Abstract
Steady shear and dynamic oscillatory measurements were used to investigate the effect of concentration, ionic strength and thermal treatment on rheological properties of soybean β-conglycinin in aqueous dispersions. SDS-PAGE and Differential Scanning Calorimetry (DSC) showed that β-conglycinin exhibited partial denaturation and formation of aggregates during isolation. Under steady shear flow, strong shear-thinning behavior was observed with increasing shear rate from 0.001 to 1200 s−1. A dispersion of β-conglycinin (≥5% w/v, without applying thermal treatment) exhibited gel-like dynamic mechanical spectra at 20°C. This suggested that β-conglycinin in aqueous dispersions showed rheological properties of typical weak gel-like (entanglement) or semi diluted polymeric solution. Weak gel network of β-conglycinin was susceptible to ionic strength, suggesting that electrostatic forces play an important role in the formation of weak gel network. These properties of β-conglycinin have practical significance for the food processors in the formulation of new products.
INTRODUCTION
Soybean proteins consist of two major components, β-conglycinin (7S) and glycinin (11S) with different structures and gel properties.[Citation1] β-Conglycinin (a major 7S soybean protein) possesses 7S form (S0 20, W = 7.2) and 9S form (S0 20, W = 10.7) at 0.5 and 0.1 ionic strength, respectively. The 7S form of β-conglycinin has three subunits per molecule. At very low ionic strength (<0.01), α subunit may dissociate from the protomer.[Citation2] Soybean protein has become an important food ingredient. Gelation is an important functional property of soybean protein, commonly used in the food industry. Soybean protein gels are most commonly achieved through heat treatment at different pH and ionic strength.[Citation3] In many food applications, however, it is not desirable to heat soybean protein products to high temperatures to induce gelation. Therefore, it is important to explore the rheology of proteins for better understanding of gelation behavior.
The globular proteins suspended in water constitute a colloidal suspension.[Citation4] Soy protein gives Newtonian dispersions in medium range concentrations (up to 6.0%). On the other hand, the aqueous suspension of soy protein is strongly shear thinning and presents viscosity values several times greater than those expected for an equivalent suspension of hard spheres. It is known in suspension rheology that electrostatic forces influence the viscosity through the first and second electro-viscous effects.[Citation5] Aqueous colloids of globular protein (β-lactoglobulin) have been shown to exhibit solid-like mechanical responses to oscillating small strains and yield stress against flow.[Citation6]
Polymer gels and networks can be divided into two main classes, chemically cross-linked materials (including bulk elastomers), and “entanglement networks.”[Citation7] However, many systems consist of chains “physically” cross-linked into networks. These are called “physical gels,” which can be divided into “strong” and “weak” gels. Both gels respond as solids at small deformations. However, the strong gels, e.g., swollen elastomers are also solids at large deformations. The weak gels are really structured fluids, so they flow almost as liquids at large deformations.[Citation8] Gels are viscoelastic materials. Therefore, dynamic rheological tests to evaluate properties of gel systems are well suited for studying the gelation behavior.[Citation9] Steady shear measurements are another type of valuable methods to investigate the rheological behaviors of entanglement networks or weak gels.[Citation6]
Although many investigations evaluated the rheological properties of globular proteins, most of these studies focused on the gelation properties by heating.[Citation10,Citation11] Some studies are on cold-set gelation of globular protein, which used preheating, high pressure or enzyme as additional methods to induce gel formation.[Citation12,Citation13] However, considering the importance of soy protein in the food industry, the specific purpose of this work was to determine rheological properties of aqueous dispersions of β-conglycinin (prepared by dialyzing at high concentration against distilled water before lyophilizing). The influence of concentration, ionic strength and thermal treatment on the rheological properties was investigated. The differences between weak gel system and heat set strong gel system of β-conglycinin are also presented.
MATERIALS AND METHODS
Materials
Commercial defatted soybean flakes {Nitrogen Solubility Index (NSI) > 80} were obtained from Xin Jia Hua Co., China. The flakes contained 55% protein (N × 6.25, dry basis). All other chemicals used in this study were of reagent grade.
