Abstract
The physicochemical, functional, and thermal properties of protein isolates obtained from two varieties of Bambara groundnut were evaluated. Proteins were isolated using alkaline extraction (isoelectric precipitation [IEP]) and micellisation techniques. IEP recorded a higher protein yield (56.3–58.2 g/100 g) than the micellised protein (MP) (14.2 – 15.6 g/100 g). A similar trend was observed for the protein content of the isolates. The isolates contained a high level of lysine, arginine, and glutamic acid compared to soy protein. Minimum solubility of the flours of the two varieties occured at pH 5. MP isolates exhibited higher solubility than the corresponding isoelectric (IEP) isolates over all pH values. The micellised protein recorded superior functional characteristics than the isoelectric isolates. The micellised isolates also showed a significantly higher (P < 0.05) foam capacity and stability, oil and water absorption properties than the isoelectric isolate. The MP of both varieties also recorded significantly higher emulsifying properties-+ than their isoelectric protein isolates. The micellised protein also had better gelation properties than the isoelectric isolate. Micellised and isoelectric isolates did not reveal major differences in the electrophoretic patterns; both isolates had three major bands at 35.0, 43.0, and 112.0 kDa. The bands in the isoelectric protein isolate however, were well defined compared with the micellised isolate. All Bambara isolates were not dissociated by 1,4-Dithiothreitol (DTT) suggesting that they do not contain subunits linked by a disulphide bond. This suggests that 7S vicilin may be the major storage protein in Bambara groundnut isolates. Differential scanning calorimetry studies (DSC) of the two varieties of bambara groundnut proteins indicated that the thermograms of the micellised isolates have a higher denaturation temperature Td (97.9–108.4°C) than their corresponding isoelectric isolates (89.5–90.6°C).
INTRODUCTION
The production of plant proteins is of growing interest to developing and developed countries alike because of its increasing food and non-food applications.[Citation1] Plant protein isolates are utilised in foods to improve nutritional quality and functionality. However, with respect to plant protein applications, their uses are almost limited to proteins from soybean seeds, neglecting other vegetable proteins. Therefore, there is the need to intensify research efforts aimed at identifying new vegetable protein sources.
Bambara groundnut (Voandzeia subterranean), is grown extensively in sub Sahara Africa and some parts of Latin America and Asia. It belongs to the family Fabaceae. According to Lacroix et al.,[Citation2] Bambara groundnut is the third most important legume after groundnut (Arachis hypogea) and cowpea (Vigna unguiculata) in Africa. It serves as a low cost protein and has 6–12 g/100 g oil, 14–24 g/100 g protein and 28–40 g/100 g carbohydrate. It is underutilized because of lack of information on its compositional analysis and possible utilization. Apart from this, the long cooking time discourages its use for the preparation of local dishes.
There is a dearth of information on the preparation and properties of the protein isolates from Bambara groundnut. Therefore, the main objective of this study was to isolate the major protein fraction from Bambara groundnut using both isoelectric precipitation and micellisation and to characterize and determine the physicochemical properties of the various proteins using SDS-PAGE and differential scanning calorimetry. This will generate data that will provide basic information for understanding the structure-function relationship and potential applications of Bambara groundnut protein in domestic and industrial food products.
MATERIALS AND METHODS
Bambara groundnut seeds were obtained from the International institute for Tropical Agriculture, International Livestock Research Institute IITA/ILRI, Ibadan-Nigeria.
Preparation of Defatted Flour
Bambara groundnut seeds were dehulled manually, using a pestle and mortal. The seeds were ground in a Christy Laboratory Mill (Cheff Food Processor, Tokyo, Japan) and thereafter sieved through a screen of 20 mesh sizes before extraction for 9h with hexane in a soxhlet apparatus (10 g/100 g w/v hexane). The defatted flour was air-dried at room temperature (approximately 28°C) and subsequently kept in air-tight plastic containers at 4°C prior to use.
Preparation of Isolates
The Bambara groundnut isolates were prepared as previously described by other workers[Citation3] with some modifications. When the protein isolate was obtained by isoelectric precipitation, it was termed isoelectric protein (IEP). The protein isolate produced by micellisation technique was termed micellisation protein (MP). The basic steps were as follows.
Slurry (1:20, flour-to-water ratio) of defatted flour in water was prepared at pH 6.37. This slurry was stirred for 2 h using a Gallenhamp magnetic stirrer (the pH was adjusted to the desired pH of 9.0 using 1 M NaOH), followed by centrifuging using a Sorvall RC5C automatic super speed refrigerated centrifuge at 10,000 × g for 30 min at 5°C. After centrifugation and recovery of supernatant, three additional extractions were carried out with half of the volume of the initial water as described above. The supernatants were pooled and the proteins precipitated at pH 5.0, the isoelectric point (IP). The precipitate formed was subsequently recovered by centrifugation at 10,000 × g for 15 min at 5°C. The precipitate was washed twice with distilled water adjusted to pH 5.0 with HCl. The precipitate was neutralised by the addition of 1 M NaOH. The final protein isolate was obtained by lyophilisation.
The MP isolate was obtained by extracting the protein from defatted meal with 0.8 M NaCl at pH 7.0 (1:10, w/v) for 2 h at 25°C. The suspension was centrifuged for 60 min at 10,000 × g at 4°C and the supernatant was concentrated by ultrafiltration to one half of its volume using a Minitan ultrafiltration system (Millipore Corp., Denvers, MA, USA) with four membranes of 10 kDa nominal molecular weight limit. The concentrated protein solution was then diluted 1:12 with cold distilled water. After stirring for 2 h at 25°C, the protein was recovered by centrifugation and then freeze-dried.
