Abstract
Recent studies have reported the biological activities of the crude extracts/purified compounds from various parts of Garcinia cowa. In the present study, the dried fruit rinds of G. cowa were extracted with hexane and chloroform and the extracts were used to evaluate their antioxidant and antimutagenic activities. Using β-carotene-linoleate-model system, at 200 ppm concentration, hexane, chloroform extracts and butylated hydroxyanisole (BHA) showed 91.7, 93.7, and 98.0% antioxidant activity, respectively, whereas, at 50 ppm concentration the radical scavenging activity was 83.3, 86.3, and 88.5%, respectively, through DPPH method. At a concentration of 5000 μg/plate, hexane extract exhibited strong antimutagenicity against the mutagenicity of sodium azide in both the tester strains of Salmonella typhimurium (TA-100 and TA-1535). Chloroform extract showed strong antimutagenicity in both the tester strains at a concentration of 2500 μg/plate and above. However, the chloroform extract exhibited higher antioxidant and antimutagenic activities than that of hexane extract. This study showed that both the extracts from the fruit rinds of G. cowa possess antioxidant and antimutagenic properties.
INTRODUCTION
Garcinia (Family Guttiferae) is a large genus of polygamous trees or shrubs distributed in tropical Asia, Africa, and Polynesia. It consists of 180 species, of which about 30 species are found in India.[Citation1] In upper Assam, G. cowa is often cultivated in homesteads for its acid fruits and is known as “Kuji thekera” locally. They can be made into jam or preserves and the sundried slices of the fruits are used in the treatment of dysentery. In Burma, young leaves are cooked and eaten as vegetables.[Citation1] However, the fruits of G. cowa are under utilized. Crude extracts from the leaves of G. cowa have been reported to possess antitumour promoting activity.[Citation2] A good number of xanthones and their derivatives have been isolated from the stem barks and latex of G. cowa.[Citation3–8] Jayaprakasha et al.[Citation9] have isolated cambogin from the fruit rinds of G. cowa. The extracts from G. pedunculata showed antioxidant and antimutagenic activities.[Citation10] It has been demonstrated that crude extracts as well as partially purified compounds from different parts of various species of Garcinia have shown the bioactivities like antimicrobial, antioxidant, antitumour-promoting, cytotoxic, etc.[Citation11–14]
A major internal threat to cellular homeostasis of the aerobic organisms arises from reactive oxygen species/intermediates and the byproducts generated from oxygen metabolism. Besides the endogenous defenses, consumption of dietary antioxidants could be an important aspect of body's defense mechanism to protect against reactive oxygen species (ROS)[Citation15] and also many antioxidants are being identified as anticarcinogens.[Citation16] It is important to note that most of the investigations regarding inhibitory effect of food component on the oxidative damages of biomolecules have been devoted to the foods of plant origin. Diets rich in fruits and vegetables have been associated with lower risk of cancer and lower dietary intake of the same doubles the risk of cancer as compared to high intake.[Citation17] Much of the research linking free radicals and cancer has centered on ROS and the role of antioxidants in the mutagenesis, promotion of tumour, and carcinogenesis.[Citation18] Since mutagens are involved in the inception and regression of several human diseases, research work related to the discovery, characterization and use of antimutagenic agents is receiving considerable attention.[Citation19] Durnev and Seredenin[Citation20] have suggested antioxidants as means of protecting the genetic apparatus. Moreover, natural antioxidants from plant extracts have attracted the attention due to consumer concern about the safety of the synthetic antioxidants in food. The present investigation explores the antioxidant and antimutagenic properties of the extracts from the dried fruit rinds of G. cowa.
MATERIALS AND METHODS
Materials
All solvents/chemicals used were of analytical grade and obtained from HIMedia, Mumbai, India. DPPH, β-carotene, were obtained from Sigma Chemical Co., (St. Louis, MO, USA). The dried fruit rinds of Garcinia cowa were obtained from Jallah in Barpeta district of Assam, India. Professor B. N. Ames, University of Berkeley, California kindly supplied Salmonella typhimurium strains, TA100 and TA1535.
