, the NFC was the highest in durum wheat dough at the highest concentration of trypsin ().
Table 5 Effect of enzyme over farinograms data of bread and durum wheat flour
Figure 4 Effect of enzyme on consistency falling and net consistency falling (NCF) after 1.5 h of incubation.
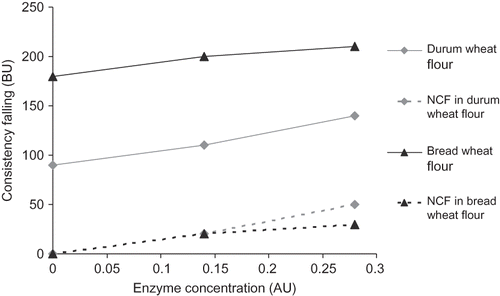
Farinographs have been used to detect rheological changes in dough[Citation36] that sometimes are related to protein composition.[Citation37] In this study, no changes were detected with short enzymatic incubations, only after 1.5 h of incubation was detected a decrease in the wheat dough consistency. The consistency loss due to excess mixing has been previously reported,[Citation38] but this interference was considered to be eliminated by the NCF calculation.
CONCLUSIONS
The addition of semipurified trypsin obtained from a fishery industry byproduct promotes changes in the physicochemical characteristics of gluten proteins. These effects can be manipulated by incubation time or enzyme concentration to obtain certain characteristics in wheat flour from different species. The strength (gluten index) of dough from durum wheat can be decreased by the addition of semi purified trypsin from S. sierra gut. In this study, an easy and effective application of a fishery byproduct was demonstrated. This application could be the focus in the mixing steps due to the effect showed by tests, which simulate mixing. In addition, the activity of the trypsin obtained in this study is highest at neutral pH as in the mixing step.
ACKNOWLEDGMENTS
This work was supported by Mexican Council of Science and Technology (CONACyT), by Grant P43056-Z given to author Ofelia Rouzaud Sández. The authors wish to thank the Spanish Scientific Research Council (CSIC) for facilities given to Francisco Cabrera Chávez.
REFERENCES
- Sapirstein , H.D. , David , P. , Preston , K.R. and Dexter , J.E. 2007 . Durum wheat breadmaking quality: Effects of gluten strength, protein composition, semolina particle size and fermentation time . Journal of Cereal Science , 45 : 150 – 161 .
- Feillet , P. and Dexter , J.E. 1996 . “ Quality requirements of durum wheat for semolina milling and pasta production ” . In Pasta and Noodle Technology , Edited by: Kruger , J.E. , Matsuo , R.R. and Dick , J.W. 95 – 131 . St. Paul, MN : AACC International .
- Quaglia , G.B. 1988 . “ Other durum wheat products ” . In Durum, Chemistry and Technology , Edited by: Fabriani , G. and Lintas , C. 263 – 282 . St. Paul, MN : AACC International .
- Ereifej , K.I. , Al-Karaki , G.N. and Hammouri , M.K. 2001 . Variability of some physico-chemical characteristics of wheat cultivars grown under arid in semiarid Mediterranean conditions . International Journal of Food Properties , 4 : 91 – 101 .
- Liu , C.Y. , Shepherd , K.W. and Rathjen , A.J. 1996 . Improvement of durum wheat pastamaking and breadmaking qualities . Cereal Chemistry. , 73 : 155 – 166 .
- Indrani , D. and Venkateswara , G. 2006 . Effect of additives on rheological characteristics and quality of wheat flour parota . Journal of Texture Studies , 37 : 315 – 338 .
- Nakamura , M. and Kurata , T. 1997 . Effect of L-ascorbic acid on the rheological properties of wheat flour-water dough . Cereal Chemistry , 74 : 647 – 650 .
- Giesecke , W.G. and Taillie , S.A. 2000 . Identifying factors affecting bromate residue levels in baked products: preliminary studies . Cereal Foods World , 45 : 111 – 120 .
- Pedersen , L. , Kaack , K. , Bergsoe , M.N. and Adler-Nissen , J. 2005 . Effects of chemical and enzymatic modification on dough rheology and biscuit characteristics . Journal of Food Science , 70 : 152 – 158 .
- Ezquerra , J.M. , García , F. and Carrillo , O. 1998 . In vitro digestibility of dietary protein sources for white shrimp (Penaeus vannamei) . Aquaculture , 163 : 123 – 136 .
- Olivas , B.H. , Ezquerra , B.J.M. , Rouzaud , S.O. and Pacheco , A. R. 2001 . Protease activity and partial characterization of the trypsin-like enzyme in the digestive tract of the tropical sierra (Scomberomorus concolor) . Journal of Aquatic Food Product Technology , 10 : 51 – 64 .
- American Association of Cereal Chemists . 2000 . Approved Methods of the AACC, Methods: 26–95, 38–10, 38–12A, 54–21 , 10th , St Paul, MN : AACC .
- Hernández , M.P. , Kanavouras , A. , Ng , P.K.W and Gavara , R. 2003 . Development and characterization of biodegradable films made from wheat gluten protein fractions . Journal of Agricultural and Food Chemistry , 51 : 7647 – 7654 .
- Guizani , N. , Rolle , R.S. , Marshall , M.R. and Wei , C.I. 1991 . Isolation, purification and characterization of a trypsin from the pyloric ceca of mullet (Mugil cephalus). Comparative Biochemistry and Physiology . B. Comparative Biochemistry , 98 : 517 – 521 .