Analysis of Flour and Protein Isolates
Moisture, crude protein, fat, ash, and crude fibre of the flours and protein isolates were determined using the standard methods described in the AOAC.[Citation4] The carbohydrate content was determined as the weight difference using moisture, crude protein, lipid, and ash content data.
Amino Acid Determination
Freeze-dried protein sample (10.0 to 11.0 mg) were hydrolysed in 10 ml, 6 mol/L HCl following the procedure of Henle et al.[Citation5] Amino acid analysis was performed with an alpha plus amino acid analyser (LKB Biochrom, Freiburg, Germany) using an ion exchange chromatograph with a sodium system. The chemicals, standards, analysis, and the conditions of running were as described in Henle et al.[Citation5, Citation6] The eluents were detected via derivatisation with ninhydrin. Peak integration was evaluated using Chromstar chromatographie software 32-bit. Dried samples from acid hydrolysis were dissolved in 0.2 mol/L Na citrate pH 2.2 and passed through a 0.20 μm membrane filter before injection into the amino acid analyser. Lysinoalanine (LAL) was also determined according to the method of Henle et al.[Citation5] using the same amino acid analyser.
Functional Properties
Protein solubility
Protein solubility was determined by the method of Sathe et al.[Citation7] with some modifications stated below. The suspension (0.2 g/100 g) of the flour or isolate in distilled water was adjusted to different pH values of between 2 and 12 using either 1 M HCl or 1 M NaOH. Percent nitrogen in each supernatant was determined by the micro Kjedahl method according to the AOAC method.[Citation4] Percent soluble protein was calculated as percent nitrogen multiplied by 6.25 on a wet basis.
Foaming Properties
The foaming characteristics of the protein isolates were investigated using the methods of Coffmann and Garcia,[Citation8] and Vani and Zayas.[Citation9] The procedure involved blending of 50 ml of a protein suspension (1 g/100 g, adjusted to the required pH) in an Ultra-Turrax homogeniser (Janke & Kunkel, Staufen, FRG) at 12,000 rev min−1 for 1 min at about 25°C and determining the volume of foam (in ml) that was present above the surface of the liquid contained in a glass cylinder of 100 ml. Foam expansion was expressed as follows:
Emulsifying Properties
The emulsifying stability index (ESI) and the emulsifying activity index (EAI) for the protein isolates were determined by the turbidimetric methods.[Citation10, Citation11] Freshly prepared emulsions (1 ml) were pipetted out at 0, and 10 min after homogenisation and serially diluted with 99 ml distilled water (100-fold) followed by 1 ml of the diluted emulsion into 39 ml (40-fold) of 1g.kg−1 SDS, resulting in a 4000-fold total dilution. Absorbance of the final dispersion was measured at 500 nm (Ultrospec 1000, UV/Visible Spectrophotometer, Pharmacia Biotech, Cambridge, England). The ESI and EAI were determined as follows:
Water and Oil Absorption Capacity
Water absorption capacity was determined using the method of Sathe and Salunkhe[Citation12] with slight modifications. Ten ml of distilled water was added to 1.0 g of the sample in a beaker. The suspension was stirred using a magnetic stirrer for 5 min. The suspension obtained was thereafter centrifuged at 4000 × g for 30 min and the supernatant measured in a 10-ml graduated cylinder. The density of water was taken as 1.0 g/cm3. Water absorbed was calculated as the difference between the initial volume of water added to the sample and the volume of the supernatant. The same procedure was repeated for oil absorption except Mazola corn oil was used instead of water.
Determination of the Gelation Concentration
The least gelation concentration was determined by the method of Sathe et al.[Citation13] Test tubes containing suspensions of 2, 4, 6, 8, up to 20 g/100 g (w/v) flour in 5 ml distilled water were heated for 1 hr in boiling water, followed by cooling in ice and further cooling for 2 hr at 4°C. The least gelation concentration was the one at which the sample did not slip when the test tube was inverted.
SDS-PAGE Electrophoresis
The molecular weight profiles for the protein fractions were established using SDS-PAGE according to the method of Lane[Citation14] with slight modification. Protein samples (1 mg/ml) were prepared in a buffer containing 4.84 g Tris, 0.03 g EDTA, 1.0 g SDS, 13.8 ml glycerol (87%) and 0.01 g orange G (pH 8.0). SDS-PAGE was carried out on a slab gel with 8 μl protein samples and the anode buffer (pH 8.9) contained 121.1 g Tris and cathode buffer (pH 8.2) contained 12.1 g Tris, 17.9 g tricine, and 1 g SDS. The separating gel was 20 g/100 g final acrylamide concentration while the stacking gel was 4 g/100 g concentration. For reduction of the protein samples, 0.5 ml of sample buffer was added to 1 mg of protein sample. Thereafter, 10 μl of 1,4-Dithiothreitol (DTT) (0.15 DTT/0.2 ml double distilled water) was added, and the solution was heated for 4 min in a water bath. After cooling, 5 μl of DTT solution and 53 μl of iodacetamide (0.2 g iodacetamide/1 ml distilled water) solution was added. The solution was stored at 4°C prior use.
The molecular weights of protein subunits for each sample were determined using the molecular weight marker from Carl Roth GmbH and SERVA electrophoresis GmbH containing the following proteins: myosin (from beef), 212 kDa; β-galactosidase (from E. coli) 118 kDa; serum albumin (from beef) 66 kDa; ovalbumin (from chicken) 43 kDa; carboanhydrase 29 kDa; trypsin inhibitor (from soybean) 20 kDa; lysozyme (from chicken) 14k Da; trypsin inhibitor (bovine lung) 6.5 kDa; and phosphorylase B 97 kDa.