Extraction Procedures
The dried fruit rinds of G. cowa were extracted as described by Jena et al.[Citation21] Sun dried fruit rinds (10–12% moisture content) of G. cowa were cut into small pieces manually. 50 g of dried fruit rinds was extracted with 200 ml of hexane and chloroform separately and successively with Soxhlet extractor for six h at 60°C. The extracts were filtered through Whatman filter paper No. 1 and concentrated under vacuum to get crude viscous extracts. Further, the extracts were dried in vacuum oven at 40°C and 25 mm Hg for 12 h for complete removal of solvents. The solvent free extracts were used for the present study.
Antioxidant Assay Using β-carotene-linoleate Model System
The antioxidant activity of hexane and chloroform extracts of G. cowa was evaluated using β-carotene-linoleate model system as described by Jayaprakasha et al.[Citation22] Briefly, 0.4 mg of β-carotene in 0.4 ml of chloroform, 40 mg of linoleic acid and 400 mg of Tween-40 (polyoxyethylene sorbitan monopalmitate) were mixed. Chloroform was removed at 40°C under vacuum and the resulting mixture was diluted with 10 ml of water and was mixed well. To this emulsion, 90 ml of oxygenated water was added. 4 ml aliquots of the emulsion were pipetted into different test tubes containing 0.2 ml of G. cowa extracts (equivalent to 50, 100, 200, and 500 ppm) and separate test tubes were prepared containing 0.2 ml of BHA (50,100, 150 and 200 ppm) in ethanol in place of the extracts. BHA was used for comparative purpose. A control containing 0.2 ml of ethanol and 4 ml of the above emulsion was prepared. The tubes were placed at 50°C in a water bath, and the absorbance at 470 nm was taken at zero time (t = 0) in a Genesys-5-UV-Visible spectrophotometer (Milton Roy, New York, USA). Measurement of absorbance was continued till the color of β-carotene disappeared (t = 120 min) in the control tubes at an interval of 15 min. A mixture prepared as above without β-carotene served as blank. All determinations were carried out in triplicate. The antioxidant activity (AA) of the extracts was evaluated in terms of bleaching of the β-carotene using the following formula:
Radical Scavenging Activity Using DPPH Method
The radical scavenging activity of hexane and chloroform extracts of G. cowa was evaluated using DPPH as described by Chidambara Murthy et al.[Citation23] Different concentrations (equivalent to 10, 25, 50, and 100 ppm) of hexane and chloroform extracts of G. cowa and BHA were taken in different test tubes. The total volume was adjusted to 500 μl by addition of MeOH. 5.0 ml of 0.1 mM methanolic solution of DPPH was added to these tubes and shaken vigorously. The tubes were allowed to stand at room temperature (27 ± 1°C) for 20 min. The control was prepared as above without extract and MeOH was used for the baseline correction. The changes in the absorbance of the samples were measured at 517 nm in a Genesys-5-UV-Visible spectrophotometer (Milton Roy, New York, USA). Radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula:
All the estimations were performed in triplicate.
Antimutagenicity Assay
The antimutagenicity of hexane and chloroform extracts from the fruit rinds of G. cowa was studied using the tester strains of S. typhimurium (TA100 and TA1535) through the standard plate incorporation test as described by Maron and Ames.[Citation24] In the antimutagenicity test, the inhibitions of mutagenicity of the sodium azide by the test samples were calculated by determining the number of His+ revertants in the plate. The test samples (625 μg, 1250 μg, 2500 μg, and 5000 μg) were assayed by plating with molten soft agar (2 ml) containing 0.5 mM of histidine/biotin and 0.1 ml of 10 h old culture of strains of S. typhimurium. Positive and negative controls were also included in each assay. Sodium azide was used as a diagnostic mutagen (1.5 μg per plate) in positive control and plates without sodium azide and without test samples were considered as negative controls. His+ revertants were counted after incubation of the plates at 37°C for 48 h. Each sample was assayed using triplicate plates and the data presented here are mean ± SD of three independent assays. The mutagenicity of sodium azide in the absence of test samples was defined as 100% or 0% inhibition. The calculation of % inhibition was done according to the formula given by Ong et al.[Citation25]:
Statistical Analysis
Student's t-tests were performed to evaluate the statistical significance of the results considering p-levels at 0.05 and below were considered significant. The values were mean ± SD of three replicates.
