Abstract
Total polyphenolics and free radical scavenging properties of extracts obtained from osmotically dehydrated, and spiced mushroom snack food with raw unprocessed mushrooms were studied. Sensorially acceptable mushroom snack was prepared in the laboratory by osmotically dehydrating Agaricus bisporous in 5% salt solution followed by addition of an adequate combination of spices and vacuum drying. The extracts of raw and dry snack-mushrooms were then evaluated for their scavenging activities against the 2,2-diphenyl-1-picryhydrazyl (DPPH) free radical and 2,2-azobis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS). The scavenging activity of free extracts of raw and dry snack mushrooms on DPPH radical were 76% and 72%, respectively. The ABTS radical scavenging activity of the free extracts of raw sample expressed as ascorbic acid equivalent antioxidant activity (AEAC) was 2.76 mg ascorbic acid equivalents/100 g and 2.67 mg ascorbic acid equivalents/100 g snack mushroom. Both free and bound polyphenolic contents in mushroom snacks were slightly higher than raw mushrooms whereas total flavonoids levels decreased marginally. No change in antioxidants status of the dry snack product was observed when dry snack stored at ambient temperature for up to 15 days. The results of this study suggested that mushroom snacks prepared using a combination of the above methods was able to retain a significant amount of antioxidants, phenols and flavonoids and mushroom snacks may prove to be an alternative nutritious snack.
INTRODUCTION
Reactive oxygen species and free radicals have attracted increasing attention over the past decade. Reactive oxygen species (ROS), which include free radicals such as superoxide anion radicals (O2 .−), hydroxyl radicals (OH·) and non free-radical species such as H2O2 and singlet oxygen (1O2), are various forms of activated oxygen. These molecules are exacerbating factors in cellular injury and aging process.[Citation1] Free radicals and other reactive oxygen species are continuously produced in vivo and can also be produced by radiation, chemical reactions and several redox reactions of various compounds that may contribute to protein oxidation, DNA damage, lipid peroxidation in living tissues and cells that leads to cancer and cardiovascular diseases.[Citation2–4] Although almost all organisms are well protected against free-radical damage by enzymes such as superoxide dismutase and catalase or by compounds such as ascorbic acid, tocopherols, and glutathione, these systems are insufficient to prevent damage entirely. Consequently, an antioxidant supplement in the human diet is important to either prevent or reduce oxidative damage.[Citation4] Recent epidemiological studies indicated that increased consumption of certain foods, such as fruits, vegetables, red wines and certain juices, provide protection against these diseases.[Citation2] This association may be attributed from the antioxidants in the foods including vitamin C, vitamin E, carotenoids, polyphenolic compounds and flavonoids, which prevent free radical damage.[Citation5]
Mushrooms are being used as traditional foods for their flavor, vegetable like texture and high medicinal properties in Asia.[Citation6] Generally, mushrooms are rich in dietary fiber, minerals, vitamins, and low in fat.[Citation7–8] Moreover, mushrooms contain various polyphenolic compounds recognized as an excellent antioxidant.[Citation9] However, due to highly perishable nature of mushroom, freshly harvested mushrooms usually need to be processed for manufacture of pickles, chutneys, and soups. Drying of mushrooms is a traditional method for preserving mushrooms in the Indian subcontinent. One of the most useful pretreatments for drying of vegetables and fruit is osmotic dehydration. Osmotic dehydration (OD) of fruits and vegetables by immersion in liquids with a water activity lower than that of food has received considerable attention in recent years as an air-drying pretreatment. This dehydration method, in which the simultaneous transfer of water out and solutes into the material takes place, can be used before air drying in order to reduce the food water content by 30 to 70% of the original amount.[Citation10] Osmotic dehydration of mushrooms influence the principal drying method, shortening of the drying process, resulting in lower energy requirements as well as higher retention of fresh like characteristics (colour, aroma, nutritional constituents, and flavor compounds).[Citation11] Using osmotic dehydration followed by vacuum drying, procedure for the preparation of a sensorially acceptable dry mushroom snack was optimized, in view of the fact that natural nutrients could be significantly lost during processing (most of the bioactive compounds are relatively unstable to heat). Overall antioxidant activities of dry mushroom snack using ABTS and DPPH radical scavenging activity, polyphenolic and flavonoid contents in the free and bound extracts of button mushroom was also compared.
