Abstract
This study investigated the antimicrobial effects of guava against E. coli O157:H7 and Salmonella in liquid medium. The minimum inhibitory concentration (MIC) and the minimum lethal concentration (MLC) values for each bacterial strain were recorded. In addition, the growth of the individual strain and a combined mixture of the strains in liquid medium over time were assessed. Guava was found to inhibit the growth of all tested strains. The MIC ranged from 200 to 700 μL/mL and the MLC was at least 500 μL/mL. The minimum effective guava extract concentration needed to show significant growth inhibition was 5%. Without guava extract, bacterial population levels reached 7.0–8.0 log CFU/mL. The addition of guava extract caused significant growth inhibition, resulting in bacterial populations remaining within 3.0 log CFU/mL during the incubation. These results indicate guava could be used as a potential effective antimicrobial agent that can be used to ensure food safety.
Keywords:
INTRODUCTION
Shiga toxin-producing Escherichia coli O157:H7 and Salmonella play a significant role in foodborne infections.[Citation1,Citation2] Escherichia coli O157:H7 is a foodborne pathogen that is frequently associated with cases of illness characterized by outbreaks of bloody diarrhoea, hæmorrhagic colitis, and hæmolytic uræmia syndrome,[Citation3,Citation4] whereas Salmonella causes salmonellosis. E. coli O157:H7 was first identified as a dangerous foodborne pathogen in 1982 when several people in Michigan and Oregon became ill with severe abdominal pain and bloody diarrhoea. This outbreak was associated with the consumption of undercooked ground beef from a fast food restaurant chain.[Citation5] In 1993, over 700 people in four states were infected with E. coli O157:H7 and four deaths were attributed to contaminated hamburgers.[Citation6,Citation7] More recently, other foods have been implicated in outbreaks of microbial contamination related illness, including fresh produce, acid or acidified food products (such as apple cider), salad dressings, and mayonnaise.[Citation8–12 Out of 17,252 laboratory-confirmed cases of foodborne illness in FoodNet surveillance areas, 38% were determined to be associated with Salmonella.[Citation13]
Food preservation processes are used by the food industry to ensure that consumers receive safe wholesome food products. Among the safest and the most effective approaches in food preservation, is the use of preservative agents that have antimicrobial activity. These antimicrobial agents can be classified into artificial and natural compounds. Artificial compounds are generally chemicals derived from organic acids such as acetic and lactic acid, whereas natural compounds are developed from plants such as garlic, onions and peppers and biopreservatives, which include compounds produced by lactic acid bacteria and probiotic cultures.[Citation14–17
Currently, consumers are paying closer attention to the risk of foodborne pathogens, and to artificial chemical preservatives often used to control these foodborne pathogens. The result of this concern has generated considerable interest in the use of natural ingredients as antimicrobial compounds as a way of avoiding consumption of perceived “unhealthy” ingredients represented by artificial chemical compounds used in food products.
The antimicrobial effects of many plant extracts have been well studied. The antimicrobial action found in plants extracts is believed to be present as a defense mechanism. For example, essential oils from plants such as chive, basil, cumin, and oregano have been shown to inhibit different foodborne pathogens.[Citation18–23 Several fruits and fruit extracts, as well as arrowroot tea extract[Citation15] and caffeine[Citation24] have been found to exhibit antimicrobial activity against E. coli O157:H7. This suggests that plants manifest relatively high levels of antimicrobial action may be sources of compounds that can be used to inhibit the growth of foodborne pathogens. Bacterial cells could be killed by the rupture of cell walls and membranes, and by the irregular disruption of the intracellular matrix when treated with plant extracts.[Citation15]
Several reports have demonstrated that guava fruits contain compounds associated with positive health benefits.[Citation25–27 Regular consumption of guava fruit significantly increases the level of high-density lipoprotein (good cholesterol) and significantly decreases serum total cholesterol, triglycerides, and blood pressures.[Citation25] Guava also contains several phytochemicals with many unique properties including high antioxidant activity.[Citation28] The objective of the present paper was to determine the antimicrobial activity of guava against Escherichia coli O157:H7 and Salmonella Typhimurium in a liquid medium.