RESULTS AND DISCUSSION
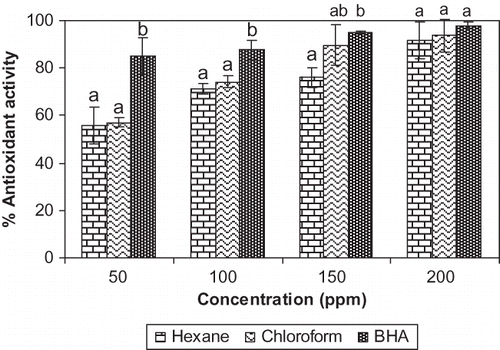
The antioxidant activity of hexane and chloroform extracts from G. cowa was assayed through β-carotene-linoleate model system at 50, 100, 150, and 200 ppm concentrations and was compared with BHA (). The addition of hexane extract, chloroform extract and butylated hydroxyanisole (BHA) at 50 ppm concentrations prevented the bleaching of β-carotene to different degrees. At 200 ppm concentration, the hexane, chloroform extracts and BHA showed 91.7 ± 7.6, 93.7 ± 7.1, and 98.0 ± 1.4% antioxidant activity respectively. The antioxidant activity of hexane and chloroform extracts was observed to increase with increase in concentration of the extracts. In case of hexane extract, the increase of antioxidant activity was statistically significant between 50 ppm and 100 ppm (p < 0.05), 150 ppm and 200 ppm (p < 0.05) and for the chloroform extract, the same parameter was significant between 50 ppm and 100 ppm (p < 0.001) and 100 ppm and 150 ppm (p < 0.05). The chloroform extract was found to possess higher degree of antioxidant activity than hexane extract at each concentration, but it was not statistically significant. In this model system β-carotene undergoes rapid discoloration in the absence of an antioxidant. This is because of the coupled oxidation of β-carotene and linoleic acid, which generates free radicals. The linoleic acid free radical formed upon the abstraction of a hydrogen atom from one of its diallylic methylene groups attacks the highly unsaturated β-carotene molecules. As a result, β-carotene is oxidized and broken down in part; subsequently the system loses its chromophore and characteristic orange colour. In the present study the discolouration of β-carotene was suppressed by hexane and chloroform extracts from G. cowa indicating their ability to inhibit the extent of β-carotene bleaching by neutralizing the linoleate free radical and other free radicals formed in the system.
Figure 1 Antioxidant activity of G. cowa fruit rind extracts at different concentrations by β-carotene linoleate method. Columns in each concentration followed by same letter are not significantly different (p ≤ 0.05).
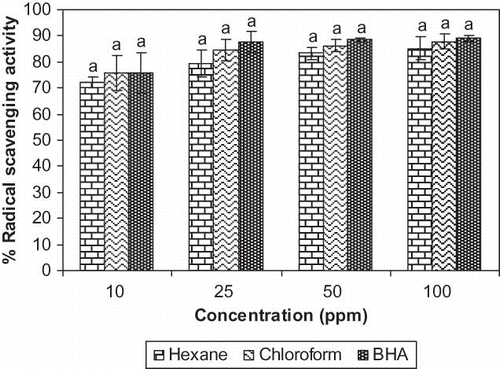
The free radical scavenging activity of the hexane and chloroform extracts from G. cowa were tested through DPPH method and the results were compared with BHA (). In the present study the free radical scavenging activity of the hexane, chloroform extracts and BHA was found to be 85.3 ± 4.51, 87.7 ± 2.88, and 89.0 ± 1.41%, respectively at 100 ppm concentration. The free radical scavenging activity of both the extracts was found to increase with the increase in concentration. In between 10 ppm and 100 ppm the increase of free radical scavenging activity was statistically significant at the level of p < 0.01 for hexane extract and p < 0.05 for chloroform extract and the same parameter was statistically significant (p < 0.01) in between 10 ppm and 50 ppm for hexane extract. However, the chloroform extract showed higher degree of free radical scavenging activity than that of hexane extract at each concentration, but statistically not significant. In essence, the antioxidants react with the stable free radical, i.e., α, α-diphenyl-β-picrylhydrazyl (deep violet colour) and convert it to α, α-diphenyl-β-picrylhadrazine with discolouration. The degree of discolouration indicates the free radical scavenging potentials of the sample/antioxidant and it has been found that known antioxidant such as cysteine, glutathione, ascorbic acid, tocopherol, polyhydroxy aromatic compounds (hydroquinone, pyrogallol, etc.) and aromatic amines (p-phenylene diamine, p-aminophenol, etc.) reduce and decolourise α, α-diphenyl-β-picrylhydrazyl by their hydrogen donating ability.[Citation27] DPPH• is free radical and accepts an electron or hydrogen radical to become a diamagnetic molecule.[Citation28] It appears that both the hexane and chloroform extracts from the fruit rinds of G. cowa possess hydrogen donating ability as antioxidant.
