Abstract
A stabilised yogurt drink (SYD) was produced by mixing yogurt and water with the galactomannans guar (GG) and locust bean (LBG) gum at concentrations of 0.02, 0.06, 0.10, 0.14, and 0.20 g/100 g. The resulting colloid mixtures' viscous behaviour and stability was investigated. Rheological characterization using oscillatory methodology revealed that dynamic properties of elastic (G′), viscous (G″) modules and complex viscosity (η*) appreciably increased with increasing addition of both gums. Changing shear stress demonstrated complex flow behaviour and revealed the shear dependency of the SYD's flow behaviour. Syneresis decreased with increasing viscosity giving 93% improvement at 0.2 g/100 g gum addition.
INTRODUCTION
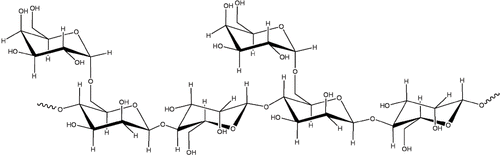
Dairy manufacturers have long been utilising ingredients such as stabilisers to improve the kinetic stability of food emulsions.[Citation1] Stabilisers are used in yogurts as a single compound or as a blend. The selection of a particular type of stabiliser is dependent on aspects such as functional properties, the effect, or mode of action of the stabiliser and the optimum concentration to be utilized. Choice of the proper type and level of hydrocolloid used is one of the most important factors in the manufacture of fermented dairy products. In applications such as yogurt-based products, it is important that the hydrocolloids do not mask the natural flavour of the product and that they are effective at the typical product pH range, i.e. 4.0–4.6.[Citation2] Galactomannans, water-soluble polysaccharides found in the seed endosperm of a variety of legumes, consist of a 1–4 linked β-D mannopyranosyl backbone partially substituted with 1–6 α-D-galactopyranosyl side groups[Citation3] (). Guar gum (GG) and locust bean gum (LBG) are the most widely used as industrial stabilisers, their side groups preventing close polymeric association and thus giving them greatly improved solubility. The degree of branching is described by mannose to galactose ratio (M/G), in LBG values are generally in the region of 3.8 to 4.0 with GG ratios reported between 1.7–2.0.[Citation4–6] GG has a symmetrical structure whereas LBG has unbranched “smooth” regions, which associate with other polysaccharides, giving LBG synergistic qualities that GG does not possess.[Citation7] Both galactomannans used are high grade; therefore, the molecular weights are expected to be in the region of 800 to 1000 KDa.[Citation6,Citation8] They are neutral polymers, and therefore, reasonably stable under an acidic environment. The viscosity of fully hydrated gum solutions at acidic pH was found to be only slightly lower than it was at neutral pH.[Citation8–Citation9]
Previous research has shown that these gums and other stabilisers have an improving effect on acidified milk drinks (AMDs).[Citation10–13] However, research to date has been conducted using rotational viscosity measurement, which is limited in that it only provides shear viscosity data. In order to understand the complex flow behaviour appropriate models, such as Power Law or Herschel-Bulkley, need to be used. The accuracy of the model depends on the suitability of it to describe the rheological behaviour of the material and an incorrectly applied model can give highly improbable results.[Citation14] The alternative powerful measuring technique of oscillation used in this research provides a more detailed understanding of the rheological behaviour of yogurt drink following gum addition.[Citation15] This provides invaluable data on parameters such as elastic or storage modulus (G′), viscous or loss modulus (G″), complex viscosity (η*), and phase angle (δ). G′ indicates the elasticity (solid-like behaviour) while G″ provides information on the viscoelasticy (liquid-like behaviour) of the material. G′ is equal to the ratio of in-phase stress to applied strain while the corresponding parameter for the out-of-phase response is G″. The relationship between G′ and G″ can be defined as:
where δ is phase lag, which is characteristic of viscoelastic behaviour. The overall response of the sample can be characterised by the complex modulus (G*)
Complex viscosity η* can be related to the complex modulus (G*) and the frequency (f) by:
Using oscillation technique the dynamic properties which describe the flow behaviour of the material are obtained from direct measurement and do not require further calculations to be under taken or assumptions to be made. Another important consideration is that oscillatory technique, especially at low shears, does not disturb the network structure of the material being investigated because it employs small alternating movements, in contrast to the one directional movements of shear viscometry.[Citation15,Citation16]
Ayran is a traditional yogurt drink widely made and drunk in Turkey, which is now being industrially manufactured and sold in readymade form in response to a rapidly increasing market demand. Buying ayran in a readymade pre-packaged form is a result of changing lifestyles and expectations. Consumers are assured of its hygiene and food safety criteria as quality standards in Turkey improve.[Citation17] However, a major problem for producers is the syneresis (serum separation) that occurs during storage (maximum of 15 days at 4°C) and the poor mouth feel afforded by low-fat content. Ayran is traditionally made by adding water to yogurt at a rate of 30–50% and salt to taste, then mixing vigorously to form a smooth drink with a texture slightly thicker than milk. Industrially it is made by fermentation of diluted milk, which has had dry matter content adjusted and been diluted with water and the final concentration is achieved by further diluting with salty water to give a product containing roughly 1.5% fat, 0.5% salt. Over addition of water or salt will reduce the products palatability and viscosity.[Citation18] To date manufacturers do not use any additives in the production of ayran as this would mean that it is could no longer be classed as the traditional drink of that name. Therefore, in this paper the production of an ayran with added stabilisers will be termed “Stabilised Yogurt Drink” (SYD). In this study the effect of the galactomannans GG and LBG, added at a range of concentrations, on the rheological and sensory properties as well as stability of the yogurt drink, was investigated.
MATERIALS AND METHODS
Materials
Yogurt provided by Pınar Süt, Pınarbaşi İzmir, Turkey. 100 g Pınar yogurt contains 5.5 g protein, 3.8 g fat, 7 g total carbohydrate, and 165 mg calcium. Gums were provided by Unipectin Ingredients AG, (Eschenz, Switzerland). Guar gum (GG) Vidogum GH 175 (Composition: Water < 13.5%, Fat < 1.5%, Protein < 7.0%, Sugars (Mono + Di-saccharides) 1.0%, Dietary Fibre > 75.0%, Crude fibres (acid-insoluble-matter) < 3.0%, Minerals and Ash < 1.2%.) Energy values without dietary fibre approx. 46 kcal /100 g or 192 kJ/100g). Locust bean gum Vidogum L175/C500 (Composition: Water 13.5%, Fat 1.5%, Protein 7.0%, Sugars (Mono + Di-saccharides) 1.0%, Dietary Fibre > 75.0%, Minerals and Ash < 1.5%.) Energy values without dietary fibre approx. 42 kcal /100g or 176 kJ /100 g.
Gum stock solutions at a concentration of 2 g/100g (w/w) GG and LBG were prepared in 100 mL media bottles. In order to maximise solubilisation of the gums, the bottles were well shaken and heated for one hour at 40°C for GG and 80°C for LBG. This is because GG will fully solubilise at lower temperatures but can denature at higher temperatures whereas LBG requires the high temperature to become fully solubilised and its functionality is not affected by this form of preparation.[38] Controlled and staggered dry gum addition and frequent shaking of the bottles ensured homogenisation of solutions. Concentrations of final solution are based on actual gum addition, which includes the intrinsic moisture content of the gum.
Sensory Evaluation
Sensory analyses were performed in a laboratory by an internal panel of 10 voluntary assessors consisting of one female and nine males aged between 21 and 40 years old. The samples were left to reach room temperature (∼20°C) before being served in 10-mL plastic cups for evaluation and were performed in the order of low to high concentration of gum. Panellists were asked to take one sip, about 5 mL, of sample in their mouth and indicate when they perceived a noticeable change in the texture, flavour, and perceived creaminess with increasing gum addition. They were instructed to rinse their mouths with tap water between each tasting. Tasting sessions were conducted twice initially at day 0, and again at day 7 with samples stored at 4°C. The effects of the stabilizers on sensory attributes of the samples were evaluated by one-way ANOVAs using SPSS version 10.