MATERIALS AND METHODS
Chemicals
Folin-Ciocalteu reagent, gallic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonicacid) (ABTS), and potassium persulfate were purchased from Sigma and all other reagents were analytical grade.
Preparation of Dry Mushroom Snack
Freshly harvested mushrooms Agaricus bisporus were obtained from the National Centre for Mushroom Research and Training (NCMRT), (Solan, Himachal Pradesh, India). The mushrooms having diameters that ranged between 4.0–4.5 cm were selected for experiments. About 500 g of button mushrooms were cleaned and cut into round pieces (5 mm thickness) and soaked in 5% (Sodium chloride) salt solution (5 min). Mushroom was vacuum dried (NSW-143, Indian Scientific instruments) for two and half hour at 45°C (system pressure 20 kPa). After drying, a pre-formulated 3 g spice mixture (coriander powder, turmeric powder, black pepper, chilly powder, cumin powder, mango powder) was sprinkled using a sprayer (glass bottle) on chips; prepared chips were packed in metalized polyester bags and stored at ambient temperature ().
Extraction
Free and bound compounds of raw and processed mushroom were extracted as described by Ganguli et al.[Citation12] For the extraction of free compounds, samples (10 g) were homogenized with 200 ml of 99% ethanol in a shaker for 24 h. The mixture was further extracted with a homogenizer for 15 min at a medium speed and filtered. The solvents used for extraction were removed using a rotary vacuum evaporator at 40°C under vacuum and redissolved into 50 mL of ethanol to be tested; the extracts were stored at −20°C until analysis. The residue from free compounds extracted was hydrolyzed with 20 ml of 4N NaOH for 1 h under nitrogen and adjusted to pH 2 with 6N HCl. The bound compounds were extracted six times with 20 ml of ethyl acetate. The organic extracts were then evaporated at 40°C to dryness, redissolved in 25 ml of distilled water and stored at −20°C until analyzed.
Determination of Free and Bound Polyphenolics
Polyphenolic contents in the free and bound extracts were determined using the Folin-Ciocalteu method[Citation14–15] with some modifications and results were expressed as mg gallic acid equivalents per 100 g of mushroom.[Citation1] Standard solution or mushroom extract (200 μl) was mixed with 2 ml of 2% sodium carbonate solution and 100 μl of a 50% Folin-Ciocalteu reagent. After incubation for 30 min at room temperature, the absorbance was measured at 750 nm. All extracts were analyzed in triplicate. Gallic acid was used as standard.
DPPH Radical Scavenging Activity
The scavenging activity of the free and bound extracts on DPPH radical was measured according to the method of Cheung et al.[Citation16] with some modifications. Aliquots of 0.8 ml of 0.2 mM DPPH ethanolic solution was mixed with 0.2 ml of the extracts. The mixture was vigorously shaken and left to stand for 10 min under subdued light. The absorbance was measured at 520 nm. The DPPH radical scavenging activity (%) was calculated by the following equation:
ABTS Radical Scavenging Activity
The scavenging activity of the free and bound extracts from mushroom on ABTS radical cation was measured according to the method of Re et al.[Citation17] with some modifications. ABTS radical cation was generated by adding 7 mM ABTS to 2.45 mM potassium persulfate solution and the mixture was left to stand over night in the dark at room temperature. The ABTS radical cation solution was diluted with distilled water to obtain absorbance of 1.4–1.5 at 414 nm (molar extinction coefficient, € = 6·104 mol −1cm −1). Diluted ABTS radical cation solution (1 ml) was added to 50 μl of the extract or ascorbic acid standard solution or distilled water. After 90 min, the absorbance was measured at 414 nm using spectrophotometer (Hitachi, U-2800). The ABTS radical cation scavenging activity was expressed as ascorbic acid equivalent antioxidant activity (AEAC) and defined as mg of ascorbic acid equivalents per 100 g of sample. The AEAC was calculated by the following equation:
Determination of Free and Bound Flavonoids
Flavonoid contents in the free and bound extracts were determined by colorimetric method described by Jia et al.[Citation18] with some modifications and results were expressed as mg catechin equivalents per 100 g of mushroom. Mushroom extract (250 μl) was mixed with 1.25 ml of distilled water and 75 μl of a 5% NaNO2 solution. After 5 min, 150 μl of a 10% AlCl3.H2O was added. After 6 min, 500 μl of 1 M NaOH and 275 μl of distilled water were added to the mixture, mixed well and intensity of pink color was measured at 510 nm. All extracts were analyzed in triplicate. Catechin was used as standard.