MATERIALS AND METHODS
Maintenance and Preparation of Cultures
Six strains of E. coli O157:H7 and two strains of Salmonella Typhimurium () were used in this study. E. coli strains were supplied by Dr. S. S. Summer, Department of Food Science, and Technology at Virginia Tech. Salmonella strains were obtained from the North Carolina A &T State University food microbiology laboratory culture collection. These strains were maintained on tryptic soy agar (TSA, Difco Laboratories, Becton Dickinson, Sparks, MD, USA) slants at 4°C and transferred to fresh tryptic soy broth (TSB) before use. Cultures of each strain were grown separately in TSB (Difco, Becton Dickinson, Sparks, MD, USA) at 37°C and transferred at 24 h intervals.
Table 1 Bacterial strains and original sources
Preparation of Guava Extract
Whole fresh guava fruits were obtained from a local international market in Greensboro, NC. The fruits were washed under running tap water and blotted with a single-use paper towel. They were then sliced into pieces and 100 g of sliced fruits were blended in a sterilized kitchen blender for 3 minutes and centrifuged at 5500 g for 45 min at 4°C. The supernatant was collected and filtered using a 0.45-μm filter (Nagle, Rochester, NY, USA) and the collected material (guava-filtered juice) was stored overnight at 4°C until used within 12 h. This guava filtered juice and used to represent guava extract.
Antibacterial Activity of Guava
An agar diffusion assay was used to determine the antibacterial activity of guava. Batches of 100 mL Brain Heart Infusion (BHI) agar with 0.5% agar and 0.2% Tween 80 were prepared and sterilized at 121°C for 15 min. The agar was placed in a water bath at 49°C, allowed to equilibrate, and then inoculated individually with a single strain of E. coli O157:H7 or Salmonella. The BHI agar (∼15 mL) was poured into Petri dishes and allowed to solidify. The wells with 7 mm in diameter were made in the center of the agar using a sterile test tube. Guava extract (0–1000 μL with 100 μL increase for each plate) was transferred into each well. Plates were then incubated for 10–12 h at 35°C. The MIC and MLC values for each strain were determined. The size of inhibition zone around the well was recorded for the plate with addition of 500 μL guava extract for each strain. The inhibition zone was measured in millimeters and the assay was carried out three times.
Preparation of Mixture Strains
Overnight samples of the individual strains were serially diluted to ∼4 log CFU/mL. Then, 1 mL of each diluted strain was combined into a sterilized empty tube to represent the combined strain mixture sample.
Growth Over Time
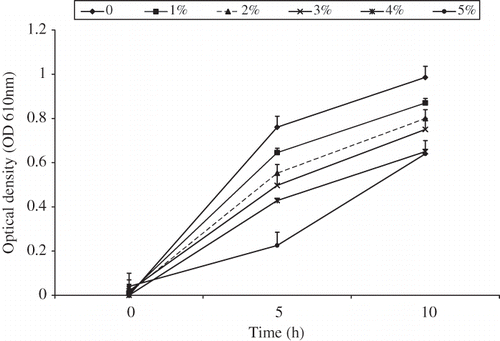
The antibacterial activity of the guava extract was tested against this combined strain mixture. Batches of sterile TSB tubes containing guava extract at six concentrations (0, 1, 2, 3, 4, and 5%) with the total volume of 10 mL were inoculated with 100 μL of the mixture strains. Samples were then incubated at 37°C and growth was monitored by measuring the optical density (OD) at 5 h interval during the incubation period using a Milton Roy Spectronic 21 Spectrophotometer (Thermo Electron Scientific Co., Madison, WI, USA) at the wavelength of 610 nm.
