Abstract
Araucaria angustifolia and Araucaria araucana are coniferous trees with large and nutritious seeds. The structure and nutritional properties of the proteins of these seeds were studied. About 70% of the proteins of the gametophyte were soluble in distilled water, although their solubility decreased drastically in raw seed flour. The protein components of both araucaria seeds were very similar; the main protein species have molecular weights of 14, 24, and 30 kDa. These proteins have a high nutritional quality, being triptophan the first limiting amino acid.
INTRODUCTION
Araucaria angustifolia and Araucaria araucana are coniferous trees with large and nutritious seeds. A. angustifolia covers areas of the South and South East of Brazil and North East of Argentina,[Citation1] whereas A. araucana is restricted to high mountain areas in the South of Argentina and Chile.[Citation2] Their seeds have been consumed from prehistoric times, especially by natives of these zones.[Citation1] In the province of Neuquén, Argentina, it is possible to eat “alfajores,” consisting in two biscuits made with araucaria seed flour, with a sweet filling between them.
The main component of araucaria seeds is the starch (about 88.0 g/100 g solids) following by protein (about 7.0 g/100 g solids). Several studies were performed on starch of A. angustifolia and A. araucana seeds,[Citation3–8] but little information is available concerning the proteins of these araucaria species. The protein expression during the seed development was studied for A. angustifolia[Citation9] and protein bodies and vacuoles containing proteins have been observed in the embryo of this seed.[Citation10] The protein content of different tissues of A. angustifolia seeds has also been analysed.[Citation11]
Several articles studied different properties of food proteins.[Citation12–14] The characterisation of proteins is important because of their nutritional and functional properties. The aim of this work was to study and compare the proteins of A. angustifolia and A. araucana seeds, through their solubility in different media, measuring the molecular weight of the protein components by electrophoresis, and analysing its amino acid content.
MATERIALS AND METHODS
Materials
The seeds of Araucaria angustifolia were obtained from the field “Manuel Belgrano” (EEA Montecarlo, INTA), San Antonio, province of Misiones, Argentina, whereas the seeds of Araucaria araucana were obtained from local shops in the province of Neuquén, Argentina.
Raw Seed Flour
In order to reduce the external microbial content, the seeds were previously washed with NaClO 5% and the remaining solution was removed with an absorbent paper. The flour of raw seeds was obtained by separating the resistant coat, and then milling the seeds. The ground seeds were dried at 80°C for 2 h, milled again (flour passed through a 0.500-mm sieve), and left at 50–55°C until the moisture content was lower than 10 g/100 g flour. The raw seed flour of A. angustifolia contained, in 100 g dry flour, 7.3 g protein (N × 6.25), 1.7 g lipid, 88.1 g carbohydrates, and 2.9 g ash. The raw seed flour of A. araucana contained, also in 100 g dry flour, 7.4 g protein, 2.2 g lipid, 87.6 g carbohydrates, and 2.8 g ash.
Protein Solubility
Gametophyte was milled during 1 min at maximal velocity with a food processor Philips Cucina HR7633 (Vedia, Bs. As., Argentina). Proteins of the gametophyte and raw seed flour from A. angustifolia and A. araucana seeds were sequentially extracted according to the Osborne classification,[Citation15] and the protein solubility was measured by the Kjeldahl method. Samples of 4.8 g of raw seed flour or 8.6 g of gametophyte were sequentially extracted twice with 30 mL of distilled water (albumins), 5% NaCl (globulins), 70% ethanol (prolamins), and 0.1 M borate buffer, pH 10 (glutelins). Samples were homogenized with an Ultra-Turrax T25 during 3 min, and centrifuged at 11000 × g for 20 min. Nitrogen determination by the Kjeldahl method was performed on 40 mL of supernatant. Results are the average of two independent determinations.