Isolation of Soybean β-conglycinin
Soybean β-conglycinin was prepared as described by Nagano et al.[Citation10] Protein was extracted by making slurry with 15-fold volumes of deionized water, adjusted to pH 7.5 with 2N NaOH and put through a nylon mesh (180-mesh size). The filtrate was collected and centrifuged (9000 g × 30 min). Dry sodium bisulfite (SBS) was added to the supernatant (0.98 g/L) and its pH was adjusted to 6.4 with 2N HCl. The mixture was kept in ice bath overnight and centrifuged (6500 g × 20 min) at 4°C. Salt concentration of supernatant was adjusted to 0.25M by the addition of solid NaCI. The pH of supernatant was adjusted to 5.0 with 2N HCI. After 1 h, insoluble fraction was removed by centrifugation (9000 g × 30 min) at 4°C. The supernatant was diluted two-fold with ice-cold water, adjusted to pH 4.8 with 2N HCl and then centrifuged (6500 g × 20 min). The obtained precipitate (β-conglycinin fraction) was adjusted to pH 7.5 with 2N NaOH, dialyzed (18% concentration) against distilled water and then lyophilized to yield soybean β-conglycinin fraction. Protein content of lyophilized powder was determined by Kjeldahl method (N × 6.25). To evaluate apparent viscosity, various concentrations of β-conglycinin (2.25, 4.5, 9.0, and 18.0% w/v corresponding to samples B, C, D, and E, respectively) were dialyzed against distilled water for 36 h at 4°C and then lyophilized. Sample A (control) was lyophilized without dialyzing. β-conglycinin dispersion (18%) formed gel at the end of dialyzing. Therefore this sample was diluted before being lyophilized.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed on a discontinuous buffered system according to the method of Laemmli[Citation14] by using 12% separating gel and 4% stacking gel. β-conglycinin solutions (6%) were diluted 30 times (ratio of protein to buffer was 1:30) by Laemmli sample buffer (25% glycerol, 2% SDS, and 0.01% Bromophenol Blue). 50 μL of β-mercaptoethanol was mixed as reducing conditions and for non-reducing conditions the samples were prepared without β-mercaptoethanol. All samples were heated for 5 min in boiling water before electrophoresis. 10 μL sample was applied to each lane. The electrophoresis was performed at 10 mA and after the sample entered the separating gel the current was adjusted up to 20 mA. The gel was stained with 0.25% Coomassie brilliant blue (R-250) in 50% trichloroacetic acid, and destained in 7% acetic acid [methanol: acetic: water : 1:1:8 (v/v/v)].
Differential Scanning Calorimetry (DSC)
Thermal characteristics of β-conglycinin were examined using a TA Q100-DSC Thermal Analyzer (TA Instruments, New Castle, DE USA), according to the procedure of Meng and Ma[Citation15] with some modifications. Approximately 2 mg of β-conglycinin samples were weighed into coated aluminum pans, and 10 μL of 50 mM phosphate buffer (pH 7) was added. The pans were hermetically sealed and heated from 20°–110°C at a rate of 5°C·min−1. A sealed empty pan was used as a reference. Incipient temperature (Ti), the endothermic peak temperature (Tp) and enthalpy of denaturation (ΔH) were computed from the thermograms by the Universal Analysis 2000 software, Version 4.1D (TA Instruments, Waters LLC). All experiments were conducted in triplicate.
Preparation of Soybean β-conglycinin Dispersions
The lyophilized β-conglycinin powders (sample A, B, C, D, and E) were used to prepare dispersions (10% w/v) to determine apparent viscosity. Protein concentration was determined by the Bradford method.[Citation16] To determine steady shear and dynamic rheological properties, soybean β-conglycinin dispersions (3, 5, 7, and 10% w/v), were prepared in distilled water and stirred for 2 h at 4°C (pH 7.5). High concentration samples (7% and 10%) were centrifuged (10,000 g × 30 min) to discard trapped air. A low centrifugal force (3000 g × 10 min) was used for low concentration samples (3% and 5%). Soybean β-conglycinin dispersions (10%) with addition of salt (0.125, 0.25, and 0.5 M) were also centrifuged at low speed. All samples were hydrated for overnight at 4°C before further investigation.
Rheological Measurements
Rheometer (Thermo HAAKE RS600, Karlsruhe, Germany) with parallel plates (d = 27.83 mm, 1 mm gap), equipped with a double-gap cylinder test fixture (DG41; inner gap = 0.25 mm; outer gap = 0.3 mm; height = 55 mm) was used. Sample (1 ml) was placed between parallel plates and covered with silicone oil to prevent water loss. The equipment was driven through the RheoWin 3 Data Manager (Thermo HAAKE, Germany). All measurements were made in triplicate. Apparent viscosities were determined at shear rate of 130 s−1. The experiment was done in CR mode (control rotation model). Shear time of 60 s was used.
Flow curves for steady shear properties were obtained by up-down stepwise controlling (steady state) program. CS mode (controlled shear stress τ) was used at 20°C. The shear stress range was chosen to allow measuring shear rates lower than 1200 s−1. The stress was held for 60 s at the end of up curve and then decreased. The corresponding shear rate was recorded. In the presence of NaCl, a time of 30 s was maintained to attain stationary flow. In the absence of NaCl, the stationary flow cannot be attained because of its thixotropic behavior. Therefore, considering the slight decrease in viscosity with time, the same time (30 s) was maintained for close approximation to stationary flow.
Dynamic rheological measurements were conducted using CD mode (strain = 0.1; ω = 0.628 rad·s−1) to determine the storage molulus (G′), loss modulus (G′′) and loss tangent (tan δ) at 20°C. To investigate effect of thermal treatment on gelation, a temperature increase ramp 2°C·min−1 from 20 to 80°C, holding for 30 min at 80°C and a temperature decrease ramp of 2°C·min−1 from 80 to 20°C was used. In order to determine linear viscoelastic range, strain sweep was performed at a shear oscillation of ω = 0.628 rad·s−1. The linear viscoelastic region was extended up to 0.2. Therefore, stain 0.1 was used in the present study. Frequency sweep tests were performed from 0.01 to 100 rad·s−1 at 20°C. Determined values of G', G” and tan δ were reproducible with an estimated error of ±20%.