Differential Scanning Calorimetry
Differential scanning calorimetry was performed according to the method described by Escamilla-Silva et al.[Citation15] using a Thermal Advantage TA Instrument Universal Analysis 2000 differential scanning calorimeter (New Castle, DE, USA), calibrated with indium. Thermograms were obtained using slurries of protein prepared on a 20 g/100 g (w/v) basis. Samples of 10–15 mg were weighed accurately to the nearest 0.01 mg on a DSC stainless steel capsule and scanned at a heating rate of 10°C/min from 30–150°C. The onset temperature (To ), denaturation temperature (Td ), and enthalpy of denaturation (ΔH) were determined using the universal analysis program version 1.9D (TA Instruments, New Castle, DE, USA).
Statistical Analysis
All values presented are means of three replicates. One-way analysis of variance (ANOVA) was carried out and differences in the means were determined at P < 0.05 (SAS[Citation16]).
RESULTS AND DISCUSSION
Proximate Chemical Composition of Flour and Protein Isolates
Results of proximate composition are summarized in . Both varieties of Bambara groundnut had similar proximate composition; the protein content of Bambara groundnut isolates compared well with soybean protein isolates. Protein yield from the two techniques is presented in . The yield of protein isolate using the IEP (56.08–58.70 g/100 g) was higher than that obtained by MP (14.40–15.60 g/100 g). This might be due to differences in the method of isolation. Sefa-Dedeh and Stanley[Citation17] reported a similar observation during the extraction of cowpea flour protein with sodium hydroxide; likewise, Okezie and Bello[Citation18] noted that the use of sodium hydroxide for pH adjustment resulted in higher protein extractability from winged bean flour. In addition, legumes are noted for the high solubility of their nitrogenous constituents in NaOH.[Citation19]
Table 1 Chemical composition of Bambara groundnut flour and protein isolates from three replicate analysis
Table 2 Protein yield and content of Bambara groundnut isolates obtained by isoelectric precipitation (IEP) or micellization (MP) and soybean isolate
Amino Acid Composition of Bambara Groundnut Protein Isolate
The amino acid composition of the two varieties of Bambara groundnut is shown in . The result is presented alongside soybean protein isolates for comparison purposes. According to Pellet and Young,[Citation20] the nutritive value of proteins depends primarily on the capacity to satisfy the needs of nitrogen and essential amino acids. Aspartic acid and glutamic acid were the most abundant amino acids found in Bambara groundnut protein isolates. The levels of some of the essential amino acids in the protein isolate were within that of the “ideal protein” (FAO[Citation21]). Bambara groundnut also contained high levels of leucine, arginine, and lysine. The high lysine content of the Bambara groundnut protein is a very important nutritional attribute that makes the legume a good supplementary protein to cereals that are known to be deficient in lysine. However, the content of sulphur-containing amino acids, such as methionine, is low. Methionine and cysteine are considered to be limiting amino acids in Phaseolus beans.[Citation22–24] The low level of methionine in Bambara groundnut protein isolate is similar to other legume seed protein reported by other studies.[Citation25, Citation26] Protein sources rich in arginine and glutamine have recently gained popularity because of the reported effects of arginine in preventing heart disease[Citation27] and of glutamine in supporting the immune system[Citation28] and improving athletic performance.[Citation29]
Table 3 Amino acid (g/100 g protein) composition of Bambara groundnut
The two varieties of Bambara groundnut protein isolates had very similar amino acid patterns. It is noteworthy that the levels of all the essential amino acids in Bambara groundnut protein isolates except methionine were higher than the FAO/WHO/UNU[Citation30] recommended pattern and that bambara groundnut is comparable to soybean in the amino acid pattern. Protein isolation by alkali at high pH has been shown to cause various cross-linkages between amino acids; the cross-linkages of major concern are lysinoalanine formation from lysine and dehydroalanine through degradation of cystine or serine, because it reduces protein digestibility.[Citation31] The isoelectric protein isolates were analysed for lysinoalanine and it was found that no lysinoalanine was produced during the isolation of Bambara protein isolate at pH 9.
Functional Properties of Bambara Groundnut Protein Isolate
Protein solubility
The pH-dependent protein solubility profile for the Bambara groundnut flours and the isolates are presented in and b. It was observed that the solubility of the flours of the two varieties were pH dependent having their minimum solubility (isoelectric point) at pH 5. Generally, the solubility decreased as the pH increased until it reached the isoelectric point; this was followed by a progressive increase in solubility with a further increase in pH. A similar observation was reported for winged bean,[Citation7] chickpea,[Citation32] and cowpea.[Citation33] The solubility profile of a protein provides some insight into the extent of denaturation or irreversible aggregation and precipitation that might have occurred during the isolation process. It is also important in foods because it modifies other properties, such as emulsification, foaming, and gelation.[Citation34, Citation35]
Figure 1 (a) Protein solubility profile for two varieties of Bambara groundnut flours. (b) Protein solubility profiles of IEP isolate and MP isolate for the two varieties of Bambara groundnut.