SYD samples were prepared at 50% (w/v) of yogurt to distilled water and / or GG and LBG gum solution to provide final gum concentrations of 0.02, 0.06, 0.10, 0.14, 0.2 0.25, 0.3, 0.4, 0.5, and 1 g/100 g (w/w) in the SYD. The fat content of the SYD was approximately 1.9%. No salt was added.
Preparation of Samples for Rheological Measurements
Gum stock solutions at a concentration of 0.4 g/100 g (w/w) GG and LBG were prepared in 100 mL media bottles as before. SYD in batches of 40 mL was prepared by mixing yogurt, distilled water, and gum solution at the relevant volumes () in 100 mL media bottles and hand shaken to provide homogenisation. Samples of SYD containing 0.02, 0.06, 0.10, 0.14, and 0.20 g/100 g GG or LBG were prepared in duplicate. Higher concentrations were not prepared as the results of the sensory evaluation made clear that above 0.25 g/100 g the addition of gums gave a negative effect on flavour and texture. The control contained no gum. Samples were stored in a refrigerator at 4°C and tested over a seven-day period.
Table 1 Stabilised yogurt drink preparation with the addition of GG and LBG
Measurement of Rheological Properties
Rheological properties were determined by dynamic (oscillation) method, using a controlled stress/strain rheometer (HAAKE RheoStress 1, Germany) with a cone and plate C35/1 geometry. This geometry was chosen due ease of gap setting, ease of loading and unloading, ease of cleaning and small sample size requirement, which all allowed for multiple runs within the short period of time in which they had to be conducted. The rheological parameters of G′, G″ and η* were analysed using Rheowin Pro Data Manager (Version 2.96) software.
Prior to all the measurements, samples were homogenised by a vortex and 0.85 mL of sample was placed on to the geometry. Measurements were performed at 20.0 ± 0.1°C to best represent the temperature at which the product is usually consumed. The temperature control was regulated by a Peltier Plate System. Initial frequency sweep experiments were carried out at 0.1 to 100 Hz and Stress sweep experiments were performed from 0.1 to 100 Pa, in order to verify the linear viscoelastic region (LVER). The frequency range of 0.1 to 10 Hz produced the LVER. Therefore, the frequency was set at 1 Hz and the stress range was set between 0.1 to 10 Pa for all measurements in order to examine the stress dependency of dynamic properties. Measurements were repeated six times, with 50 data points collected for each sample.
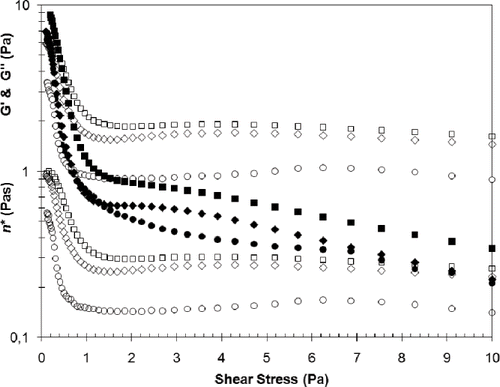
Results of G′, G″ and η* are reported at 1.05 Pa as at this value of shear stress, shear thinning reached its maximum. Above this value of shear stress, the colloid solution network structure that has been formed would be over disturbed (). The shear rate (γ) can be estimated from η* readings and the chosen shear stress (σ) using the formula:
Figure 2 Rheological parameters for SYD with 0.2 g/100 g GG; G′ (□), G″(▪) and η* (□), SYD with 0.2 g/100 g LBG; G′ (⋄), G″(♦) and η* (⋄) and Control; G′ (○), G″(•) and η* (○), after storage at 4°C for seven days. Measurements were made at frequency 1 Hz using a stress sweep test at 20°C.
At a shear stress of 1.05 Pa the shear rate is in the region of 5s-1. The results were analysed using MS Excel to calculate the mean and standard deviations.