RESULTS AND DISCUSSION
Composition of β-Conglycinin
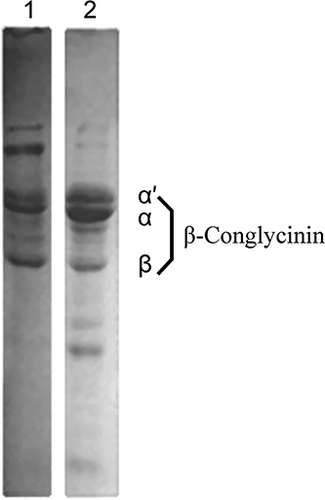
The composition and purity of soybean β-conglycinin was evaluated by SDS-PAGE (). The relative content of soybean β-conglycinin was higher than 82% as evaluated by comparing the optical density, indicating the effectiveness of the preparation method used in the present study. Protein content of this soybean β-conglycinin was 89% (dry basis), as determined by the Kjeldahl method (N × 6.25). It was observed that some protein components did not enter the separating and stacking gels in the absence of β-mercaptoethanol (2-ME), indicating the presence of aggregate (, Lane 1). This kind of aggregates could be disrupted in the presence of 2-ME (, Lane 2), suggesting that the disulfide bonds are important for the formation of the aggregates. Thus SDS-PAGE under unreduced conditions revealed that β-conglycinin exhibited formation of aggregates during isolation. The gel chromatography analysis using Sepharose CL-6B column eluted with 50 mM phosphate buffer (pH 7.6, 0.2M NaCl) confirmed the presence of aggregates in β-conglycinin fractions (data not shown here).
Differential Scanning Calorimetry (DSC) Measurements
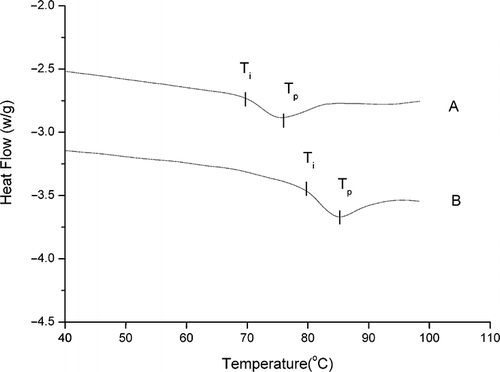
Typical DSC thermograms of soybean β-conglycinin in 50 mM phosphate buffer (pH 7.0) and its related DSC characteristics are shown in . The results showed that β-conglycinin exhibited one main endothermic peak. The endothermic peak temperature (Tp) was 75.58°C, which was consistent with previous literature reports.[Citation10,Citation17] Differential Scanning Calorimetry (DSC) showed that β-conglycinin exhibited partial denaturation during isolation. Incipient temperature (Ti) and endothermic peak temperature (Tp) and enthalpy of denaturation (ΔH) were increased with increasing ionic strength (). Previously it was also reported that endothermic temperatures in DSC heating curves shift to higher temperatures with increasing scanning rate and ionic strength.[Citation17] The Tp and ΔH data, representing thermal stability of the protein and proportion of undenatured protein in a sample, or extent of ordered structure,[Citation18] indicated that the presence of NaCl improved the thermal stability of the protein.
Effect of Concentration
Effect of concentration (during dialyzing) on apparent viscosity
The concentrations of β-conglycinin during dialyzing have significant effect on the apparent viscosity of β-conglycinin in aqueous dispersion. Sample A (without dialyzing) was used as control. Dialyzed samples (B, C, D, and E corresponding to 2.25, 4.5, 9, and 18% concentration of β-conglycinin during dialyzing, respectively) were evaluated for apparent viscosity. Sample E formed gel at the end of dialyzing. Thus dialyzing at sufficient high concentration (18% before lyophilized, pH 7.4) induced phenomenon of weak gel in Sample E. Highest apparent viscosity was observed in Sample E (1.598 Pas) followed by Sample D (0.1069 Pas), Sample C (0.00886 Pas), Sample A (0.0139 Pas) and sample B (0.006977 Pas). The apparent viscosity (η), at = 130 [1·s−1] increased with the increase in concentration of β-conglycinin during dialyzing. However, apparent viscosity increased slightly from 2.25% (Sample B) to 9% (Sample D) and increased sharply between 9% (Sample D) and 18% (Sample E).