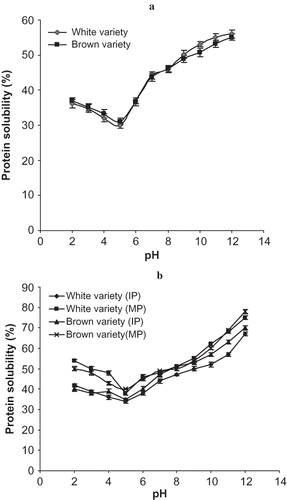
There was a significant difference in the solubility of IEP and MP isolates. Micellised protein isolates exhibited higher solubility than the corresponding isoelectric isolates over all pH values. Previous studies had shown that micellised protein isolate was superior to isoelectric protein isolate in terms of solubility for safflower[Citation36] and chickpea.[Citation26] High solubility is related to the presence of a low number of hydrophobic residues, elevated charge, electrostatic repulsion, and ionic hydration occurring at pH above and below the isoelectric pH.[Citation37]
Foaming Properties
The foaming capacity and stability of Bambara groundnut protein isolates are presented in . The micellised isolates (MP) showed a significantly higher foam capacity and stability in the two varieties of Bambara groundnut and soybean isolate than the isoelectric isolate (IEP). However, soy isolate exhibited better foaming capacity than Bambara isolate. Similar studies on micellised and isoelectric protein of safflower proteins gave similar results.[Citation38] Differences in the foaming properties of the MP and IEP isolates might be due to the relative degree of denaturation of the isolates by the two techniques.[Citation39] This observation was supported by results from the DSC studies of the protein reported in this study. The foaming properties of bambara groundnut isolate from this study indicates that it may serve as a potential replacement for better-known proteins in food applications requiring high foamability and stability, such as cakes, breads, marshmallow, whipped toppings, ice cream, and desserts.
Table 4 Functional properties of Bambara groundnut protein isolate in comparison with soybean protein isolate
Emulsifying Properties
The emulsifying properties of the two varieties of Bambara groundnut isolates are presented in and . The emulsifying properties were determined using the two indices of emulsifying activity index (EAI) and emulsifying stability index (ESI). The EAI reflects the ability of the sample to rapidly adsorb at the water-oil interphase during the formation of emulsion, thereby preventing flocculation and coalescence; while ESI reflects the ability to maintain a stable emulsion over a period by preventing the flocculation and coalescence of the oil globules.[Citation40] The micellised protein (MP) of both varieties showed significantly (P < 0.05) higher emulsifying activity than their isoelectric protein isolates (IEP). For the micellised protein isolate, the EAI ranged from 130–138 m2/g compared with isoelectric protein isolate, which had EAI values between 120 and 126 m2/g.
Figure 2 (a) Emulsifying activity index of isoelectric isolate (IEP) and micellisation isolate (MP) of Bambara groundnut and soy protein isolate. (b) Emulsion stability index of isoelectric isolate (IEP) and micellisation isolate (MP) of Bambara groundnut and soy protein isolate (color figure available online).
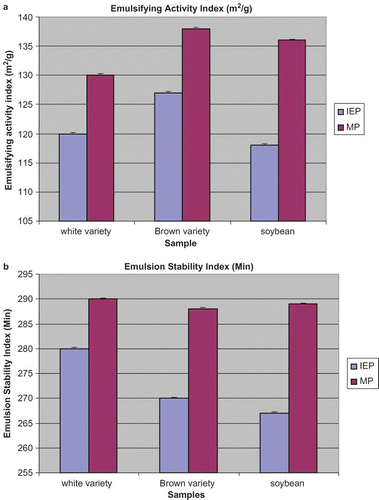
Emulsifying properties of protein depends on the hydrophilic-lipophilic balance. At the oil-water interphase, the protein orients lipophilic residues to the oil phase and hydrophilic residues to the aqueous phase. The net charge of the lipophilic-hydrophilic interphase determines the emulsifying properties. The higher EAI and ESI in Bambara groundnut micellised protein might be attributed to higher solubility of the isoelectric isolate compared with micellised isolate. Similar studies showed that micellised faba bean, chickpea, and fenugreek protein isolates exhibited better emulsifying properties than isoelectric protein isolates.[Citation41–44] Bambara groundnut protein isolate had higher EAI and ESI than soybean isolate. The EAI and ESI values of the isolates obtained in this study could serve as potential ingredients in many food formulations, such as salad dressing, sausages, comminuted meats, ice creams, cake batters, and mayonnaise.
Water and Oil Absorption Capacity
The water and oil absorption capacity of Bambara groundnut micellised isolate and isoelectric isolate in comparison with soy isolate is presented in . The water absorption capacity ranged from 5.8 g H20/g sample to 6.0 g H20/g sample for the isoelectric protein isolate, and 6.5 g H20/g sample to 6.7 g H20/g sample for the micellised protein isolate. The micellised protein isolate had a higher water absorption capacity than the isoelectric protein isolate. Isoelectric precipitation disrupts the structure of the proteins thereby limiting the interaction between the proteins and surrounding aqueous system and reducing the water absorption capacity. In contrast, in the micellised protein isolate there is exposure of a more polar and ionic group allowing greater interaction with the surrounding water by means of hydrogen bonding; other factors that affect the protein-water interaction lead to an increase in the water absorption capacity.[Citation45, Citation46]
Apart from this, the micellised technique of extraction favours conformational changes in the protein molecules, which expose previously buried side chains, thereby making them available to interact with water.[Citation47] Knorr[Citation48, Citation49] reported that the water uptake of spray-dried potato protein concentrates was significantly affected by coagulation method, pH, and their interaction although no consistent trends could be observed.