Syneresis
SYD samples were stored in graduated 10 mL centrifuge tubes (10-cm height, 1-cm diameter, and 1 mm = 0.1 mL) in a refrigerator at 4°C and the level of syneresis read at days 0, 3, and 7. pH measurements were conducted using an inoLab® Cond Level3 pH meter from the same samples.
RESULTS AND DISCUSSIONS
Sensory Evaluation
Creaminess in acidified milk drinks (AMDs) appears to be largely determined by sensory viscosity; it can thus be manipulated effectively by addition of thickeners.[Citation19] Sensory analysis was conducted to determine which levels of gum addition were acceptable to consumers. Panellists were asked to indicate when they perceived a negative effect on the texture or flavour and improvement in creaminess of the SYD. There was no significant detection of any unpalatable change in texture or flavour for the samples containing gum preparations up to and including 0.2 g/100 g for both GG and LBG (p < 0.05). The panellists indicated improved perceived creaminess in samples with 0.06 to 0.2 g/100 g gum addition with no preference between GG and LBG (p < 0.05). Mouth feel can be affected by the microstructure of particulates in liquid foods and changes in viscosity due to applied shear stress in the mouth, even over very narrow viscosity ranges.[Citation10] The change in texture afforded by thickeners added to low-fat foods can itself affect the perceived odour and flavour through increased perceived thickness.[Citation13] Low level addition of GG has been shown to offset the sour flavours in AMDs[Citation12] and increased levels of exopolysaccharides to increase taste perception through increased viscosity.[Citation20] The sensory properties of dairy products can to some extent be predicted from rheological parameters, high psuedoplasticity of lactic beverages were found to give an increase in acceptability by sensory panellists.[Citation14]
Panellists unanimously agreed that at 0.25 g/100 g gum addition they could detect flavour and texture change. These effects were accentuated as the concentrations of the gums increased. They found it difficult to swallow the samples above 0.5 g/100 g. At 1 g/100 g addition, it developed a yogurt like texture, which made it almost impossible to drink. Other researchers reported similar findings where hydrocolloids provided an oily mouth feel at 0.25 g/100 g addition; however, at 0.1 g/100 g they did not affect taste or odour but did increase consistency.[Citation11] Overly high level of stabiliser addition to dairy products will give a negative mouth feel and flavour.[Citation10,Citation21] It has been shown that increasing the viscosity of a food to a level that will slow the diffusion of components from the product matrix to the taste and olfactory receptors depresses the flavour and aroma.[Citation22]
Rheological Data
Rheological parameters of G′ and G″ as well as η* of SYDs increased, with increasing galactomannan addition ( and ; ). Oscillatory rheology data revealed that viscous component predominates over elastic modulus (G″ > G′) exhibiting non-Newtonian behaviour. Increase in viscosity has also been reported with the addition of hydrocolloids to AMDs.[Citation11,Citation13] Although G′, G″ and η* values for the samples of both gum additions are similar there is a consistent difference between their behaviour at low and high concentrations. These measured parameters are higher for samples containing LBG up to a concentration of 0.1 g/100 g. However, at the levels of 0.14 and 0.2 g/100 g addition they are greater for GG. At day seven, samples with 0.2 g/100 g GG addition gave the G′, G″ and η* values of 1,12 Pa, 2,21 Pa, and 0,34 Pas respectively where as LBG addition could only achieve 0,86 Pa, 1,57 Pa and 0,29 Pas at the same concentration.
Table 2 Rheological parameters (G′, G″, and η*) of SYD samples at different levels of GG addition
Table 3 Rheological parameters (G′, G″, and η*) of SYD samples at different levels of LBG addition
Figure 3 (a) Complex viscosity (η*) of SYD samples at different concentrations of GG addition. Samples were stored at 4°C for a seven-day period. Frequency at 1 Hz and shear stress 1.05 Pa using a stress sweep test at 20°C. Day 0 (⋄), Day 3 (□), and Day 7 (Δ). (b) Complex viscosity (η*) of SYD samples at different concentrations of LBG addition. Samples were stored at 4°C for a seven-day period. Frequency at 1 Hz and shear stress 1.05 Pa using a stress sweep test at 20°C. Day 0 (⋄), Day 3 (□), and Day 7 (Δ).