In general, the β-conglycinin has a dimmer conformation (10S) at low ionic strength (I < 0.2) and pH 4.8–11.0.[Citation19] At very low ionic strength (I < 0.01), individual subunits of β-conglycinin may dissociate from the protein, β-conglycinin may be transformed into individual subunits during the dialyzing process. These subunits are not thermo-stable and are easily denatured and aggregated during the subsequent dialyzing process, thus resulting in the formation of a weak gel network. Once these lyophilized β-conglycinin consisting of native globular and aggregates are hydrated, they will exhibit some characteristics of weak gel. Although lyophilizing had some effect on protein aggregates, it was the dialyzing process that induced gelation of β-conglycinin. The partially denatured soybean protein may exhibit some unique characteristics, which may have good potential to be applied in the protein-based formulations.[Citation20]
Effect of concentration on steady shear behavior
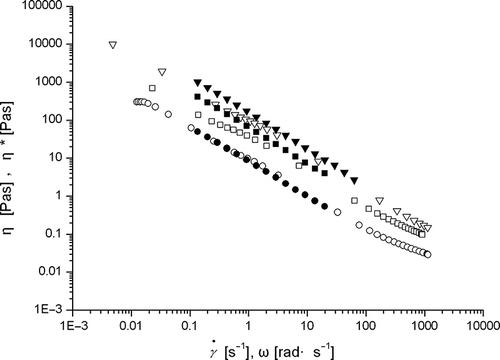
The steady shear flow properties of β-conglycinin dispersions of various concentrations (3, 5, 7, and 10%) are presented in . The dispersion of β-conglycinin (3% w/v) exhibited a characteristic behavior of polymeric solution with the presence of viscosity plateau at a small shear rate. At higher shear rates, the viscosity tends to decrease with the increase in shear rate. There was a sharp increase in low shear rate viscosity when the concentration was increased from 3–5%. All dispersions tested in the present study exhibited a shear thinning behavior. Under steady shear flow, strong shear thinning behavior was observed with increasing shear rate from 0.001 to 1200 s−1. The steady flow properties of β-conglycinin are typical of biopolymer dispersions.[Citation7]
Figure 3 Steady shear viscosity of β-conglycinin in aqueous dispersions (20°C), as a function of shear rate. Diamonds represent 3%, circles 5%, squares 7% and triangles 10% concentration of β-conglycinin. The filled represents down curve, while the empty up curve.
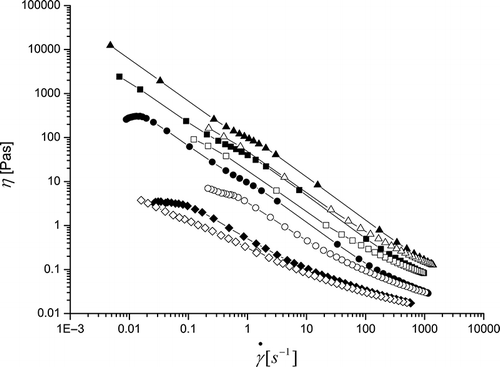
Generally, commercial soyprotein isolate (SPI) dispersions show considerable thixotropy behaviors at protein concentrations higher than 6%, while at lower concentration values, they always exhibit Newtonian behavior.[Citation21] Thixotropy behavior became more obvious, when the protein concentration was increased, due to increase in interaction among protein molecules. The down curves of all samples did not match the up curves with the presence of a hysteresis loop, showing a time-dependent flow property and a thixotropy behavior. This may be due to a partial breakdown of the weak network formed upon the application of shear. The time-dependent behavior was observed at lower concentrations (3% in our case). However, hysteresis loop was observed at higher concentrations (about 8–9%).[Citation21] This difference may be attributed to the formation of gel-like network in the present study. However, sample preparation or pre-shear history also has an influence on the steady flow behavior of biopolymer or its mixture.[Citation22]
Effect of concentration on dynamic rheological behavior
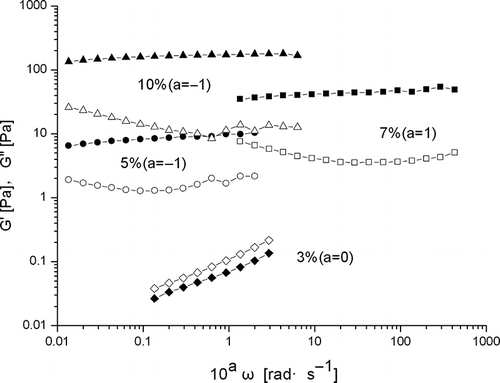
The frequency dependence of dynamic mechanical moduli (storage modulus G′ and loss modulus G′′) of β-conglycinin dispersions (3, 5, 7, and 10%) is shown in . At a protein concentration of 3%, the mechanical moduli (G′ and G′′) were highly dependent upon the oscillatory frequency ω, and G′′ predominated G′, indicating that the solution was a viscous liquid.[Citation7] The frequency dependence decreased with the increase in protein concentration from 3 to 10%. The slight frequency dependence of the moduli and relatively large value of tan δ (= G′′/G′ > 0.1) are typical of so-called weak gels.[Citation6] A gel should exhibit solid-like mechanical spectrum (G′ > G′′) throughout the experimental accessible frequency range and there is little frequency dependence of the moduli.[Citation23] A dispersion of β-conglycinin (≥5% w/v, without applying thermal treatment) exhibited gel-like dynamic mechanical spectra. This suggests that β-conglycinin in aqueous dispersions showed dynamic rheological properties of typical weak gel-like (entanglement) or semi diluted polymeric solution. The storage modulus (5, 7, and 10% β-conglycinin) was larger than the loss modulus in the entire frequency range examined. These phenomena are typical gel-like mechanical moduli patterns as a function of ω.[Citation7] Some colloidal dispersions (globular protein) also exhibit weak gel-like mechanical spectra even when they do not look like solids to the eye.[Citation24] It was observed that the critical concentration to form a weak gel-like structure was about 5% ().