The oil absorption capacity of the protein isolates ranged from 6.2 g oil/g sample to 6.9 g oil/g sample for the isoelectric isolate and from 6.8 g oil/g sample to 7.2 g oil/g sample for the micellised protein isolate. Micellised protein isolate (MP), therefore, exhibited higher oil absorption than the isoelectric protein isolate. This might be due to the effect of extraction technique on this property. Earlier authors[Citation26, Citation50] have reported higher oil absorption capacity in pigeon pea and cowpea micellised protein isolate compared with their isoelectric isolate. The oil absorption capacity of Bambara groundnut in the present study was higher than those of micellised and isoelectric protein isolates from chickenpea,[Citation26] cowpea isolates,[Citation17] and adzuki bean.[Citation42]
Oil absorption capacity is the ability of fat to bind non polar side chains of proteins.[Citation7] However, the mechanism of oil absorption is not clearly understood, although various theories, such as physical entrapment of oil and also involvement of surface area, size of macromolecule, charge, and hydrophobicity, have been shown to affect oil absorption.[Citation33] Thus, any change in any of these parameters would affect the oil absorption capacity. Alterations in conformation caused during protein isolation may also result in more oil binding sites in protein structure.[Citation51]
Water absorption capacity is a useful indication of whether isolates can be incorporated into aqueous food formulations especially those involving dough formulations, while the oil absorption capacity may determine whether the protein material will perform well as meat extenders or analogues. The results obtained from the current investigation indicated that the isolates would be potentially useful in flavour retention, improvement of palatability, and extension of shelf life in meat products through reduction of moisture and fat loss.
Least Gelation Concentration
The least gelation concentration of Bambara groundnut protein isolates is presented in . The MP had better gelation properties than the IEP. The lower water absorption capacity exhibited by the isoelectric protein isolate may be responsible for the high least gelation concentration and weak gel formed by the IEP isolate compared with micellised protein isolate. Lopez de Ogara et al.[Citation52] earlier reported the interrelationship between the gelation properties of chickpea protein and water absorption capacity. The least gelation concentration of Bambara groundnut protein isolate in the present study was comparable to those of cowpea and pigeon pea.[Citation50]
Gel Electrophoresis (SDS-PAGE)
The electrophoretic patterns of Bambara proteins isolated by isoelectric precipitation and micellisation techniques are presented in . It was carried out in the presence and absence of a reducing agent (1, 4-Dithiothreitol [DTT]). This is to distinguish between those polypeptide chains that are linked by disulphide bridges and free polypeptide chains. Soybean isolate was included in the study for comparison. Soybean isolate under non-reduced condition had about 19 subunits ranging from 14.0–212.0 kDa with three major bands at 24.0, 31.0, and 43.0 kDa (Lane 2). However, under reduced condition (with DTT) it revealed more prominent bands (Lane 8), the band around 130 kDa disappeared to give a band at 97 kDa. In addition, two pronounced bands appeared at 68 and 66 kDa and another three bands appeared between 14 and 16 kDa. This indicates that soy isolate aggregates were formed via disulphide bonds.[Citation53] Derbyshire et al.[Citation54] earlier reported that the parent subunit in soybean belongs to the legumin-like 11S type storage proteins, which are characterized by disulphide, linked α-β paired subunits.
Figure 3 Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) patterns of Bambara groundnut and soybean protein isolates: Lanes 2, 3, 4, 5, and 6 carried out without Dithiothreitol (DTT). Lanes 1, 7, and 13—Standard reference protein; Lane 2—Soy protein isolate (IEP); Lane 3—Bambarra isolate (IEP), white variety; Lane 4—Bambarra isolate (IEP), brown diversity; Lane 5—Bambarra isolate (MP), white variety; Lane 6—Bambarra isolate (MP), brown variety. Lanes 8, 9, 10, 11, and 12 with Dithiothreitol (DTT): Lane 8—Soy protein isolate (IEP); Lane 9—Bambarra isolate (IEP), white variety; Lane 10—Bambarra isolate (IEP), brown diversity; Lane 11—Bambarra isolate (MP), white variety; Lane 12—Bambarra isolate (MP), brown variety (color figure available online).
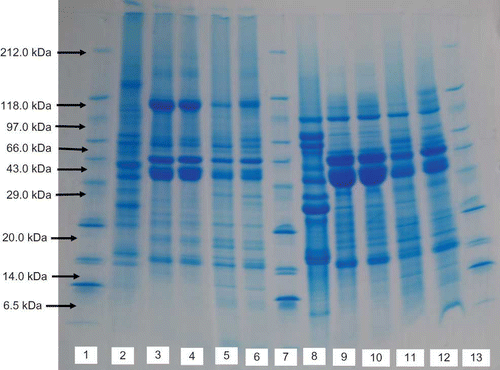
Observation of the polypeptide patterns of the Bambara protein isolates did not reveal major differences in the electrophoretic patterns of micellised and isoelectric isolates. Both isolates have three major bands at 35.0, 43.0, and 112.0 kDa. The bands in the isoelectric protein isolate, however, were well defined compared with the micellised isolate. A similar observation was reported by Ordorica-Falomir et al.[Citation55] on electrophoretic patterns of safflower protein isolates.
Not all protein loaded appeared to enter the gel under non-reducing condition compared with the gel under reduced condition. They appeared as aggregate bands on the top of the gel. This resulted in a pronounced increase in the width of all the major bands in the reduced gel.
All Bambara protein isolates were not dissociated by DTT, suggesting that they do not contain subunits linked by disulphide bond and suggesting that 7S vicilin may be the major storage protein in Bambara groundnut isolates. Vicilin are glycoprotein devoid of disulphide bonds and are frequently non-covalently associated in trimers or even hexamers with molecular weights of 200.0 ± 50.0 kDa.[Citation56]
The result from this study is in contrast with the work of Soottawat et al.[Citation57] on Bambara groundnut protein extract. The authors reported an appearance of a new band under reducing condition. Differences in our observations might be due to the nature of the sample used. The authors used seed extract rather than the protein isolate, which was used for the present study. In addition, there were no differences in the electrophoretic pattern of the white and brown varieties of Bambara groundnut.