Sensory Evaluation
Sensory quality of the dry mushroom-snack following preparation was evaluated by a panel of ten trained judges. Two differently coded samples, i.e., snack 1 and snack 2, were given to 10 panelists for sensory evaluation. The panelists were asked to give the scores for each sample, for different quality attributes like color, surface character, texture, taste, mouthfeel, and overall quality by assigning scores as follows. Color: 1 = brownish/whitish, non-uniform and 9 = golden brown, uniform; surface character: 1 = rough and 9 = smooth; texture: 1 = hard/brittle/grittiness and 9 = crisp; taste: 1 = off-flavor/dislike and 9 = pleasant to mouth; Mouthfeel: 1 = residual taste/formation of lump in mouth and 9 = easy break down/clean mouth feel. The overall quality score (45) was taken as the combined score of all previous attributes.
Statistical Analysis
The results were reported as means ± standard deviation (SD). The significance of differences among means among treatment means as determined by a one-way analysis of variance (ANOVA) (SAS version 8.1; SAS Institute, Cary, NC, USA) with a significant level of 0.05.
RESULTS AND DISCUSSION
Sensory scores shown in indicate that the consistency and the overall acceptability of mushroom snack which improved significantly (P < 0.01) when the osmotic dehydration time was 5 min. Although an increase of time from 5 to 10 min resulted in lowering the time for vacuum drying, salty taste persisted even after addition of spices resulting in poor acceptability. Riaiz et al.[Citation19] reported that blanching of mushroom slices in water at 80°C for 2 min was adequate to inactivate enzymes, additional heat treatment for blanching leads to a substantial loss of nutrients. Dehydrated blanched mushrooms were reported to be superior in nutritional and sensory characteristics to dehydrated unblanched mushrooms.[Citation19] Similar results were obtained in this study. Blanching of foodstuffs is a thermal treatment, mainly applied in vegetable and fruit processing, with the primary goal of inactivation of the enzymes responsible for color, flavour, and textural changes. It is commonly required (prior to different subsequent process) for moderately reducing microbial load, cleansing of the product, and an enhancement of the bioavailability of some food constituents.[Citation20]
Table 1 Mean sensory score of dry mushroom snack
Osmotic treatments of foods (on account of mass transfer, improvements in the physical–chemical, nutritional and sensory properties) have been viewed upon as an important processing technique for enhancing shelf life, optimization of further processes. Application of osmotic dehydration in conjunction to other processes is expected to introduce innovative products in the food market with health beneficial properties.[Citation21] This method has been applied to Agaricus bisporous for producing a low cost, acceptable snack food.