Figure 4 Frequency dependence of β-conglycinin in aqueous dispersions (20°C, strain = 0.1). Diamonds represent 3%, circles 5%, squares 7% and triangles 10% concentration of β-conglycinin. The filled represents G′, while the empty G′′.
The rheological properties of fluids are associated with their internal structure. Cox and Merz rule can provide some information about the nature of simple polymeric solutions.[Citation7,Citation25] The empirical Cox-Merz rule EquationEq. (1) states that values of the dynamic viscosity (η*) and the steady shear viscosity (η) should be identical at a comparable time scale of observation when the angular frequency ω is equal to the steady-shear rate , as long as the structure that determines the mechanical properties of the material remains intact under steady flow.
Dynamic and steady-shear viscosities for β-conglycinin dispersions are presented in . Test was not carried out for 3% concentration because it exhibited very low modulus as observed in . In case of β-conglycinin dispersion (5%), values of η* overlapped with those of η at any comparable angular frequency and shear rate, which is typical of concentrated polymer solution. However, β-conglycinin dispersion (5%) exhibited weak-gel like properties in dynamic rheological test. It was observed that 5% concentration was the critical concentration for the weak-gel formation. Therefore, β-conglycinin dispersion (5%) may show some properties of polymer solution and observe Cox-Merz rule. However, β-conglycinin dispersions (7 and 10%) exhibited gel-like properties () and values of dynamic viscosity (η*) were larger than those of steady shear viscosity (η). A similar relation between steady and dynamic properties has been observed in mixture of tomato juice and soy protein.[Citation26] In case of β-conglycinin dispersions (7% and 10%), the violation of Cox-Merz rule confirms that this system is a weak gel system.
Effect of Ionic Strength
Effect of ionic strength on steady shear behavior
Preliminary experiments were conducted and found that a stronger gel network was formed from 10% of β-conglycinin solution than from lower concentrations (data not shown). It was also difficult to remove the air trapped in the solutions or dispersions at higher protein concentrations, e.g., 15–20%. Thus, in order to study the effect of ionic strength on steady flow properties, an optimum concentration of β-conglycinin (10%) was selected. Sample without NaCl was used as control. The effect of addition of different concentrations of NaCl (0.125, 0.25, and 0.5M) on steady flow properties of β-conglycinin in aqueous dispersions is evident from . The steady flow properties were remarkably affected by the presence of NaCl. The presence of NaCl decreased the steady shear viscosity, especially at low shear rates. An obvious plateau at low shear rates was observed for 0.125M and 0.25M NaCl (), indicating Newtonian behavior. However, at higher shear rates, shear thinning still predominated. These are typical behaviors of a semi-dilute macromolecular solution.[Citation7]
Figure 6 Steady shear viscosity of β-conglycinin (10% w/v) in aqueous dispersions at 20°C, as a function of shear rate. Triangles represent without NaCl, squares 0.125M, circles 0.25M and diamonds 0.5M concentration of NaCl. The filled represents down curve, while the empty up curve.
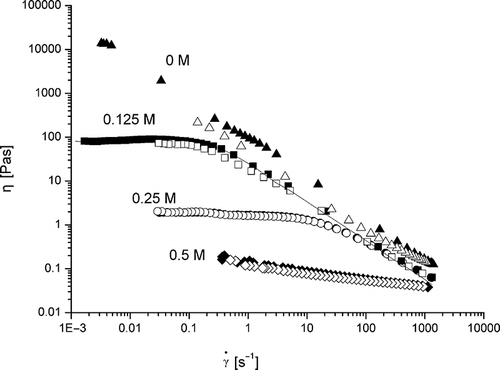
Weak gel network of β-conglycinin was susceptible to ionic strength, suggesting that electrostatic forces play an important role in the formation of weak gel network. The presence of NaCl also decreased the extent of thixotropy. The globular protein exhibits more compact conformation in the presence of salt due to reduction of diffusion double layers. Apart from the electrical forces, the hydration repulsive force (non-DLVO repulsive forces) may be considered in the calculation of protein–protein interaction energy, in order to achieve a satisfactory prediction of high viscosity values obtained experimentally.[Citation5] However, high voluminosities (volume per unit dry weight) obtained with soybean protein might be explained by considering a quaternary structure in which large particles are built by the aggregation of polymers (or sub particles).[Citation27]
The Carreau model EquationEq. (2) was used to fit the shear rate dependence of steady shear viscosity of β-conglycinin (10% w/v) in the presence of different concentrations of NaCl. In the case of 0.125M NaCl, the fitting curve of Carreau model is demonstrated in . This equation describes the variation of viscosity (η) with shear rate , which can predict accurately the behavior of many polymeric solutions. This model contains three parameters: (1) zero shear viscosity, ηo; (2) λ is a characteristic time, which is the reciprocal of the critical shear rate calculated from the intersection of the power-law region and the constant viscosity region in the flow curve; (3) n is power-law behavior index.[Citation28] It is evident from the fitting parameters for the model () that Carreau model could be well applied to describe the shear rate dependence of steady shear viscosity (with a correlation coefficient of 0.9601–0.9975).