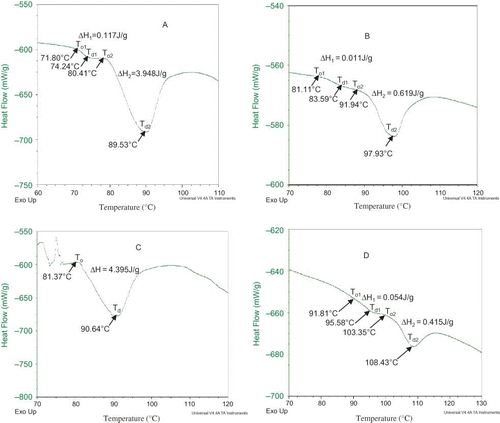
DSC Thermograms of Bambara Groundnut Protein Isolates (IEP and MP)
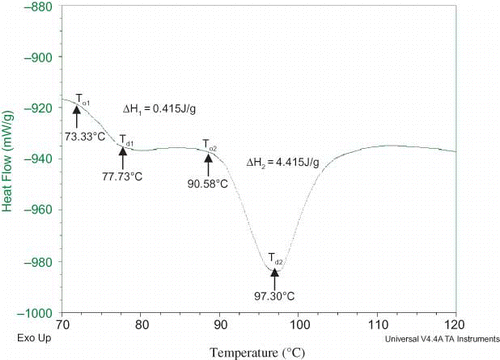
The DSC thermograms of the Bambara groundnut protein isolates are shown in , while the DSC of the soybean isolate is shown in . In both varieties, the thermograms of the micellised isolates have higher denaturation temperature (Td ) than their corresponding isoelectric isolates. Denaturation temperature is the temperature at which transitions occur and it is a measure of thermal stability. The low Td of the isoelectric isolate could be attributed to the extreme conditions of pH utilized in its isolation. It appeared that alkaline extraction (pH 9.0) and isoelectric precipitation at pH 5 resulted in structural rearrangements, which encompassed a wide molecular weight distribution. Cordero-de-los-Santos et al.[Citation58] reported similar results in amaranth protein isolates produced by isoelectric precipitation or by micellisation. They reported that MP had well defined, narrow, and symmetrical peaks, which indicated a homogenous, and less denatured protein population. According to Arntfield and Murray,[Citation59] and Biliaderis[Citation60] high Td and To indicate that a less heat stable group of proteins has been denatured during the process. If a protein isolate is already partly denatured, the heat of transition or enthalpy (ΔH) will decrease and if it is completely denatured no endothermic transition will appear. Soybean isolate also showed two endothermic peaks with Td of 77.73°C for the first peak and 97.30°C for the second peak, and compared very well with Bambara groundnut isolates. The information on protein thermal properties is useful for food processing strategies and heat-processing design.[Citation61]
CONCLUSION
Bambara groundnut legume flours and isolates are good sources of protein and are rich in essential amino acids, except sulphur-containing amino acids. This implies that the groundnut is useful as a supplementary protein source to most cereals and viable raw material for the food industry. Summarily, the micellised protein isolates had better functional characteristics than the isoelectric isolates. Both isolates possessed high water and oil absorption capacities. This suggests its potential usefulness in flavour retention, improvement of palatability, and extension of shelf life in meat products. The isolates also had good foaming capacity and stability. Since foam contributes to smoothness, lightness, flavour dispersions, and palatability, the results obtained from the study indicate that the isolates could serve as replacements of better-known proteins in food applications that require high foam capacity and stability, such as cakes, breads, marshmallow, whippings, toppings, ice creams, and desserts. The high emulsifying activity and stability of the Bambara groundnut isolates indicate that they could be used as ingredients in many food formulations, such as salad dressing, comminuted meats, ice creams, cake batters, and mayonnaise. The integrity of the isolates was preserved better by micellisation than by isoelectric precipitation. This effect was evident from DSC thermograms, which showed less denaturation in terms of the denaturation temperature Td and the heat of denaturation ΔH of the micellised sample compared with the isoelectric isolate. The result on gel electrophoresis (SDS-PAGE) revealed that Bambara protein are devoid of disulphide bonds; hence, the protein might consist mainly of 7S globulin with a marginal amount of 11S globulin.
ACKNOWLEDGMENTS
YAA is grateful to the Alexander von Humboldt AvH Foundation for their support through the Georg Foster Fellowships. The assistance of Frau Dr. Yvonne Schneider and Frau Dr. Böhme Birgt is acknowledged.
REFERENCES
- Marcello , D. and Gius , C. 1997 . Legume seeds: Protein content and nutritional value . Field Crops Research , 53 : 31 – 45 .
- Lacroix , B.N. , Assocumou , R.S. and Sangwan , L. 2003 . Effect of vitro direct shoot regeneration systems in Bambara groundnut (Vigna substeranea L) . Plant Cell Report , 21 : 1153 – 1158 .
- Paredes-Lopez , O. , Ordorica-Falomir , C. and Carabez-Trejo , A. 1988 . Production of safflower protein isolates: Physicochemical characterization . Food Sci & Technol , 21 : 328 – 333 .
- 1990 . AOAC Official Methods of Analysis , 15th , Washington, DC : Association of Official Analytical Chemists .
- Henle , T. , Walter , I. , Krause , H. and Klostermeyer , H. 1991 . Efficient determination of individual Maillard compounds in heat-treated milk products by amino acids analysis . Int Dairy J , 1 : 125 – 135 .
- Henle , T. , Schwarzenbolz , U. and Klostermeyer , H. 1997 . Detection and quantification of pentosidine in foods . Z. Lenbensm Unters Forsch , 204 : 95 – 98 .
- Sathe , S.K. , Deshpande , S.S. and Salunkhe , D.K. 1982 . Functional properties of winged bean (Psophocarpus tetragonolobus L., DC) proteins . J Food Sci , 47 : 503 – 507 .