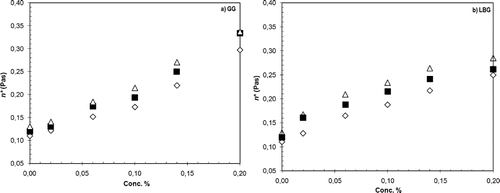
GG has a greater degree of branching (approximately twice that of LBG) which will prevent close polymer associations, especially at higher concentrations, providing more surface area and so greater resistance to shear. The higher degree of branching could also accommodate room for casein micelles to be held. It has been reported that GG at concentrations higher than 0.15–0.2% in casein micellar dispersions influenced depletion flocculation, phase separation of the system and micelle sedimentation.[Citation23] There was an increase in viscosity over time with values for all concentrations being higher at day 7 than day 0. This can be attributed to improvement of network formation within the diluted yogurt system but could also be accentuated by the increasing synergy between the galactomannan chains and casein micelles.[Citation23] Higher viscosities of GG and milk protein solutions compared with gum and water solutions have been reported, indicative of an interaction between the proteins and hydrocolloids.[Citation24]
Yogurts are generally considered as non-thixotropic fluids because their organic structure can be broken by shear and once destroyed it will never be rebuilt.[Citation25] It has been reported that many polymeric materials, and particularly yogurt, display significant shear thinning.[Citation26,Citation27] However, yogurt drinks have been shown to have the flow behaviour of both pseudoplasticity, increased shear thinning with increasing shear stress, and thixotropy.[Citation18,Citation28,Citation29] Thixotropy is the property of some non-Newtonian pseudoplastic fluids to show a time-dependent change in viscosity; a decrease in viscosity over time at a constant shear rate. The addition of partially hydrolysed Guar Gum (PHGG) to low-fat yogurt samples leads to a shift in the viscoelastic behaviour of these yogurts towards more viscous-like materials although the yogurt formulations of PHGG incorporated samples appear to form gel-like structures alongside the casein matrix.[Citation30] Other research has shown that at low gum concentrations, milk protein and gum solutions possessed Newtonian flow behaviour. However, at higher concentrations, the flow behaviour was pseudoplastic.[Citation24]
For both SYDs and control samples the viscous characteristics of the colloid solutions is dominant at lower shear stresses (). It was reported by Koksoy and Kilic,[Citation11] at only two concentrations of gum addition, that the increase in concentration showed increasing pseudoplasticity. However the greater range of concentrations and oscillatory technique used in this research has shown that the relationship is more complex (). As the shear increases to over 1Pa it can be seen that shear thinning (pseudo-plastic) behaviour is dominant, though there was the tendency towards shear thickening (dilatant) at levels above 2Pa shear stress. This phenomenon is more apparent as the concentration of the gums in the solution increases. However when shear stress reaches beyond 5Pa, pseudoplastic flow behaviour is again more dominant. This demonstrates the shear dependency of the SYD colloid mixtures flow behaviour. Although it has been reported that adding salt to yogurt drink changes its flow characteristics to Newtonian behaviour at shear rates in the range of 5 – 20 s−1[Citation18] it was found in this work that studying at high shear rates will give this effect regardless of increasing dilution or added salt. It is suggested that work on yogurt drinks is more accurate if the oscillatory method is used at shear rates below 5 s−1.