Table 1 Rheological parameters of the Carreau model
Effect of ionic strength on dynamic rheological behavior
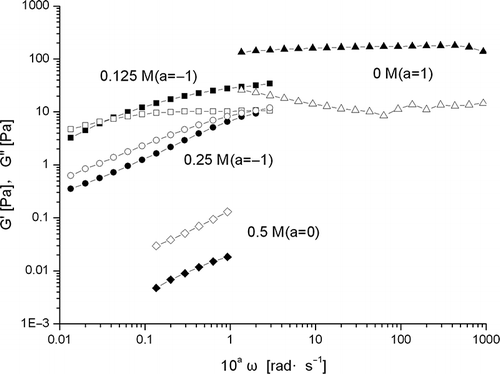
The dynamic frequency dependence of β-conglycinin (10% w/v) in aqueous dispersions in the presence of various concentrations of NaCl (0M, 0.125M, 0.25M and 0.5M) is shown in . In the presence of 0.125M NaCl, the dispersions behaved as semi diluted macromolecular solutions or more entangled semi diluted solutions,[Citation7] since G′′ predominated over G′. Then there was a crossover of G′ and G′′ at a frequency of 0.3 rad·s−1. However, after the crossover, or at higher frequency values, the G′ gradually predominated the G′′, suggesting the gel-like behavior at high frequency. In case of 0.25M NaCl, the dispersions also appeared as a semi diluted polymer solution, since G′′ predominated over G′ at frequencies in the range of 0.1–30 rad·s−1. However, in case of 0.5M NaCl, the dispersions behaved as dilute macromolecular solutions,[Citation7,Citation29] where G′ is much less than G′′. These results are also in agreement with that of steady flow measurements presented in .
Effect of Thermal Treatment
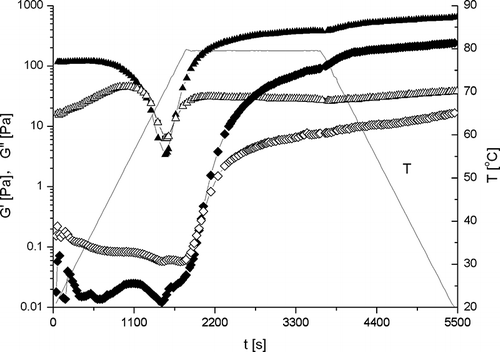
Thermal gelation of β-conglycinin (10% w/v) in aqueous dispersion with and without 0.5M NaCl is shown in . A cycle of heating-holding-cooling (20°C → 80°C-holding for 30 min-80°C → 20°C) was applied to study the thermal gelation. In the absence of NaCl, the mechanical moduli (G′ and G′′) of β-conglycinin gel remarkably declined when heating up to about 70°C, indicating “melting” or breaking up the gel structure. However, further heating and holding at 80°C resulted in the formation of gel with much stronger strength. However, β-conglycinin dispersion in the presence of 0.5M NaCl followed a typical gelation process upon heating and subsequent cooling cycle. The strength of gels in the presence of NaCl was remarkably less than that of without NaCl. The effects of salts on protein structure involve two mechanisms: the electrostatic shielding effects and ion-specific effects in hydrophobic interactions. These results also confirmed that hydrophobic interactions play an important role in the formation of β-conglycinin gels.[Citation30] The effects of salts on the hydrophobic interactions in protein was investigated by Damodaran and Kinsella.[Citation31] They demonstrated that binding affinity for the ligand (2-nonanone) decreased with increasing concentration of NaSCN, while the binding affinity increased with increasing concentration of NaCl. These results suggest that NaSCN weakens and NaCl strengthens hydrophobic interactions. However, the weakness of the gel strength in the present case was mainly contributed to the fact that the number of functional groups involved in the gel network formation was reduced, due to the stabilizing effect of NaCl on the conformation of β-conglycinin.[Citation30]
Figure 8 Thermal gelation profiles of β-conglycinin (10% w/v) in aqueous dispersions. Triangles represent without NaCl, diamonds with 0.5M NaCl. The filled represents G′, while the empty G′′.