Figure 2 Radical scavenging activity of G. cowa fruit rind extracts at different concentrations by DPPH method. Columns in each concentration followed by same letter are not significantly different (p ≤ 0.05).
In the present study, the results obtained from both the antioxidant assay methods showed that the extracts from the fruit rinds of G. cowa are free radical inhibitors and act as primary antioxidants that react with free radicals. Recently, Jayaprakasha et al.[Citation10] have reported the antioxidant activity of the hexane and chloroform extracts from the fruit rinds of another species of Garcinia such as G. pedunculata. Crude extracts of various plant parts of G. atroviridis including fruits exhibited antioxidant activity.[Citation11] Garcinia is a rich source of secondary metabolites including xanthones and most of the xanthones have phenolic functional groups on a linear tricyclic ring.[Citation29] Phenolics compounds are well known antioxidants from plant sources with remarkable hydrogen or electron donating capacity.[Citation30] Several xanthones with antioxidative properties have been reported in other species of Garcinia such as G. subelliptica and G. mangostana.[Citation14,Citation31] We have isolated a xanthone such as cambogin from the fruit rinds of G. cowa.[Citation9]
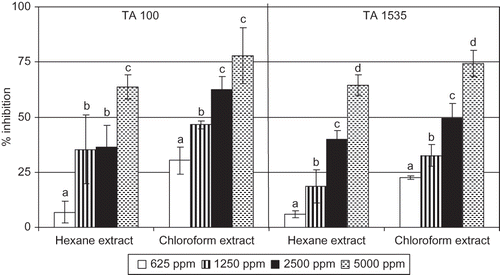
Several studies have revealed that certain chemical carcinogens produce active oxygen and free radicals during carcinogenic process.[Citation32] Active oxygen scavengers reduce mutation induced by various mutagens.[Citation33] Hochstein and Atallah[Citation34] have suggested that compounds, which possess antioxidant activity, can inhibit mutation and cancer because they scavenge free radicals or induce antioxidant enzymes. Fruits and vegetables are the major sources of antimutagens and/or anticarcinogens.[Citation35] Dietary supplementation of antioxidants present in fruits and vegetables are thought to decrease free radical attack on DNA and hence protect against mutation that causes cancer.[Citation36] Fergusan[Citation37] have suggested that intake of antimutagens and anticarcinogens in everyday life would be the most effective procedure for preventing human cancer and genetic diseases by means of increasing consumption of fruits and vegetables. Ames test has been widely used to assess the antimutagenic activity of various compounds and plant extracts.[Citation26,Citation38] The antimutagenicity of the hexane and chloroform extracts from the fruit rinds of G. cowa against sodium azide was evaluated by means of the Ames test using two strains of S. typhimurium (TA100 and TA1535). The degree of antimutagenicity of hexane and chloroform extracts from G. cowa was found to be dose dependent in the range of weak to strong against the mutagenicity of sodium azide in both the strains of Salmonella tester strains (). In case of, hexane extract, strong antimutagenicity (above 40%) effect was shown at 5000 μg/plate for both the tester strains. On the other hand, chloroform extract showed strong antimutagenicity effect at and above 2500 μg/plate for both the tester strains. In case of TA100, the increase of antimutagenicity of hexane and chloroform extracts was statistically significant between the concentration of 625 and 1250 μg/plate (p < 0.05) and 2500 and 5000 μg/plate (p < 0.05). But the same parameter for the chloroform extract was significant between the concentration of 625 and 1250 μg/plate (p < 0.05) and 1250 and 2500 μg/plate (p < 0.05). In case of TA1535, the increase of antimutagenicity of hexane and chloroform extracts was statistically significant between the concentration of 625 and 1250 μg/plate (p < 0.05), 1250 and 2500 μg/plate (p < 0.05) and 2500 and 5000 μg/plate (p < 0.01). The chloroform extract showed higher degree of antimutagenicity than that of hexane extract against the mutagenicity of sodium azide at each concentration in both the tester strains, but is was statistically significant at 625 μg/plate (p < 0.01) and 2500 μg/plate (p < 0.05) in case of TA100 and at 625 μg/plate (p < 0.001) in case of TA1535. Shankaranarayan et al.