- Coffman , C.W. and Garcia , V.V. 1977 . Functional properties and amino acid content of the protein isolate of Mung bean flour . J Food Technol , 12 : 473 – 484 .
- Vani , B. and Zayas , J.F. 1995 . Foaming properties of selected plant and animal proteins . J Food Sci , 60 : 1025 – 1028 .
- Pearce , N. and Kinsella , F. 1978 . Emulsifying properties of proteins: Evaluation by turbidimetric technique . J Agric Food Chem , 26 : 716 – 723 .
- Wu , V. , Hettiarachchy , S. and Qi , M. 1998 . Hydrophobicity, solubility and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration . JAOCS , 75 : 450 – 845 .
- Sathe , S.K. and Salunkhe , D.K. 1981 . Functional properties of great northern bean (Phaseolus vulgaris) proteins: Emulsion, foaming, viscosity and gelation properties . J Food Sci , 46 : 71 – 75 .
- Sathe , S.K. , Ponte , J.G. , Rangnekar , P.D. and Salunkhe , D.K. 1981 . Effects of addition of great northern bean flour and protein concentrates on rheological properties of dough and baking quality of bread . Cereal Chem , 8 : 97 – 100 .
- Lane , L.A. 1978 . Simple method for stabilizing protein-sulf-hydryl groups during SDS-gel electrophoresis . Anal Biochem , 86 : 655 – 664 .
- Escamilla , M.E. , Guzman-Maldonado , S.H. , Cano-Medinal , A. and Gonzalez-Alatorre , G. 2003 . Simplified process for the production of sesame protein concentrate: Differential scanning calorimetry and nutritional, physicochemical, and functional properties . J Sci Food Agric , 83 : 972 – 979 .
- SAS . 1990 . Statistical Analysis Systems Users Guide , Cary, NC : SAS Institute . Vol. I and II, Version 6
- Sefa Dedeh , S. and Stanley , D. 1979 . Cowpea proteins 1. Use of response surface methodology in predicting cowpea (Vigna unquiculata) protein extractability . J Agric Food Chem , 27 : 1238 – 1243 .
- Okezie , B.O. and Bello , A.B. 1988 . Physicochemical and functional properties of winged bean flour and isolates compared with soy isolate . J Food Sci , 53 : 450 – 454 .
- Smith , C.R. , Earle , F.R. , Wolff , I.A and Jones , R. 1959 . Comparison of solubility characteristics of selected seed proteins . J Agric Food Chem , 7 : 132 – 136 .
- Pellet , P.L. and Young , V.R. Nutritional evaluation of protein foods . Report of working group sponsored by the International Union of Nutritional Sciences and the United Nations University World Hunger Programme .
- FAO/WHO Protein Quality Evaluation . 1991 . Food and Agricultural Organisation of the United Nations 66 Rome, , Italy
- Baldi , G. and Salamini , F. 1973 . Variability of essential amino acid content in seeds of Phaseolus species . Theoretical and Applied Genetics , 43 : 75 – 78 .
- Kanamori , M. , Ikeuch , T. , Ibuki , F. , Kotaru , M. and Kan , K.K. 1982 . Amino acid composition of protein fractions extracted from Phaseolus beans and the field bean (Vicia faba L.) . J Food Sci , 47 : 1991 – 1994 .
- Laurena , A.C. , Rodriquez , F.M. , Sabino , N.G. , Zamora , A.F. and Mendosa , E.T. 1991 . Amino acid composition, relative nutritive value and in vitro protein digestibility of several Philippine indigenous legumes . Plant Foods for Human Nutrition , 41 : 59 – 68 .
- Sosulski , F.W. and McCurdy , A.R. 1987 . Functionality of flours, protein fractions and isolates from field peas and faba beans . J Food Sci , 52 : 1010 – 1014 .
- Paredes-Lopez , O. , Ordorica-Falomir , C. and Olivares-Vazquez , M.R. 1991 . Chickpea protein isolates physicochemical, functional and nutritional characterization . J Food Sci , 56 : 726 – 729 .
- Pszczola , D.E. 2002 . Genes and diet. The specialized role ingredients may play . Food Technol , 54 : 82 – 84 .
- Oomah , B.D. 2001 . Flaxseed as a functional food source . J Sci Food Agric , 81 : 889 – 894 .
- Blenford , D.E. 1996 . Use of amino acid and peptides in sports nutrition . International Food Ingredients , 3 : 20 – 23 .
- FAO/WHO/UNU . Energy and protein requirements. Report of a joint meeting, WHO, Geneva . Technical Report Series No. 724 . 1985 .
- Donna , M. , Klockeman , R.T. and Kevin , A.S. 1997 . Isolation and characterization of defatted canola meal protein . J Agric Food Chem , 45 : 3867 – 3870 .
- Sanchez-Vioque , R. , Clement , A. , Vioque , J. , Bautista , J. and Millan , F. 1999 . Protein isolates from chickpea (Cicer arietinum) chemical composition, functional properties and characterization . Food Chem , 64 : 243 – 273 .
- Prinyawiwatkul , W. , Beuchat , L.R. , McWatters , K.H. and Philips , R.D. 1997 . functional properties of cowpea (Vigna unguiculata) flour as affected by soaking, boiling and fungal fermentation . J Agric Food Chem , 45 : 480 – 486 .
- Kinsella , J. 1976 . Functional proteins of proteins in foods: A survey . J Food Sci , 37 : 219 – 280 .
- Hettiarachchy , N.S. , Griffin , V.K. and Gananasambabdam , R. 1996 . Preparation and functional properties of a protein isolate from defatted wheat germ . Cereal Chem , 73 : 363 – 367 .