In order to analyse the protein solubility in media that disrupt different kind of bonds, samples of gametophyte (0.15 g) and raw seed flour (0.06 g) were dispersed in 3.5 mL of the following media: distilled water (DW), pH 8.0 buffer (0.086 M Tris, 0.09 M glycine, 4 mM Na2EDTA) (B), and the same buffer containing 0.5% SDS and 8 M urea (BSU)[Citation16]. Samples were homogenized with an Ultra-Turrax T25 during 1.5 min, and 1 mL of each sample was centrifuged at 15800 × g for 30 min. Protein concentration was determined with a Beckman DU 650 Spectrophotometer (California, USA) at 280 nm with an apparent E 1% 1cm of 36.12 and 48.27 for the gametophyte of A. angustifolia and A. araucana, respectively, and 58.54 and 42.39 for the raw seed flour of A. angustifolia and A. araucana, respectively The apparent E 1% 1cm was determined by measuring the absorbance at 280 nm and the protein content by the Kjeldahl method (N × 6.25) in the same protein solution. Solubility values are the average of at least 5 determinations.
Electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli,[Citation17] using a linear gradient separating gel (5–15% in polyacrylamide) without stacking gel, with an acrylamide:bisacrylamide ratio of 75:2. Samples of gametophyte, embryo or raw flour of A. angustifolia and A. araucana seeds were dispersed in distilled water (about 1% protein), and 1 mL of each sample was centrifuged at 15800 × g for 30 min. Supernatants (100 μL) were diluted 1:1 with a pH 8.0 buffer (0.01 M Tris-HCl, 0.001 M EDTA, 1% SDS, and about 30% glycerol), with 10% ß-ME. Samples were heated in a boiling water bath for 5 min before electrophoresis. Bands were stained with 0.2% Coomassie Brilliant Blue R-250 in methanol:distilled water:acetic acid 10:10:4, and destained with ethanol:acetic acid:distilled water 25:10:65. Low molecular weight standards from Sigma were used to estimate the molecular weight of the protein components.
Amino Acid Analysis
The determination of Asp, Glu, Ser, His, Gly, Thr, Arg, Ala, Tyr, Val, Phe, Leu, Ile, Lys and Pro was performed on 0.1 g sample treated with 6N HCl at 110°C during 24 h. Other portion of the sample was treated with performic acid to oxidize Cys and Met, and then the determination continued as before. Finally, in order to determine Trp, a third portion of the sample was treated with NaOH 4.2N at 110°C during 22 h. The hydrolysates were filtered through a Nylon membrane (0.45 μm pore) and derivatized with Oftaldehyde/9-fluorenylmethyl chloroformate (OPA/FMOC) in borate buffer, pH 9.9. Chromatographer was performed with a Hypersyl 5μm column in a high performance liquid chromatograper (Hewlett Packard 1100 series, California, USA), equipped with a fluorescent detector (Hewlett Packard 1046A, Germany). Measures were performed in duplicate with external standards (Sigma-Aldrich, Steinhein, Germany).
The amino acid content was expressed as milligrams of amino acid per gram protein. An amino acid score was computed for each essential amino acid as (milligrams of amino acid in 1 g of test protein/milligrams of amino acid in a reference protein) × 100. The lowest amino acid score predicted the first–limiting amino acid. The reference protein used was that established by FAO/WHO.
Statistics
In order to compare data of A. angustifolia and A. araucana, a test for difference between two variances was performed, followed by a two-sample t test of media comparison for equal or non-equal variances, as corresponded,[Citation18] using the Excel computer program.
RESULTS AND DISCUSSION
Protein Solubility
Proteins of the gametophyte from A. angustifolia and A. araucana seeds were sequentially extracted according to the Osborne classification, and the protein solubility was measured by the Kjeldahl method. Both araucaria seeds presented about 70% albumins, 8% globulins, and little amounts of protein soluble in 70% ethanol (prolamins) or in borate buffer, pH 10 (glutelins). When this analysis was performed in raw flour, an important decrease in the protein solubility in distilled water (albumins) and dilute saline solution (globulins) was observed, especially in A. angustifolia. These results are shown in . Simultaneously, an increase in the protein solubility in borate buffer was observed, mainly in A. angustifolia ().