[Citation39] have reported that the extracts from the pericarp of the fruits of G. mangostana possess antimutagenic activity. Garcinone E, a xanthone derivative isolated from the fruits of G. mangostana found to be cytotoxic against hepatocellular carcinoma cell lines.[Citation40] Xanthones such as euxanthone, 1, 5-dihydroxy-8-methoxyxanthone isolated from the roots of Vismia guaramirangae and xanthone derivatives such as eustoimin and demethyleustomin isolated from the aerial parts of Centauium erythraea display considerable antimutagenic properties in S. typhimurium tester strains.[Citation41,Citation42]
CONCLUSION
The results of our present study indicated that the hexane and chloroform extracts from the fruit rinds of G. cowa possessed marked antioxidant and antimutagenic activities. It appears that antimutagnicity of the extracts are ascribed to their extent of antioxidant activities. Recently Negi et al.[Citation43] have reported the antibacterial activity of the hexane and chloroform extracts from the fruit rinds of G. cowa against foodborne pathogens and spoilage bacteria. Moreover, Nanditha et al.[Citation44] have used the hexane and chloroform extracts from the fruit rinds of G. cowa as natural antioxidants for the preparation of biscuits replacing synthetic antioxidant. Therefore, the extracts from G. cowa have scope for the possible use as food biopreservative as well as nutraceutical. Furthermore, Jena et al.[Citation45] reported that the fruit rinds of G. cowa are rich in (-)-hydroxycitric acid (HCA), which is a natural antiobese agent and after extracting these bioactive fractions with hexane and chloroform, the spent can be used for HCA extraction so that a value addition could be possible for this underutilized species of Garcinia. As the xanthones are the prevalent bioactive compounds in this species, further studies are warranted to identify the compounds present in both the extracts from the fruit rinds of G. cowa.
ACKNOWLEDGMENTS
We wish to thank Professor B. N. Ames, University of Berkeley, Berkeley, California, for supplying Salmonella strains, Dr. V. Prakash, Director, Dr. K. K. Sakariah, former Head, and Dr. M. C. Varadaraj, Head, Human Resource Development, Central Food Technological Research Institute, Mysore, for professional support.
REFERENCES
- Council of Scientific and Industrial Research (CSIR) . 1956 . The Wealth of India (Raw Materials) , Vol. IV , 99 – 108 . New Delhi, , India : CSIR .
- Murakami , A. , Jiwajiinda , S. , Koshimizu , K. and Ohigashi , H. 1995 . Screening for in vitro anti-tumour promoting activities of edible plants from Thailand . Cancer Letters , 95 : 137 – 146 .
- Lee , H.H. and Chan , H.K. 1977 . 1,3,6-Trihydroxy-7-methoxy-8-(3,7-dimethyl-2,6-octadienyl) xanthone from Garcinia cowa . Phytochemistry , 16 : 2038 – 2040 .
- Na Pattalung , P. , Thongtheeraparp , W. , Wiriyachitra , P. and Taylor , W.C. 1994 . Xanthones from Garcinia cowa . Planta Medica , 60 : 365 – 368 .
- Likhitwitayawuid , K. , Phadungcharoen , T. and Krungkrai , J. 1998 . Antimalarial xanthones from Garcinia cowa . Planta Medica , 64 : 70 – 72 .
- Likhitwitayawuid , K. , Phadungcharoen , T. , Mahidol , C. and Ruchirawat , S. 1997 . 7-O-Methylgarcinone E from Garcinia cowa . Phytochemistry , 45 : 1299 – 1301 .
- Mahabusarakam , W. , Chairerk , P. and Taylor , W.C. 2005 . Xanthones from Garcinia cowa Roxb . Latex. Phytochemistry , 66 : 1148 – 1153 .