- Paredes-Lopez , O. and Ordorica-Falomir , C. 1986 . Production of safflower protein isolates: Composition, yield and protein quality . J Sci Food Agric , 37 : 1097 – 1103 .
- Moure , A. , Dominguez , H. and Parajo , J. 2006 . Ultrafiltration of industrial waste liquors from the manufacture of soy protein concentrates . J Chem Technol Biotechnol , 81 ( 7 ) : 1252 – 1258 .
- Paredes-Lopez , O. and Ordorica-Falomir , C. 1986 . Functional properties of safflower protein isolates: Water absorption, whipping and emulsifying characteristics . J Sci Food Agric , 37 : 1104 – 1108 .
- Kinsella , J.E. 1979 . Functional properties of soy proteins . JAOCS , 56 : 242 – 258 .
- Subagio , A. 2006 . Characterization of hyacinth bean (Lablab purpureus L., sweet) seeds from Indonesia and their protein isolate . Food Chem , 95 : 65 – 70 .
- Abdel-Aal , E.M. , Shehata , A.A. , El-Mahdy , A.R. and Youssef , M.M. 1986 . Extractability and functional properties of some legume proteins isolated by three different methods . J Sci Food Agric , 37 : 553 – 559 .
- Tjahjadi , C. , Lin , S. and Breene , W.M. 1988 . Isolation and characterization of adzuki bean (Vigna angularis cv. Tanaka) proteins . J Food Sci , 53 : 1438 – 1443 .
- Nakai , S. 1983 . Structure-function relationships of food proteins with emphasis on the importance of protein hydrophobicity . J Agric Food Chem , 31 : 676 – 683 .
- Voutsina , L.P. , Cheung , E. and Nakai , S. 1983 . Relationships of hydrophobicity to emulsifying properties of heat denatured proteins . J Food Sci , 48 : 26 – 32 .
- Lawal , O.S. and Adebowale , K.O. 2004 . Effect of acetylation and succinylation on solubility profile, water absorption capacity, oil absorption capacity and emulsifying properties of mucuna (Mucuna pruriens) protein concentrate . Nahrung/Food , 48 : 129 – 136 .
- Lawal , O.S. and Adebowale , K.O. 2005 . An assessment of changes in thermal and physicochemical parameters of Jack bean (Canavalia ensiformis) starch following hydrothermal modifications . European Food Res and Technol , 22 : 631 – 638 .
- Hutton , C.W. and Campbell , A.M. 1981 . “ Water and fat absorption ” . In Protein Functionality in Foods , Edited by: Cherry , J.P. 177 – 200 . Washington, DC : American Chemical Society .
- Knorr , D. 1980 . Protein recovery from food wastes: The influence of various methods of protein coagulation upon yield, quality and properties of potato protein concentrates . J Food Sci , 45 : 1183
- Knorr , D. 1983 . Recovery of functional proteins from food processing wastes . Food Technol , 37 : 71
- Mwasaru , A.M. , Muhammad , K. , Bakar , J. and Che Man , Y. 1999 . Effect of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeon pea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. II: Functional properties . Food Chem , 67 : 445 – 452 .
- El-Adawy , T.A. 2000 . functional properties and nutritional quality of acetylated and succinylated mung bean protein isolate . Food Chem , 70 : 83 – 91 .
- Lopez de Ogara , M.C. , Delgado de Layno , M. , Pilosof , A.M. and Macchi , R.A. 1992 . Functional properties of soy protein isolates as affected by heat treatment during isoelectric precipitation . JAOCS , 69 : 184 – 187 .
- Li , X. , Li , Y. , Hua , Y. , Qiu , A. , Yang , C. and Cui , S. 2007 . Effect of concentration, ionic strength and freeze-drying on the heat-induced aggregation of soy proteins . Food Chem , 104 : 1410 – 1417 .
- Derbyshire , E. , Wright , D.J. and Boulter , D. 1976 . Legumin and Vicillin storage proteins of legume seeds . Phytochemistry , 15 : 3 – 24 .
- Ordorica-Falomir , C. , Paredes-Lopez , O. and Pena , R.J. 1990 . Production of safflower protein isolates, gel filtration and electrophoretic patterns . Food Sci & Technol , 23 : 122 – 125 .
- Pernollet , J.C. and Mosse , J. 1983 . “ Structure and location of legume and cereal seed storage ” . In Seed Proteins , Edited by: Daussant , J. , Mosse , J. and Vaughan , J. 155 – 192 . New York : Academic Press .
- Soottawat , B. , Wonnop , V. and Paiboon , T. 2000 . Isolation and characterization of trypsin inhibitors from some Thai legume seeds . J Food Biochem , 24 : 107 – 127 .
- Cordero-de-los-Santos , M.Y. , Osuna-Castro , J.A. , Borodanenko , A. and Paredes-Lopez , O. 2005 . Physicochemical and functional characterization of amaranth (Amaranth hypochondriacus) protein isolates obtained by isoelectric precipitation and micellisation . Food Sci Technol Int , 11 : 269 – 280 .
- Arntfield , S.D. and Murray , E.D. 1981 . The influence of processing parameters on food protein functionality. I: Differential Scanning calorimetry as an indicator of protein denaturation . Canadian Inst of Food Sci and Technol J , 14 : 289 – 294 .
- Biliaderis , C.G. 1983 . Differential scanning calorimetry in food research—A review . Food Chem , 10 : 239 – 265 .
- Ju , Z.Y. , Hettiarachchy , N.S. and Rath , N. 2001 . Extraction, denaturation and hydrophobic properties of rice flour proteins . J Food Sci. , 66 : 229 – 232 .