Effect of Treatment on Total Antioxidant Properties
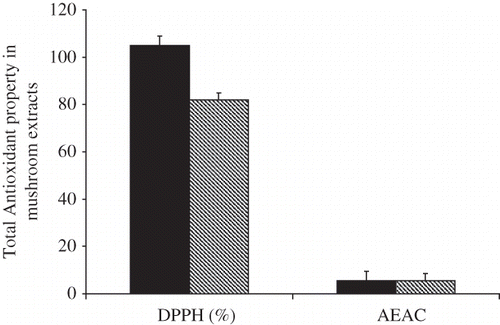
An important factor of plant food is their antioxidant properties; therefore, it is necessary to consider the overall effect of processing on total antioxidant profile of mushroom snacks. The antioxidant activities of the raw and treated mushroom as determined by scavenging DPPH radical are presented in . The DPPH radical scavenging activity (%) of the free extracts of the raw sample showed 76%. After processing, the DPPH radical scavenging activities of free extracts did not changed considerably 72%. The DPPH radical scavenging activity of bound extract decreased notably relative to those of raw mushroom.
Figure 2 Effect of processing on DPPH radical scavenging activity in free and bound mushroom extracts (n = 3). Free ![]()
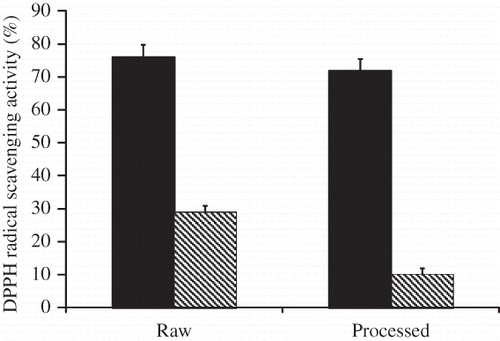
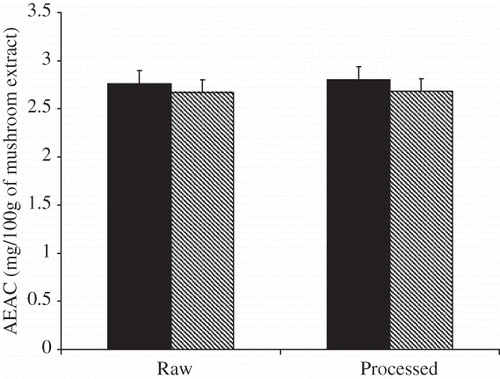
The total antioxidant activities of the raw and treated mushroom, as determined by scavenging ABTS radical, are presented in . The ABTS radical scavenging activity of the free extracts of the raw sample expressed as ascorbic acid equivalent antioxidant activity (AEAC) and defined as the mg of ascorbic acid equivalents per 100 g of mushroom showed 2.76 mg ascorbic acid equivalents/100 g sample. After treatment, the AEAC values did not change significantly (P > 0.05) and were 2.67 mg ascorbic acid equivalents/100 g sample, respectively. The ABTS radical scavenging activity of the bound extracts were 2.8 mg ascorbic acid equivalents/100 g sample in raw sample. After treatment, the ABTS radical scavenging activities showed small increase to a value of 2.68 mg ascorbic acid equivalents/100 g sample.
Effect of Treatment on Total Antioxidant Properties
Effects of treatment on the total polyphenolics of mushroom extracts are shown in . The free polyphenolics of raw mushroom (expressed as mg of gallic acid equivalents per 100 g of sample), was 10.09 mg gallic acid/100 g and 16.29 mg gallic acid /100 g snack mushroom, respectively. The bound polyphenolic compounds were 14.57 mg gallic acid /100 g of snack mushroom as compared to 5.43 mg gallic acid /100 g of raw mushroom.