- Shen , J. and Yang , J.S. 2006 . Two new xanthones from the stems of Garcinia cowa . Chem. Pharm. Bull. , 54 : 126 – 128 .
- Jayaprakasha , G.K. , Jena , B.S. , Jagan Mohan Rao , L. and Varadaraj , M.C. A process for isolation of cambogin from Garcinia cowa . Indian Patent 2004, 519/DEL/04 .
- Jayaprakasha , G.K. , Negi , P.S. and Jena , B.S. 2006 . Antioxidative and antimutagenic activities of the extracts from the rinds of Garcinia pedunculata . Innovative Food Science and Emerging Technology , 7 : 246 – 250 .
- Mackeen , M.M. , Ali , A.M. , Lajis , N.H. , Kawazu , K. , Hassan , Z. , Amran , M. , Habash , M. , Mooi , L.Y. and Mohamed , S.M. 2000 . Antimicrobial, antioxidant, antitumour-promoting and cytotoxic activities of different plant part extracts of Garcinia atroviridis griff. ex T. anders . Journal of Ethnopharmacology , 72 : 395 – 402 .
- Adegoke , G.O. , Kumar , M.V. , Sambaiah , K. and Lokesh , B.R. 1998 . Inhibitory effect of Garcinia kola on lipid peroxidation in rat liver homogenate . Indian Journal of Experimental Biology , 36 : 907 – 910 .
- Asano , J. , Chiba , K. , Tada , M. and Yoshii , T. 1996 . Cytotoxic xanthones from Garcinia hanburyi . Phytochemistry , 41 : 815 – 820 .
- Minami , H. , Kuwayama , A. , Yoshizawa , T. and Fukuyama , Y. 1996 . Novel prenylated xanthones with antioxidant property from the wood of Garcinia subelliptica . Chem. Pharm. Bull. , 44 : 2103 – 2106 .
- Willet , W.C. 1994 . Diet and health: what should we eat? . Science , 264 : 532 – 537 .
- Ames , B.N. 1983 . Dietary carcinogens and anticarcinogens: oxygen radicals and degenerative diseases . Science , 221 : 1256 – 1263 .
- Ames , B.N. , Shigenaga , M.K. and Hagen , T.M. 1993 . Oxidants, antioxidants and degenerative diseases of aging . Proceedings of National Academy of Sciences USA , 90 : 7915 – 7922 .
- Sun , Y. 1990 . Free radicals, antioxidant enzymes and carcinogenesis . Free Radical Biology and Medicine , 8 : 583 – 599 .
- Lupulescu , A. 1986 . Inhibition of DNA synthesis and neoplastic cell growth by vitamin A (retinal) . Journal of National Cancer Institute , 77 : 149 – 156 .
- Durnev , A.D. and Seredenin , S.B. 1990 . Antioxidants as means of protecting the genetic apparatus . Pharmaceutical Chemistry Journal , 24 : 71 – 82 .
- Jena , B.S. , Jayaprakasha , G.K. , Negi , P.S. and Sakariah , K.K. 2007 . A Process for the isolation of antioxidant and antibacterial fraction from Garcinia pedunculata . Indian Patent , IN200200358
- Jayaprakasha , G.K. , Singh , R.P. and Sakariah , K.K. 2002 . Antioxidant activity of Grape seed (Vitis vinefera) extracts on peroxidation models in vitro . Food Chemistry , 73 : 285 – 290 .
- Chidamabara Murthy , K.N. , Singh , R.P. and Jayaprakasha , G.K. 2002 . Antioxidant activity of grape pomace in vitro and in vivo models . Journal of Agricultural Food Chemistry , 50 : 5909 – 5914 .
- Maron , D.M. and Ames , B.N. 1983 . Revised methods for the Salmonella mutagenicity test . Mutation Research , 113 : 173 – 215 .
- Ong , T. , Wong , W.Z. , Stewart , J.D. and Brockman , H.E. 1986 . Chlorophyllin: a potent antimutagen against environmental and dietary complex mixture . Mutation Research , 173 : 111 – 115 .
- Ikken , Y. , Morales , P. , Martinez , A. , Marin , M.L. , Haza , A.I. and Cambero , M.I. 1999 . Antimutagenic effect of fruit and vegetable ethanolic extracts against N-nitrosamines evaluated by the Ames test . Journal of Agricultural Food Chemistry , 47 : 3257 – 3264 .