Effect of Guava Extract on the Survival and Growth of E. coli O157:H7 and Salmonella in Liquid Medium
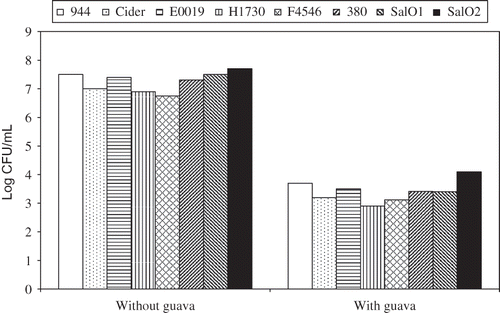
Batches of 100 mL sterilized BHI were prepared and divided into two portions. Guava extract was added at 5 % (v/v) to one portion. The other BHI broth portion without extract was treated as control. Samples were then inoculated with overnight bacterial strains to achieve a final inoculum level of ∼3 log CFU/mL. The samples were then incubated at 37°C for 24 h. A 0.1 mL aliquot of each sample was removed at the end of incubation period, serially diluted in 0.1% peptone water (Bacto peptone, Becton Dickinson, Sparks, MD, USA), and spread plated in duplicate on prepared BHI agar. The inoculated plates were then incubated at 35°C for 24 h. The colonies were counted to determine the bacterial population in each sample.
Statistical Analysis
Experiments were conducted three times to determine if addition of guava extract has significant influence on bacterial growth. The Statistical Analysis System (SAS) version 6.0 computer statistical package (SAS Institute, Cary, NC, USA) was used for the analysis of data with Duncan's multiple range to determine significant differences (P < 0.05).
RESULTS AND DISCUSSION
Antibacterial Activity of Guava
Our laboratory has been working with different methods to determine the antimicrobial activity of natural ingredients including fruits and vegetables. Our approach is based on three steps: (1) initial screening for the antimicrobial activity using agar plate assay, (2) measuring the MIC and MLC values for each ingredient with each bacterial strain, and (3) determining the inhibitory effect over time. These are the most common standard methods used in food microbiology laboratories.
The antimicrobial activity of guava extract against E. coli O157:H7 and Salmonella was assessed using agar diffusion assay. A clear zone of inhibition for all tested strains was observed indicating that guava extract possess such activity. presents the zone of growth inhibition for the tested strains. Results demonstrated that E. coli O157:H7 was more sensitive to guava extract compared to Salmonella. The zone size ranged from 9.52 mm to 10.34 mm, whereas the zone size for Salmonella ranged from 7.80 mm to 7.99 mm.
Table 2 MIC, MLC, and zone of growth inhibition for E. coli O157:H7 and Salmonella in the presence of guava extract on BHI agar plate
The minimum inhibitory concentration (MIC) was defined as the lowest concentration of guava liquid that inhibited bacterial growth. The minimum lethal concentration (MLC) was defined as the lowest concentration at which no growth was observed after incubation at 37°C for up to three days. also shows the MIC and MLC data for the guava extract for the tested strains. In the case of E. coli O157:H7, the MIC ranged from 200–400 μL, whereas the MLC ranged from 500–800 μL. The MIC and MLC for Salmonella were 500–700 μL and at least 900 μL respectively. Recent work in our laboratory has shown that chive extract has antimicrobial activity against Salmonella. The MIC for the chive extract ranged from 180–300 μL with an average 235 μL and the MLC ranged from 650–900 μL with an average 747 μL.[Citation18]
Growth Over Time
shows the survival and growth of the mixture strains in the presence of guava extract in BHI broth during the incubation at 37°C. In the samples without guava extract, the bacteria continued to grow and reached an OD of 0.8 within 5 h. The addition of guava extract (1%) caused a slight growth inhibition, when the guava extract concentration was increased to 2% BHI or greater, significant growth inhibition was observed (P < 0.05). The presence of guava extract (1–3%) significantly retarded lowered the growth of the combined strain mixture during the incubation period compared to samples without the guava extract (P < 0.05). At higher concentrations (4–5%), the inhibition effect of bacteria growth was much stronger (P < 0.001).
Effect of Guava Extract on the Survival and Growth of E. coli O157:H7 and Salmonella in Liquid Medium
The antimicrobial effect of guava extract was assessed using E. coli O157:H7 and Salmonella in BHI broth containing 5% guava extract (). Without guava extract, bacterial population levels reached approximately 7.0–8.0 log CFU/mL. The addition of guava extract significantly inhibited the growth of all strains of bacteria, as the bacterial population remained within 3 log CFU/mL during the incubation at 37°C. This indicates that 5% guava extract exhibited a strong inhibition against all tested strains.