Figure 4 Effect of processing on polyphenolics content in free and bound mushroom extract (n = 3). Free ![]()
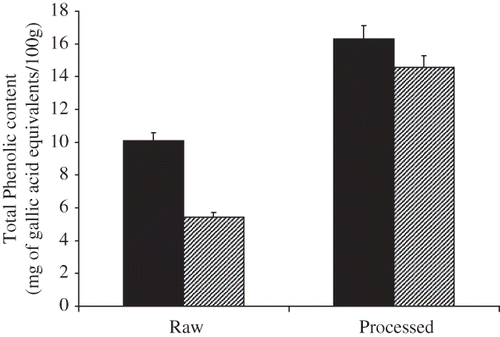
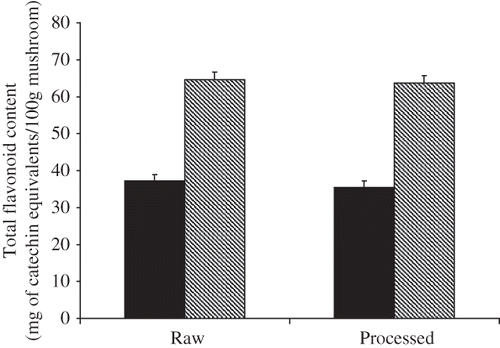
Total flavonoid content of snack and raw mushrooms are shown in . Free flavonoids of raw mushroom (expressed as mg of (+)-catechin equivalents per 100 g sample), were 37.12 mg/100 g raw mushroom and 35.42 mg/100 g snack mushroom. Bound flavonoid content of raw mushroom was 64.69 mg/100 g and 63.72 mg/100 g snack mushroom respectively. It has been suggested polyphenols directly contributes to antioxidant activity[Citation22] since compounds have inhibitory effects on mutagenesis and carcinogenesis in humans, when up to 1.0 g daily ingested from a diet rich in fruits and vegetables.[Citation23] The results obtained in the present study indicate that significant changes especially reductions in polyphenolics, flavonoids, and antioxidant properties of snack mushrooms did not occur following processing (). A small increase in polyphenolic level was observed in extracts of snack mushrooms might resulted from the temperature treatment (disruption of the cell wall, release of antioxidant compounds from insoluble portion of mushroom, and in turn, increasing the pool of bioaccessible antioxidant compounds). Results in this study were similar to those reported by Choi et al.,[Citation24] where temperature mediated increase in both polyphenolics and antioxidant properties were observed in Shitake (Lentinus edodes) mushrooms; a higher temperature (121°C) was however used in that study.
CONCLUSION
A simple process for preparation of Agaricus bisporous snack food was prepared by sequential application of osmotic dehydration and vacuum drying. Total polyphenolics, flavonoids, and antioxidant properties in snack mushroom did not change significantly for at least a period of 15-day storage at ambient temperature. Although more studies are required for commercializing this product, the above study, suggests that mushrooms snacks may serve as a good alternative for currently existing snack foods-since it retains a significant amount of polyphenolics and antioxidants; combined with other commonly used snack foods it may provide beneficial health effects to the consumers economically.
ACKNOWLEDGMENTS
The authors thank the Director, Thapar University, Patiala for providing adequate infrastructure and financial support.
REFERENCES
- Gulcin , I. , Tel , A.Z. and Kirecci , E. 2008 . Antioxidant, Antimicrobial, Antifungal, and Antiradical Activities of Cyclotrichium Niveum (BOISS.) Manden and Scheng . International Journal of Food Properties , 11 : 450 – 471 .
- Halliwell , B. 1996 . Antioxidants in human health and disease . Annual Reviews of Nutrition , 16 : 33 – 49 .
- Morrissey , P.A. and O'Brien , N.M. 1998 . Dietary antioxidants in health and disease . International Dairy Journal , 8 : 463 – 472 .
- Yang , J.H. , Lin , H.C. and Mau , J.L. 2002 . Antioxidant properties of several commercial mushrooms . Food Chemistry , 77 : 229 – 235 .
- Chang , R. 1996 . Functional properties of edible mushrooms . Nutrition Reviews , 54 : S91 – S93 .
- Diplock , A.T. , Charleux , J.L. , Crozier-Willi , G. , Kok , F.J. , Rice-Evan , C. and Roberfroid , M. 1998 . Functional food science and defense against reactive oxidative species . British Journal of Nutrition , 80S : S77 – S112 .