Table 1 Soluble protein content (g soluble protein/100 g total protein) of gametophyte and raw seed flour of A. angustifolia and A. araucana seeds, extracted with different media. Values not followed by the same letter are different at the 5% level
The protein solubility of gametophyte and raw flour from A. angustifolia and A. araucana seeds was also analysed by using media that disrupt different kind of bonds: distilled water (DW), pH 8 buffer (B), and pH 8 buffer containing SDS and urea (BSU). In this assay, the protein extraction was not sequential but the extraction was performed independently with each medium. The protein solubility was estimated by the absorbance at 280 nm, because some of the media contain nitrogen; thus, protein determination by the Kjeldahl method was not possible. The values of protein solubility in distilled water () were similar to those obtained by the Kjeldahl method. The protein solubility of A. araucana in distilled water and pH 8.0 buffer was higher than that of A. angustifolia. The protein solubility of raw flour in distilled water and pH 8 buffer was lower than the corresponding to gametophyte, but the protein solubility of raw flour in BSU was about 85%, suggesting that non covalent bonds, such as hydrogen and hydrophobic bonds, which can be disrupted by urea and SDS, were formed during the drying process. Most of the gametophyte proteins are soluble in distilled water; because of that, it was assumed that probably they are globular proteins. When globular proteins are heated, as in the preparation of raw seed flour, hydrophobic residues that were inside in the native molecule can be exposed, producing an aggregation of proteins through hydrophobic bonds and a decrease in the solubility.
Table 2 Soluble protein content (g soluble protein/100 g total protein) of gametophyte and raw seed flour of A. angustifolia and A. araucana seeds, extracted with different media. Values not followed by the same letter are different at the 5% level. DW: distilled water; B: buffer, pH 8.0; BSU: buffer, pH 8.0, containing SDS and urea
Electrophoresis
shows the electrophoretic patterns of gametophyte, embryo and raw seed flour protein components of A. angustifolia and A. araucana. The electrophoretic profiles of the protein components extracted with different media from embryo and gametophyte of araucaria seeds, presented the same main bands: two intense bands of 14 and 24 kDa, and a third band of 30 kDa. These results agree with those obtained by Pighín et al.,[Citation11] who found the same electrophoretic pattern in different tissues of the mature seed of A. angustifolia. Samples extracted with different media showed mainly quantitative differences; thus, only distilled water extracts were shown in . Only the main bands, and very slight, were observed in raw seed flour extracts, in agreement with the data of protein solubility presented earlier.
Figure 1 Electrophoretic patterns of protein components of embryo, gametophyte and raw flour of araucaria seeds. (a) Raw flour of A. araucana; (b) raw flour of A. angustifolia; (c) gametophyte of A. araucana; (d) gametophyte of A. angustifolia; (e) embryo of A. araucana; (f) embryo of A. angustifolia.
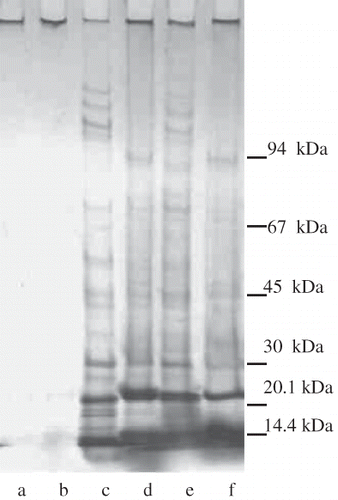
Wendt dos Santos et al.[Citation9] studied the protein expression during the seed development of A. angustifolia, and observed the presence of proteins in a wide range of apparent molecular weights, from plus 76 kDa to below 17.5 kDa. Highly abundant proteins with a size below 36 kDa were detected in mature seeds, corresponding to the accumulation of storage proteins.[Citation9] These results are in accordance with the electrophoretic patterns obtained in the present work. Some differences between A. angustifolia and A. araucana electrophoretic patterns were observed in minor bands, as the presence of a protein component of about 95 kDa in the gametophyte of A. angustifolia, which was not observed in A. araucana, whereas A. araucana presented four protein components of molecular weight higher than 100 kDa, that were not observed in A. angustifolia ().