Other factors, such as fat content, temperature and the presence of other ions will interact with the galactomannans to affect the rheological properties of the SYD.[Citation26,Citation31] Calcium ions are known to affect the performance of stabilisers but in the presence of a salt possessing the power to sequester calcium ions, the molecules of stabilizer are capable of forming a network of linkages between themselves and the milk constituent(s).[Citation32] Fruit concentrate was also found to increase lactic beverage susceptibility to shear stress.[Citation14] It is suggested that decrease in intrinsic viscosity, with increasing sucrose concentration, of GG and LBG, is because of increased competition for water, which enhances the extent of polymer contraction.[Citation33] The absence of salt in the SYD of this research may have added greater stability to its structure. It has been shown that the addition of salt does reduce the aggregation of casein particles and apparent viscosity of AMDs.[Citation18,Citation34] It has been suggested that inorganic ions in general can affect the protein-protein interactions between casein micelles during acid gel formation by altering the repulsive hydration forces between protein surfaces.[Citation35]
Syneresis
Following the fermentation of milk, the casein micelles, and the fat globules covered by adsorbed casein, aggregate and form a three-dimensional network of chains and clusters, which is partly destroyed by the shear forces of homogenization applied to the system in the course of acid milk drink preparation. AMDs tend to form, upon ageing, a clear layer at the top due to aggregation and precipitation of casein and fat globules clusters which reform because of van der Waals and electrostatic attraction forces since their pH is close to the isoelectric point of the protein. [Citation12] At initial sample preparation, no syneresis was observed, however within hours a film of surface liquid was observed on the control. There was a consistent decrease in pH values in all samples over the seven days, from 4.3 to 4.1, because of Lactic acid bacterial activity. Change in pH will not affect the behaviour of galactomannans as they are neutral polymers and therefore do not influence pH nor is their viscosity influenced by changing pH, except at very high values.[Citation36] Addition of the stabilisers show a significant effect on syneresis ( and ), especially at 0.2 g/100 g, where the amount of separation at day 3 was nil and at day 7 was reduced by 93% for both gums when compared to control. This is in agreement with the rheological findings where increase in G′ values indicate increased gel strength making the SYD less susceptible to rearrangements within its network and therefore reducing shrinkage and syneresis. However, at lower levels of galactomannan addition there is still some separation with an improvement of only 64% for GG and 57% for LBG at 0.1 g/100g gum addition. At the lowest levels of addition LBG provided slightly more stability against syneresis than GG however at the higher levels GG shows an improved performance. This appears to be reflected in the findings of dynamic viscosity, which are higher for LBG than GG at low levels of addition, but above 0.1 g/100 g the opposite is true.
Figure 4 Amount of syneresis measured during storage (mm). SYD samples at different concentrations (from L to R: control, 0.02, 0.06, 0.10, 0.14, 0.20 g/100 g) of GG and LBG additions. Samples were stored in centrifuge tubes (V =14 mL and r =15 mm) at 4°C for a seven-day period.
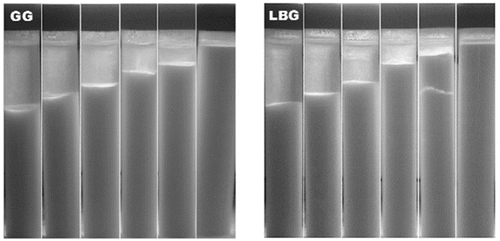
Table 4 Syneresis (mL 10 mL−1) in SYD samples with increasing concentration of GG and LBG addition
CONCLUSION
Galactomannans were chosen for this study because they are neutral in flavour and so were not expected to affect final product palatability. They are also uncharged and therefore unaffected by change in solution pH. Additionally LBG is native to Turkey although currently produced on a small scale, it has the potential for much greater production. It was found in this research that the addition of both GG and LBG to yogurt drink, up to and including a concentration of 0.2 g/100 g, increases the dynamic parameters of G′, G″, and η*. This results in greater stability, which is confirmed by a decrease in syneresis by 93% for both gums, at 0.2 g/100g gum addition. This level of gum addition does not have a negative effect on texture or flavour. The increase in viscosity at low levels of addition can be said to produce a perceived creaminess, which makes the drink more palatable though it is lacking salt. The oscillatory methodology used in this research indicates that the behaviour of yogurt drinks, which is generally described as pseudoplastic, in fact demonstrates more complexity over a wide range of shear stresses. A better understanding of flow behaviour is essential for designing of pumping systems and equipment.