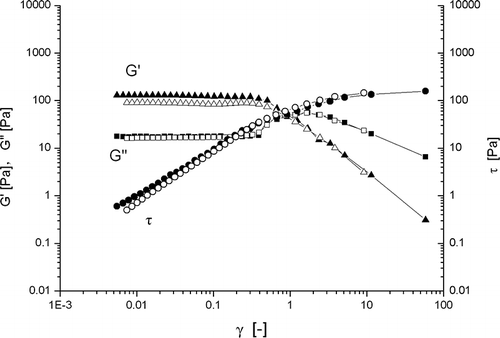
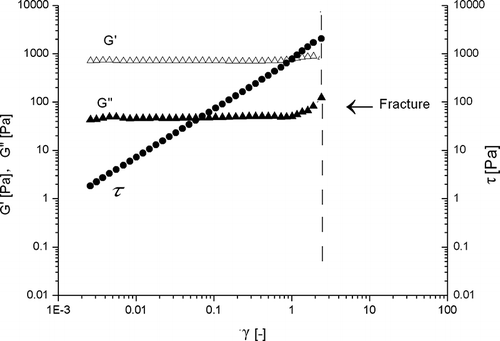
Large deformation properties (dynamic stress sweep) of β-conglycinin (with out heating) are presented in . With increasing magnitude of shear stress the linear strain region was observed up to less than 0.3 (). However, with heating (20°C to 80°C at 2°C/min, holding for 30 min at 80°C and cooling to 20°C at 2°C/min), the viscoelastic region was extended up to more than ∼2 (). A remarkable difference between these two types of gels appeared when the measurements were repeated. The gel with out heating never fractured even when a very large deformation was applied under the dynamic shear stress (τ) of 200 Pa (). These results were according to Matsumoto and Okubo.[Citation32] They described that due to absence of permanent cross-links; a weak gel system should flow but never rupture under large deformations. The heat-set gel, on the other hand, fractured at strain higher than 2 (). Therefore, it was impossible to gain useful information by repeating the same measurement. The results were similar to those of κ-carrageenan weak gel reported by Ikeda and Nishinari.[Citation6] The values of the modulus determined at the second-run measurements were slightly lower than those at the first-run measurements (). In both cases, no further significant change in the spectrum was noticeable between the second and third-run measurements (data not shown), confirming that gel-like mechanical characteristics were not lost even when extremely large deformations were applied. The results suggested that β-conglycinin in aqueous dispersions (10% w/v) behaved as weak gel-like structure even with out heating. It is well-known that hydrogen bonds are weakened with increasing temperature. To investigate the contribution of hydrogen bonds to the formation of β-conglycinin weak gel, the storage modulus G′ of the weak gel was observed as a function of a temperature cycle, 20–70–20°C at 2°C/min (data not showed). With increasing temperatures from 20 to 70°C, the storage modulus G′ decreased, then cooling from 70 to 20°C, the storage modulus G′ recover their initial level. These results suggest that the increase in the storage modulus G′ of β-conglycinin gel in the cooling process is mainly due to the formation of hydrogen bonds and was a thermally reversible reaction. Nagano et al.[Citation28] had also observed thermally reversible reaction while reheating the heat-set gel after cooling. However, the thermally reversible reaction was only contributed by the cooling process (after temperature holding at 80°C for 30 min). Neither heat-setting nor holding process had significant effect.
CONCLUSIONS
β-conglycinin prepared by dialyzing at high concentration exhibited weak gel-like properties in aqueous dispersion at ambient temperature. β-conglycinin showed melting and thermal gelation process during temperature ramp from 20 to 80°C, indicating the transition from weak gel to solution and then formation of a strong gel just like the conventional heat-setting process. These characteristics of β-conglycinin are useful in the preparation of different food products with improved textural properties. The storage modulus (5, 7, and 10% β-conglycinin) was larger than the loss modulus in the entire frequency range examined. The storage modulus was increased with the increase in concentration of β-conglycinin. The moduli exhibited slight frequency dependence and relatively large value of tan δ (= G′/G′′ > 0.1), which were typical of so-called weak gels. The violation of Cox-Merz rule (7% and 10% β-conglycinin) also confirmed that this system is a weak gel system. Weak gel network of β-conglycinin was remarkably affected by ionic strength. It suggested that electrostatic forces play an important role in the formation of weak gel network. However, covalent, hydrogen and hydrophobic bonds might not be excluded, especially for the stability of the aggregates. The detailed mechanism involved in the formation of these networks needs further investigation.
ACKNOWLEDGMENTS
This work is part of the research projects of Chinese National Natural Science Fund (serial number: 20436020), sponsored by the NNSF of China.
REFERENCES
- Morr , C.V. 1990 . Current Status of Soy Protein Functionality in Food Systems . J. Am. Oil Chem. Soc. , 67 : 265 – 271 .
- Iwabuchi , S. , Watanabe , H. and Yamauchi , F. 1991 . Thermal Denaturation of β-Conglycinin. Kinetic Resolution of Reaction Mechanism . J. Agric. Food Chem. , 39 : 27 – 33 .
- Kang , I.J. and Lee , Y.S. 2005 . Effects of beta-conglycinin and glycinin on thermal gelation and gel properties of soy protein . Food Science and Biotechnology , 14 : 11 – 15 .
- Hunter , R. 1992 . Foundations of colloid science , 66 – 69 . Oxford : Clarendon Press .
- Berli , C.L.A. , Deiber , J.A. and Añón , M.C. 1999 . Connection between rheological parameters and colloidal interactions of a soy protein suspension . Food Hydrocolloids , 13 : 507 – 515 .
- Ikeda , S. and Nishinari , K. 2001 . Weak Gel-Type Rheological Properties of Aqueous Dispersions of Nonaggregated k-Carrageenan Helices . J. Agric. Food Chem. , 49 : 4436 – 4441 .
- Ross-Murphy , S.B. 1995 . Structure–property relationships in food biopolymer gels and solutions . Journal of Rheology , 39 : 1451 – 1463 .