Amino Acid Analysis
The amino acid composition (mg/g protein) of raw flour from A. angustifolia and A. araucana seeds is shown in . The essential amino acids content of other proteins of high biological value, as well as the FAO/WHO reference content of essential amino acids, was also included in the . Significant differences (5% level) between A. angustifolia and A. araucana were found in the Ala, Tyr, Leu, Met, and Phe contents. Data of allow authors to estimate the chemical score of the amino acids, taking the FAO/WHO requirements as a standard. The first-limiting amino acid was Trp for both A. angustifolia and A. araucana raw seed flour. Data of show that the araucaria protein has a high nutritional quality, similar to that of soy protein. The essential amino acid patterns of both araucaria proteins were similar to that of soy protein isolate, being the araucaria protein richer in Val, aromatic and sulfur-containing amino acids. On the other hand, araucaria protein presented a lower content of Ile, Leu, Lys, and Trp than casein.
Table 3 Amino acid composition (mg/g protein) of A. angustifolia and A. araucana raw flour proteins. Values not followed by the same letter are different at the 5% level. The essential amino acid composition of casein powder and soy protein isolate, and the suggested requirements of essential amino acids (more than 1 year old) of the FAO/WHO and Institute of Medicine of the National Academy of Sciences (USA) were also included
The amino acid composition can explain the functional properties of these proteins, such as their solubility. Taking into account the molecular weight of each amino acid, and considering equimolar mixtures of Glu/Gln and Asp/Asn, it is possible to estimate the proximate molar composition of these proteins, and the proportion of hydrophobic to hydrophilic residues. shows the amino acid composition in moles%. Results show that about 25% of the amino acid residues are Asx + Glx. These amino acid residues are charged or can form hydrogen bonds with molecules of water, which may contribute to the water solubility observed in these proteins. The hydrophobic amino acid residues represented about 37% of the total residues. The ß-lactoglobulin A and the α-lactalbumin B, two globular whey proteins, contain 25% and 28% of residues of Asx + Glx, respectively, and 46% and 33% of hydrophobic amino acid residues, respectively.[Citation19]
Table 4 Amino acid composition (moles%) of A. angustifolia and A. araucana seed protein
CONCLUSIONS
The main protein components of A. angustifolia and A. araucana seeds showed molecular weights lower than 35 kDa, and the differences between the two seeds were in minor protein components of higher molecular weight. Most of the proteins of both seeds were albumins, although their solubility decreased drastically during the preparation of seed flour, probably due to the formation of non covalent bonds during drying. These proteins have a high nutritional value, similar to that of soy protein. This fact, in addition with their high starch content, could make these seeds an energetic food of high nutritional value.
ACKNOWLEDGMENT
Authors would like to thank Nelly Bauer and Gustavo and Cruz de Antueno for their collaboration in the present work. This investigation was supported by grant of the CONICET (PIP N° 5339). P.A. Conforti has a grant and C.E. Lupano is member of the Researcher Career of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
REFERENCES
- Cordenunsi , B.R. , Menezes , E.W. , Genovese , M.I. , Colli , C. , Gonçalves de Souza , A. and Lajolo , F.M. 2004 . Chemical composition and glycemic index of brazilian pine (Araucaria angustifolia) seeds . Journal of Agricultural and Food Chemistry , 52 : 3412 – 3416 .
- Cardemil , L. and Riquelme , A. 1991 . Expression of cell wall proteins in seeds and during early seedling growth of Araucaria araucana is a response to wound stress and is developmentally regulated . Journal of Experimental Botany , 42 : 415 – 421 .
- Wosiacki , G. and Cereda , M.P. 1985 . Characterization of pinhão starch. Part I. Extraction and properties of the starch granules . Starch/Stärke , 37 : 224 – 227 .