- Blois , M.S. 1958 . Antioxidant determinations by the use of a stable free radical . Nature , 181 : 1199 – 1200 .
- Soares , J.R. , Dins , T.C.P. , Cunha , A.P. and Ameida , L.M. 1997 . Antioxidant activity of some extracts of Thymus zygis . Free Radical Research , 26 : 469 – 478 .
- Bennet , G.J. and Lee , H.H. 1989 . Xanthones from Guttiferae . Phytochemistry , 28 : 967 – 998 .
- Uriquiaga , I. and Leighton , F. 2000 . Plant polyphenols, antioxidants and oxidative stress . Biological Research , 33 : 55 – 64 .
- Mahabusarakam , W. , Proudfoot , J. , Taylor , W. and Croft , K. 2000 . Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin . Free Radical Research , 33 : 643 – 659 .
- Yen , G.C. and Chen , H.Y. 1995 . Antioxidant activity of various tea extracts in relation to their antimutagenicity . Journal of Agricultural Food Chemistry , 43 : 27 – 32 .
- Kim , S.B. , Kim , I.S. , Yeum , D.M. and Park , Y.H. 1991 . Mutagenicity of Maillard reaction products from d-glucose-amino acid mixtures and possible roles of active oxygens in mutagenicity . Mutation Research , 254 : 65 – 69 .
- Hochstein , P. and Atallah , A.S. 1988 . The nature of oxidant and antioxidant systems in inhibition of mutation and cancer . Mutation Research , 202 : 363 – 375 .
- Nakamura , Y.K. , Suganuma , E. , Kuyama , N. , Sato , K. and Ohtsuki , K. 1998 . Comparative bio-antimutagenicity of common vegetables and traditional vegetables in Kyoto . Bioscience, Biotechnology and Biochemistry , 62 : 1161 – 1165 .
- Duthie , S.J. , Ma , A. , Ross , M.A. and Collins , A.R. 1996 . Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes . Cancer Research , 15 : 1291 – 1295 .
- Ferguson , L.R. 1994 . Antimutagens as cancer chemopreventive agents in the diet . Mutation Research , 307 : 395 – 410 .
- Botting , K.J. , Young , M.M. , Pearson , A.E. , Harris , P.J. and Ferguson , L.R. 1999 . Antimutagens in food plants eaten by Polynesians: micronutrients, phytochemicals and protection against bacterial mutagenicity of the heterocyclic amine 2-amino-3-methylimidazo [4, 5-f] quinoline . Food Chemical and Toxicology , 37 : 95 – 103 .
- Shankaranarayan , D. , Gopalakrishnan , C. and Kameshwaran , L. 1979 . Pharmacological profile of mangostin and its derivatives . Archives of International Pharmacological Therapie , 239 : 257 – 269 .
- Ho , C.K. , Huang , Y.L. and Chen , C.C. 2002 . Garcinone E, a xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines . Planta Medica , 68 : 975 – 979 .
- Monache , F.D. , Mac-Quhae , M.M. , Monache , G.D. , Bettolo , G.B.M. and Delima , R.A. 1983 . Xanthones, xanthonolgnoids and other constituents of the roots of Vismia guaramirangae . Phytochemistry , 22 : 227 – 232 .
- Schimmer , O. and Mauthner , H. 1996 . Polymethoxylated xanthones from the herb of Centaurium erythraea with strong antimutagenic properties in Salmonella typhimurium . Planta Medica , 62 : 561 – 564 .
- Negi , P.S. , Jayaprakasha , G.K. and Jena , B.S. 2008 . Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria . LWT-Food Science and Technology , 41 : 1857 – 1861 .
- Nanditha , B.R. , Jena , B.S. and Prabhasankar , P. 2009 . Influence of natural antioxidants and their carry-through property in biscuit processing . Journal of Science Food and Agriculture , 89 : 288 – 298 .
- Jena , B.S. , Jayaprakasha , G.K. and Sakariah , K.K. 2002 . Organic acids from leaves, fruits and rinds of Garcinia cowa . Journal of Agricultural and Food Chemistry , 50 : 3431 – 3434 .