- Kavanagh , G.M. and Ross-Murphy , S.B. 1998 . Rheological characterization of polymer gels . Prog. Polym. Sci. , 23 : 533 – 562 .
- Mezger , T.G. 2002 . The rheology handbook: for users of rotational and oscillatory rheometers Edited by: Zorll , U. Hannover, , Germany
- Nagano , T. , Hirotsuka , M. , Mori , H. , Kohyama , K. and Nishinarit , K. 1992 . Dynamic Viscoelastic Study on the Gelation of 75 Globulin from Soybeans . J. Agric. Food Chem. , 40 : 941 – 944 .
- Gosal , W.S. and Ross-Murphy , S.B. 2000 . Globular protein gelation: Current Opinion in Colloid & Interface Science , 5 : 188 – 194 .
- Apichartsrangkoon , A. 2003 . Effects of high pressure on rheological properties of soy protein gels . Food Chemistry , 80 : 55 – 60 .
- Tang , C.H. , Wu , H. , Chen , Z. and Yang , X.Q. 2006 . Formation and properties of glycinin-rich and β-conglycinin-rich soy protein isolate gels induced by microbial transglutaminase . Food Research International , 39 : 87 – 97 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Meng , G.-T. and Ma , C.-Y. 2001 . Thermal properties of Phaseolus angularis (red bean) globulin . Food Chemistry , 23 : 453 – 460 .
- Bradford , M.M. 1976 . A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding . Anal. Biochem. , 72 : 248 – 254 .
- Riblett , A.L. , Herald , T.J. , Schmidt , K.A. and Tilley , K.A. 2001 . Characterization of β-Conglycinin and Glycinin Soy Protein Fractions from Four Selected Soybean Genotypes . J. Agric. Food Chem. , 49 : 4983 – 4989 .
- Arntfield , S.D. and Murray , E.D.C. 1981 . Inst. Food Sci. Technol. J. , 14 : 289 – 294 .
- Thanh , V.H. and Shibasaki , K. 1979 . Major Proteins of Soybean Seeds. Reversible and Irreversible Dissociation of β-Congly cinin . J. Agric. Food Chem. , 27 : 805 – 809 .
- Wagner , J.R. , Sorgentini , D.A. and Añón , M.C. 2000 . Relation between Solubility and Surface Hydrophobicity as an Indicator of Modifications during Preparation Processes of Commercial and Laboratory-Prepared Soy Protein Isolates . J. Agric. Food Chem. , 48 : 3159 – 3165 .
- Wagner , J.R. , Sorgentini , D.A. and Añón , M.C. 1992 . Effect of Physical and Chemical Factors on Rheological Behavior of Commercial Soy Protein Isolates: Protein Concentration, Water Imbibing Capacity, Salt Addition, and Thermal Treatment . J. Agric. Food Chem. , 40 : 1930 – 1937 .
- Leng , X.J. and Turgeon , S.L. 2007 . Study of the shear effects on the mixture of whey protein/ polysaccharides-2: Application of flow models in the study of the shear effects on WPI/ polysaccharide system . Food Hydrocolloid , 21 : 1014 – 1021 .
- Almdal , K. , Dyre , J. , Hvidt , S. and Kramer , O. 1993 . Towards a phenomenological definition of the term ‘gel’. Polym . Gels Networks , 1 : 5 – 17 .
- Inoue , H. and Matsumoto , T. 1996 . Viscoelastic characterization of solid-like structure in aqueous colloids of globular proteins . Colloids Surf. , 109 : 89 – 96 .
- Cox , W.P. and Merz , E.H. 1958 . Correlation of dynamic and steady viscosities . Journal of Polymer Science , 28 : 619 – 622 .
- Tiziani , S. and Vodovotz , Y. 2005 . Rheological effects of soy protein addition to tomato juice . Food Hydrocolloids , 19 : 45 – 52 .
- Boulet , M. , Britten , M. and Lamarche , F. 1998 . Voluminosity of some food protein in aqueous dispersions at various pH and ionic strengths . Food Hydrocolloids , 12 : 443 – 441 .
- Tiu , C. , Moussa , T. and Carreau , P.J. 1995 . Steady and dynamic shear properties of non-aqueous drag-reducing polymer solutions . Rheologica Acta , 34 : 586 – 600 .
- Puppo , M.C. and Añón , M.C. 1999 . Rheological properties of acidic soybean protein gels: Salt addition effect . Food Hydrocolloids , 13 : 167 – 176 .
- Nagano , T. , Hirotsuka , M. , Mori , H. , Kohyama , K. and Nishinarit , K. 1994 . Effect of Heating and Cooling on the Gelation Kinetics of 75 Globulin from Soybeans . J. Agric. Food Chem. , 42 : 1415 – 1419 .
- Damodaran , S. and Kinsella , J.E. 1981 . The Effects of Neutral Salts on the Stability of Macromolecules . J. Biol. Chem. , 256 : 3394 – 3398 .
- Matsumoto , T. and Okubo , T. 1991 . Viscoelastic investigation of crystal-liquid transition in concentrated monodisperse lattices . J. Rheol. , 35 : 135 – 148 .