- Manzi , P. , Aguzzi , A. and Pizzoferrato , L. 2001 . Nutritional value of mushrooms widely consumed in Italy . Food Chemistry , 73 : 321 – 325 .
- Mattila , P. , Konko , K. , Eurola , M. , Pihlava , J.M. , Astola , J. and Vahteristo , L. 2001 . Contents of vitamins, mineral elements, and some polyphenolic compounds in cultivated mushrooms . Journal of Agricultural and Food Chemistry , 49 : 2343 – 2348 .
- Ishikawa , Y. , Morimoto , K. and Hamasaki , T. 1984 . Falvoglaucin, a metabolite of Eurotium chevalieri, its antioxidation and synergism with tocopherol . Journal of American Oil Chemists Society , 61 : 1864 – 1868 .
- Gonzlez-Martnez , C. , Chfer , M. , Xue , K. and Chiralt , A. 2006 . Effect of the Osmotic Pre-Treatment on the Convective Air Drying Kinetics of Pear Var. Blanquilla . International Journal of Food Properties , 9 : 541 – 549 .
- Beaudry , C. 2001 . Evaluation of drying methods on osmotically dehydrated cranberries , Montreal, QC : Unpublished M.Sc. thesis . Department of Agriculture and Biosystems Engineering, McGill University
- Ganguli , A. , Ghosh , M. and Singh , N. 2007 . Antioxidant Activities and Total Phenolics of Pickles Produced from the Edible Mushroom, Agaricus bisporous . Journal of Culinary Science & Technology , 5 : 51 – 57 .
- Dewanto , V. , Wu , X. , Adom , K.K. and Liu , R.H. 2002a . Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity . Journal of Agriculture and Food Chemistry , 50 : 3010 – 3014 .
- Dewanto , V. , Wu , X. and Liu , R.H. 2002b . Processed sweet corn has higher antioxidant activity . Journal of Agriculture and Food Chemistry , 50 : 4959 – 4964 .
- Turkoglu , A. , Duru , M.E. and Mercan , M. 2007 . Antioxidant and Antimicrobial Activity of Russula delica Fr: An Edible Wild Mushroom . Eurasian Journal of Analytical Chemistry , 2 : 1306 – 1357 .
- Cheung , L,M , Cheung , P.C.K. and Ooi , V.E.C. 2003 . Antioxidant activity and total polyphenolics of edible mushroom extracts . Food Chemistry , 81 : 249 – 255 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice- Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Jia , Z. , Tang , M. and Wu , J. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Riaiz , R.A. , Khan , S.M. and Bhatia , M.A. 1991 . Effect of blanching and storage on the quality of dehydrated oyster mushrooms . Mushroom Journal of Tropics , 11 : 39 – 44 .
- Udensi , E.A. , Ukozor , A.U.C. and Ekwu , F.C. 2005 . Effect of fermentation, blanching, and drying temperature on the functional properties of Cassava flour . International Journal of Food Properties , 8 : 171 – 177 .
- Blanda , G. , Cerretani , L. , Bendini , A. , Cardinali , A. and Lercker , G. 2008 . Phenolic content and antioxidant capacity versus consumer acceptance of soaked and vacuum impregnated frozen nectarines . European Journal of Food Research & Technology , 227 : 191 – 197 .
- Duh , P.D. , Tu , Y.Y. and Yen , G.C. 1999 . Antioxidant activity of water extracts of harn jyur Chyrsanthemum morifolium (Ramat) . Food Science and Technology , 32 : 269 – 277 .
- Tanaka , M. , Kuei , C.W. , Nagashima , Y. and Taguchi , T. 1998 . Application of antioxidative maillrad reaction products from histidine and glucose to sardine products . Nippon Suisan Gakkaishi , 54 : 1409 – 1414 .
- Choi , Y. , Lee , S.M. , Chun , J. , Lee , H.B. and Lee , J. 2006 . Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom . Food Chemistry , 99 : 381 – 387 .