ACKNOWLEDGMENTS
Author is grateful to Assoc. Prof. Dr. Ahmet Kayacier and Safa Karaman of Erciyes University, Food Engineering Department, for their help and the use of rheological facilities. Special thanks to Hülya Yaman and Fatih Isleyen, AIBU for preliminary experiments and final thanks to Mrs Sara Ann Wigglesworth for editing.
REFERENCES
- Dickinson , E. 2003 . Hydrocolloids at interfaces and the influence on the properties of dispersed systems . Food Hydrocolloids. , 17 : 25 – 39 .
- Lal , S.N.D. , O'Connor , C.J. and Eyres , L. 2006 . Application of emulsifiers/stabilizers in dairy products of high rheology . Adv. Colloid Interface Sci. , 123–126 : 433 – 437 .
- Dea , I.C.M. and Morrison , A. 1975 . Chemistry and interactions of seed galactomannans . Adv. Carbohydr. Chem. Biochem. , 31 : 241 – 312 .
- Dea , I.C.M. 1979 . “ Interaction of ordered polysaccharide structures. Synergism and freeze-thaw phenomena ” . In Polysaccharides in Fd , Edited by: Blanshard , J.M.V. and Mitchell , J.R . 229 – 248 . Butterworths : London .
- Fernandes , P.B. , Gonçalves , M.P. and Doublier , J.L. 1991 . A rheological characterization of kappa-carrageenan/galactomannan mixed gels: A comparison of locust bean gum samples . Carbohyr. Polym. , 16 ( 3 ) : 253 – 274 .
- Kök , M.S. , Hill , S.E and Mitchell , J.R. 1999 . A comparison of the rheological behaviour of crude and refined locust bean gum preparations during thermal processing . Carbohyr. Polym. , 38 : 261 – 265 .
- Sand , R. 1982 . “ Structure and Conformation of Hydrocolloids ” . In Food Hydrocolloids , Edited by: Glicksman , M. Vol. 1 , 19 – 46 . Boca Raton : CRC Press .
- Kök , M.S. , Hill , S.E. and Mitchell , J.R. 1999 . Viscosity of galactomannans during high temperature processing: influence of degradation and solubilisation . Food Hydrocolloids. , 13 : 535 – 542 .
- Wang , Q. , Ellis , P.R. and Ross-Murphy , S.B. 2000 . The stability of guar gum in an aqueous system under acidic conditions . Food Hydrocolloids , 14 : 129 – 134 .
- Gallardo-Escamilla , F.J. , Kelly , A.L. and Delahunty , C.M. 2007 . Mouthfeel and flavour of fermented whey with added hydrocolloids . Int. Dairy J. , 17 : 308 – 315 .
- Köksoy , A. and Kılıc¸ , M. 2004 . Use of hydrocolloids in textural stabilization of a yoghurt drink, ayran . Food Hydrocolloids , 18 : 593 – 600 .
- Paraskevopoulou , A. , Athanasiadis , I. , Blekas , G. , Koutinas , A.A. , Kanellaki , M. and Kiosseoglou , V. 2003 . Influence of polysaccharide addition on stability of a cheese whey kefir-milk mixture . Food Hydrocolloids , 17 : 615 – 620 .
- Wendin , K. and Solheim , R. 1997 . Allmere T. and Johansson Flavour and texture in sourmilk affected by thickeners and fat content . Food Qual. Pref. , 8 ( 4 ) : 281 – 291 .
- Penna , A.L.B. , Sivieri , K and Oliviera , M.N. 2001 . Relation between quality and rheological properties of lactic beverages . J. Food Eng. , 49 ( 1 ) : 7 – 13 .
- Weitz , D. , Wyss , H. and Larsen , R. 2007 . Oscillatory rheology: Measuring the viscoelastic behaviour of soft materials . GIT Lab. J. Eur. , 11 ( 3–4 ) : 68 – 70 .
- Sun , A. and Gunasekaran , S. 2009 . Yield Stress in Foods: Measurements and Applications . Int. J. Food Prop. , 12 ( 1 ) : 70 – 101 .