CONCLUSIONS
The results in this study revealed that guava as natural ingredient could be used to improve the safety and quality of several consumable products. Guava extra at only a 5% concentration level has a significant inhibitory effect on both E. coli O157:H7 and Salmonella indicating that guava extract could be used as natural ingredient to control the growth of these pathogens. Future work should concentrate on examining the effect of guava extract against other pathogens such as Listeria monocytogenes, and investigating in combination with organic acids or essential oils possible synergistic effects on bacterial growth inhibition.
ACKNOWLEDGMENTS
The authors wish to thank Dr. C. S. Turner, Associate Dean for Research for her support and Dr. K. J. Gruber for his review and comments on this manuscript. Support for this research work was provided by the Cooperative State Research, Education, and Extension Service of the United States Department of Agriculture, Project No. NC.X-173-5-02-170-1, Agricultural Research Program, North Carolina Agricultural and Technical State University.
REFERENCES
- Kumar , H.S. , Karunasagar , I. , Karunasagar , I. , Teizou , T. , Shima , K. and Yamasaki , S. 2004 . Characterization of shiga toxin-producing Escherichia coli (STEC) isolated from seafood and beef . FEMS Microbiology Letters , 233 : 173 – 178 .
- Tabe , E.S. , Oloya , J. , Doetkott , D.K. , Bauer , M.L. , Gibbs , P.S. and Khaitsa , ML. 2008 . Comparative effect of direct-fed microbials on fecal shedding of Escherichia coli O157:H7 and Salmonella in naturally infected feedlot cattle . Journal of Food Protection , 71 : 539 – 544 .
- Riley , L.W. , Remis , R.S. , Helgerson , S.D. , McGee , H.B. , Wells , J.G. , Davis , B.R. , Hebert , R.J. , Olcott , E.S. , Johnson , L.M. , Hargrett , N.T. , Blake , P.A. and Cohen , M.L. 1983 . Hemorrhagic colitis associated with a rare Escherichia coli serotype . The New England Journal of Medicine , 308 : 681 – 685 .
- Remis , R.S. , MacDonald , K.L. , Riley , L.W. , Puhr , N.D. , Wells , J.G. , Davis , B.R. , Blake , P.A. and Cohen , M.L. 1984 . Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7 . Annals of Internal Medicine , 101 : 624 – 626 .
- Mead , P. and Griffin , P.M. 1998 . Escherichia coli O157:H7 . Lancet , 352 : 1207 – 1212 .
- Centers for Disease Control . 1993 . Update: multistate outbreak of Escherichia coli 0157:H7 infections from hamburgers-western United States, 1992–1993 . Morbidity and Mortality Weekly Report , 42 : 258 – 263 .
- Vogt , R.L. and Dippold , L. 2005 . Escherichia coli O157:H7 outbreak associated with consumption of ground beef, June–July 2002 . Public Health Reports , 120 : 174 – 178 .
- Erickson , J.P. , Stamer , J.W. , Hayes , M. , McKenna , D.N. and van Alstide , L.A. 1995 . An assessment of Escherichia coli O157:H7 contamination risks in commercial mayonnaise from pasteurized eggs and environmental sources, and behavior in low-pH dressings . Journal of Food Protection , 58 : 1059 – 1064 .
- Centers for Disease Control and Prevention . 1996 . Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice—British Columbia, California, Colorado, and Washington, October 1996 . Morbidity and Mortality Weekly Report , 45 : 975
- Smittle , R.B. 2000 . Microbiological safety of mayonnaise, salad dressings, and sauces produced in the United States: a review . Journal of Food Protection , 63 : 1144 – 1153 .
- Fang , T.J. 2005 . “ Bacterial contamination of ready-to-eat foods: concern for human toxicity ” . In Reviews in food and nutrition toxicity , Edited by: Preedy , V.R. and Watson , R.R. 143 – 171 . London : Taylor & Francis Group .