- Simpson , B.K. and Haard , N.F. 1985 . Characterization of the trypsin fraction from cunner (Tautogolabrus adspersus) . Comparative Biochemistry and Physiology , 80B : 475 – 480 .
- Erlanger , B.F. , Kokowski , N. and Cohen , W. 1961 . The preparation and properties of two new chromogenic substrate of trypsin . Archives of Biochemistry and Biophysics , 95 : 271 – 278 .
- Bradford , M.M. 1976 . A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye-binding . Analytical Biochemistry , 72 : 248 – 254 .
- Laemmli , U.K. 1970 . Cleavage of structural protein during assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- García , C.F.L. 1992 . Protease inhibition in theory and practice . Biotechnology Education , 3 : 145 – 150 .
- Nielsen , P.M. , Petersen , D. and Dambmann , C. 2001 . Improved method for determining food protein degree of hydrolysis . Journal of Food Science , 66 : 642 – 646 .
- Bonet , A. , Caballero , P. , Rosell , C.M. and Gómez , M. 2005 . Microbial transglutaminase as a tool to restore the functionality of gluten from insect damaged wheat . Cereal Chemistry , 82 : 425 – 430 .
- Aja , S. , Pérez , G. and Rosell , C.M. 2004 . Wheat damage of Aelia spp. and Erygaster spp.: Effects on gluten and water soluble compounds released by gluten hydrolysis . Journal of Cereal Science , 39 : 187 – 193 .
- Klomklao , S. , Benjakul , S. , Visessanguan , W. , Kishimura , H. and Simpson , B. K. 2007 . 29 kDa trypsin from the pyloric ceca of Atlantic bonito (Sarda sarda): Recovery and characterization . Journal of Agricultural and Food Chemistry , 55 : 4548 – 4553 .
- Kurtovic , I. , Marshall , S.N. and Simpson , B.K. 2006 . Isolation and characterization of a trypsin fraction from the pyloric ceca of chinook salmon (Oncorhynchus tshawytscha) . Comparative Biochemistry and Physiology. Part B: Biochemistry & Molecular Biology , 143 : 432 – 440 .
- Guyonnet , V. , Tluscik , F. , Long , P.L. , Polanowski , A. and Travis , J. 1999 . Purification and partial characterization of the pancreatic proteolytic enzyme trypsin, chymotrypsin and elastase from the chicken . Journal of Chromatography A , 852 : 217 – 225 .
- Kishimura , H. , Klomklao , S. , Benjakul , S. and Chun , B.S. 2008 . Characteristics of trypsin from the pyloric ceca of walleye Pollock (Theragra chalcogramma) . Food Chemistry , 106 : 194 – 199 .
- Linares , E. , Larré , C. , Lemeste , M. and Popineau , Y. 2000 . Emulsifying and foaming properties of gluten hydrolysates with an increasing degree of hydrolysis: Role of soluble and insoluble fractions . Cereal Chemistry , 77 : 414 – 420 .
- Kong , X. , Zhou , H. and Qian , H. 2007 . Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates . Food Chemistry , 102 : 759 – 763 .
- Sivri , D. , Sapirstein , H.D. , Köksel , H. and Bushuk , W. 2002 . Effect of wheat bug (Eurygaster maura) protease on glutenin protein . Cereal Chemistry , 76 : 816 – 820 .
- Atwell , W.A. 2001 . “ Composition of commercial flour ” . In Wheat flour , Edited by: Atwell , W. A. 27 – 45 . St. Paul, MN : Eagan Press Handbook Series .
- Copeland , R.A. 2000 . Enzymes. A Practical Introduction to Structure, Mechanism, and Data Analysis , 2nd , 178 New York : John Wiley & Sons, Inc .
- Hoseney , R.C. 1979 . Dough forming properties . Journal of the American Oil Chemists' Society , 49 : 78A – 81A .
- Wang , J.S. , Zhao , M.M. , Bao , Y. , Hong , T. and Rosella , C.M. 2008 . Preparation and characterization of modified wheat gluten by enzymatic hydrolysis-ultrafiltration . Journal of Food Biochemistry , 32 : 316 – 334 .
- Gaines , C.S. , Frégeau , J. , Vander Kant , C. and Morris , C.F. 2006 . Comparison of methods for gluten strength assessment . Cereal Chemistry , 83 : 284 – 286 .
- Troccoli , A. , Borrelli , G.M. , De Vita , P. , Fares , C. and Di Fonzo , N. 2000 . Durum wheat quality: A multidisciplinary concept . Journal of Cereal Science , 32 : 99 – 113 .
- Sroan , B.S. and Kaur , A. 2004 . Effect of antioxidants on farinograph and amylograph characteristics of wheat flour . International Journal of Food Properties , 7 : 379 – 391 .
- Islas , A. , MacRitchie , F. , Gandikota , S. and Hou , G. 2005 . Relationship of protein composition and dough rheological measurements with breadmaking performance of wheat flours . Revista Fitotecnia Mexicana , 28 : 243 – 251 .
- Feillet , P. , Guinet , R. , Morel , M. H. and Rouau , X. 1996 . “ La Masa, Formación y Desarrollo ” . In La Panificación Aspectos Socioeconómicos, Materias Primas, Agentes de Fermentación, Tecnología, Calidad Edited by: Montagud . 245 – 295 . Barcelona