- Berry, B. Agri-Food Past, Present & Future Report Turkey. Agri-Food Trade Service, 2007 http://www.ats.agr.gc.ca/europe/3756_e.htm (Accessed: 12 January 2009 ).
- Köksoy , A. and Kılıc¸ , M. 2003 . Effects of water and salt level on rheological properties of ayran, a Turkish yoghurt drink . Int. Dairy J. , 13 ( 10 ) : 835 – 839 .
- Janhøj , T. , Frøst , M.B. and Ipsen , R. 2008 . Sensory and rheological characterization of acidified milk drinks . Food Hydrocolloids , 22 : 798 – 806 .
- Duboc , P. and Mollet , B. 2001 . Applications of exopolysaccharides in the dairy industry . Int. Dairy J. , 11 ( 9 ) : 759 – 768 .
- Teles , C.D. and Flôres , S.H. 2007 . The influence of additives on the rheological and sensory properties of non-fat yogurt . Int. J. Dairy Tech. , 60 ( 4 ) : 270 – 276 .
- Delwiche , J. 2004 . The impact of perceptual interactions on perceived flavor . Food Qual. Pref. , 15 : 137 – 146 .
- Bourriot , S. , Garnier , C. and Doublier , J.-L. 1999 . Phase separation, rheology, and microstructure of micellar casein–guar gum mixtures . Food Hydrocolloids. , 13 : 43 – 49 .
- Schmidt , K.A and Smith , D.E. 1992 . Rheological Properties of Gum and Milk Protein Interactions . J. Dairy Sci. , 75 : 36 – 42 .
- Schramm , G. 1994 . “ A Practical Approach to Rheology and Rheometry ” . In HAAKE Rheometers, Federal Republic of Germany 250
- Keogh , M.K. and O'Kennnedy , B.T. 1998 . Rheology of Stirred Yogurt as Affected by Added Milk Fat, Protein and Hydrocolloids . J. Food Sci. , 63 ( 1 ) : 108 – 112 .
- Ramaswamy , H.S. and Basak , S. 1991 . Rheology of stirred yogurts . J. Texture Stud. , 22 : 231 – 241 .
- Butler , F. and McNulty , P. 1995 . Time dependent rheological characterisation of buttermilk at 58oC . J. Food Eng. , 25 ( 4 ) : 569 – 580 .
- Oliviera , M.N. , Sodini , I. , Remeuf , F. and Corrieu , G. 2001 . Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria . Int. Dairy J. , 11 ( 11/12 ) : 935 – 942 .
- Brennan , C.S. and Tudorica , C.M. 2008 . Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: comparative study of the utilisation of barley beta-glucan, guar gum and inuli . Int. J. Food Sci. Tech. , 43 : 824 – 833 .
- Kristensen , D. , Jensen , P.Y. , Madsen , F. and Birdi , K.S. 1997 . Rheology and surface tension of selected processed dairy fluids: Influence of temperature . J. Dairy Sci. , 80 ( 10 ) : 2282 – 2290 .
- Tamine , A.Y. and Robinson , R.K. 1999 . Yoghurt Science and Technology , 26 Cambridge : Woodhead Publishing Limited .
- Richardson , P.H. , Willmer , J. and Foster , T.J. 1998 . Dilute solution properties of guar and locust bean gum in sucrose solutions . Food Hydrocolloids , 12 ( 3 ) : 339 – 348 .
- Schkoda , P. , Hechler , A. and Kessler , H.G. 1999 . Effect of minerals and pH on rheological properties of lactic beverages . Int. Dairy J. , 9 ( 3–6 ) : 269 – 273 .
- Bringe , N.A. and Kinsella , J.E. 1991 . Effects of cations and anions on the rate of the acidic coagulation of casein micelles: the possible role of different forces . J. Dairy Res. , 58 : 195 – 209 .
- Goycoolea , F.M. , Richardson , R.K. , Morris , E.R. and Gidley , M.J. 1995 . Stoichiometry and conformation of xanthan in synergistic gelation with locust bean gum or konjac glucomannan -Evidence for heterotypic binding . Macromolecules , 28 : 8308 – 8320 .