- Bobe , G. , Thede , D.J. , Ten Eyck , T.A. and Bourquini , L.D. 2007 . Microbial levels in Michigan apple cider and their association with manufacturing practices . Journal of Food Protection , 70 : 1187 – 1193 .
- Dargatz , D.A. , Fedorka-Cray , P.J. , Ladely , S.R. and Ferris , K.E. 2000 . Survey of Salmonella serotypes shed in feces of beef cows and their antimicrobial susceptibility patterns . Journal of Food Protection , 63 : 1648 – 1653 .
- Ibrahim , S.A. and Bezkorovainy , A. 1993 . Inhibition of Escherichia coli by bifidobacteria . Journal of Food Protection , 56 : 713 – 715 .
- Kim , S. and Fung , D.Y. 2004 . Antibacterial effect of crude water-soluble arrowroot (Pueraiae radix) tea extracts on food-borne pathogens in liquid medium . Letters in Applied Microbiology , 39 : 319 – 325 .
- Ibrahim , S.A. , Yang , H. and Seo , C.W. 2008 . Antimicrobial activity of lactic acid and copper on growth of Salmonella and Escherichia coli O157:H7 in laboratory medium and carrot juice . Food Chemistry , 109 : 137 – 143 .
- Rahman , M.S. , Al-Sheibani , H.I. , Al-Riziqi , M.H. , Mothershaw , A. , Guizani , N. and Bengtsson , G. 2006 . Assessment of the anti-microbial activity of dried garlic powders produced by different methods of drying. International . Journal of Food Properties , 9 ( 3 ) : 503 – 513 .
- Ibrahim , S.A. , Tse , T. , Yang , H. and Fraser , A. 2009 . Antibacterial activity of a crude chive extract against Salmonella in culture medium, beef broth, and chicken broth . Food Protection Trends , 29 ( 3 ) : 155 – 160 .
- Ozcan , M. and Erkman , O. 2001 . Antimicrobial activity of the essential oils of Turkish plant spices . European Food Research and Technology , 212 : 658 – 660 .
- Negi , P. , John , S.K. and Rao , P.U. 2002 . Antimicrobial activity of mango sap . European Food Research and Technology , 214 : 327 – 330 .
- Alzoreky , N.S. and Nakahara , K. 2003 . Antibacterial activity of extracts from some edible plants commonly consumed in Asia . International Journal of Food Microbiology , 80 : 223 – 230 .
- Burt , S.A. and Reinders , R.D. 2003 . Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7 . Letters in Applied Microbiology , 36 : 162 – 167 .
- Kotzekidou , P. , Giannakids , P. and Boulamatsis , A. 2008 . Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and fate of inoculated pathogens in chocolate . LWT-Food Science and Technology , 41 : 119 – 127 .
- Ibrahim , S.A. , Salameh , M.M. , Phetsomphou , S. , Yang , H. and Seo , C.W. 2006 . Application of caffeine, 1,3,7- Trimethylxanthine, to control Escherichia coli O157:H7 . Food Chemistry , 99 : 645 – 650 .
- Singh , R.M. , Rastogi , S.S. , Singh , R. , Ghosh , S. and Niaz , M.A. 1992 . Effects of guava intake on serum total and high-density lipoprotein cholesterol levels and on systemic blood pressure . The American Journal of Cardiology , 70 : 1287 – 1291 .
- Olajide , O.A. , Awe , S.O. and Makinde , J.M. 1999 . Pharmacological studies on the leaf of Psidium guajava . Fitoterapia , 70 : 25 – 31 .
- Ibrahim , S.A. , Yang , H. , Niedziela , C.E. and Tse , T.S.F. 2004 . “ Antimicrobial effect of guava and guava extracts on Escherichia coli O157:H7 in liquid medium (Abstract No. 114C-1) ” . In Institute of Food Technologist Annual Meetings and Food Expo Las Vegas , NV unpublished.
- Chen , H.Y. and Yen , G.C. 2007 . Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves . Food Chemistry , 101 : 686 – 694 .