- Cereda , M.P. and Wosiacki , G. 1985 . Characterization of pinhão starch. Part II. Rheological properties of the pastes . Starch/Stärke , 37 : 404 – 407 .
- Wosiacki , G. , Grossa , P. and Cereda , M.P. 1989 . Characterization of pinhão starch. Part III: Hydration of the granules and susceptibility to enzymatic hydrolysis . Starch/Stärke , 41 : 327 – 330 .
- Waghorn , J.J. , del Pozo , T. , Acevedo , E.A. and Cardemil , L.A. 2003 . The role of two isoenzymes of α-amylase of Araucaria araucana (Araucariaceae) on the digestion of starch granules during germination . Journal of Experimental Botany , 54 : 901 – 911 .
- Conforti , P.A. and Lupano , C.E. 2007 . Starch characterisation of Araucaria angustifolia and Araucaria araucana seeds . Starch/Stärke , 59 : 284 – 289 .
- Conforti , P.A. and Lupano , C.E. 2008 . Comparative study of the starch digestibility of Araucaria angustifolia and Araucaria araucana seed flour . Starch/Stärke , 60 : 192 – 198 .
- Wendt dos Santos , A.L. , Wiethölter , N. , El Gueddari , N.E. and Moerschbacher , B.M. 2006 . Protein expression during seed development in Araucaria angustifolia: transient accumulation of class IV chitinases and arabinogalactan proteins . Physiologia Plantarum , 127 : 138 – 148 .
- Panza , V. , Láinez , V. , Maroder , H. , Prego , I. and Maldonado , S. 2002 . Storage reserves and cellular water in mature seeds of Araucaria angustifolia . Botanical Journal of the Linnean Society , 140 : 273 – 281 .
- Pighín , D , Panza , V and Maldonado , S. 2007 . Evaluación del contenido de proteínas en semillas de Araucaria angustifolia . Proceedings of the XI Congreso Argentino de Ciencia y Tecnología de Alimentos (CYTAL) . September 12–14 2007 , Buenos Aires , Argentina . pp. 1 – 8 .
- Resh , J.J. and Daubert , C.R. 2002 . Rheological and physicochemical properties of derivatized whey protein concentrate powders . International Journal of Food Properties , 5 : 419 – 434 .
- Guo , X. and Yao , H. 2006 . Fractionation and characterization of tartary buckwheat flour proteins . Food Chemistry , 98 : 90 – 94 .
- Sahu , R.K. and Prakash , V. 2008 . Mechanism of prevention of aggregation of proteins: a case study of aggregation of α-globulin in glycerol . International Journal of Food Properties , 11 : 613 – 623 .
- Osborne , T.B. 1907 . The proteins of the wheat kernel , Washington , DC : Carnegie Institute Washington . Publ. 84.
- Shimada , K. and Cheftel , J.C. 1988 . Texture characteristics, protein solubility, and sulphydryl group/disulfide bond contents of heat-induced gels of whey protein isolate . Journal of Agricultural and Food Chemistry , 36 : 1018 – 1025 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Zar , J.H. 1996 . Biostatistical analysis , 3rd , 123 – 177 . New Jersey : Prentice Hall .
- Swaisgood , H.E. 1982 . “ Chemistry of milk protein ” . In Developments in dairy chemistry – 1 , Edited by: Fox , P.F. 1 – 59 . London : Applied Science Publishers .
- Wang , X-.S. , Tang , C-.H. , Yang , X-.Q. and Gao , W-.R. 2008 . Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins . Food Chemistry , 107 : 11 – 18 .
- Hambraeus , L. 1982 . “ Nutritional aspects of milk proteins ” . In Developments in dairy chemistry – 1 , Edited by: Fox , P.F. 289 – 313 . London : Applied Science Publishers .
- Suarez , M.M. and López , L.B. 2005 . Alimentación saludable , 110 Buenos Aires , , Argentina : Akadia .