Abstract
Artichoke polyphenol oxidases (PPOs) were obtained by (NH4)2SO4 precipitation using ascorbic acid, polyvinylpyrrolidone, and Triton X-100. The PPO content of artichoke head (AHPPO) was 8820 units (mg protein)−1 as compared with 3370 units (mg protein)−1 in artichoke leaves-and-stem (ALSPPO) by using catechol as a substrate. The substrates of both AHPPO and ALSPPO are o-diphenols, such as catechol, pyrogallol, and L-DOPA. Optimum pH and temperature of both PPOs were determined. AHPPO had higher thermal stability than ALSPPO. Also, Tm (the midpoint of thermal inactivation) and t1/2 (half-life) values were determined. Km and Vmax of both PPOs were observed to be similar. Twelve inhibitors were tested and their I50 values were determined. The most effective inhibitors were found to be potassium cyanide, ascorbic acid, L-cysteine, and thiourea. Sodiumdodecylsulfate, urea, and cupric sulfate caused an increase about 20–30% in the PPO activity.
INTRODUCTION
Enzymatic browning of fresh fruits and vegetables is mainly due to oxidation of natural phenolic compounds into quinones, which, in turn, are polymerized to dark pigments. Enzymatic browning that occurred when tissue was damaged during storage and processing of raw fruits[Citation1, Citation2] and vegetables is often undesirable and responsible for unpleasant sensory qualities and losses in nutrient quality.[Citation3] The main enzyme involved in this reaction is polyphenol oxidase (PPO). Polyphenol oxidase catalyse two different reactions by using molecular oxygen: the hydroxylation of monophenols to o-diphenols (monophenolase activity), and the further oxidation of o-diphenols to o-quinones (diphenolase activity).[Citation4] PPO has been widely studied in many fruits and vegetables to determine what the mechanism of browning, which results in the loss of their marketability, is. Some of these investigated plant tissues are kiwi,[Citation5] pears,[Citation6, Citation7] banana,[Citation8, Citation9] head lettuce,[Citation10] dog rose,[Citation11] potato,[Citation12] eggplant,[Citation13] Jerusalem artichoke,[Citation14] quince,[Citation15] bean sprouts,[Citation16] celery,[Citation17, Citation18] broccoli florets,[Citation19] grape,[Citation20] pawpaw.[Citation21]
Artichoke (Cynara scolymus L.) is cultivated in Europe and America, and it is largely used in the Mediterranean diet and in folk medicine. The artichoke head, an immature flower, constitutes the edible part of this vegetable. The artichoke is not only a good food, known for its pleasant bitter taste, but also an interesting and widespread herbal drug. Artichoke leaf extracts are widely used alone or in association with other herbs for embittering alcoholic and soft drinks and to prepare herbal teas or herbal medicinal products.[Citation22] Artichoke is characterized by its phenolic acid constituents, including caffeic acid, mono- and di-caffeoyl quinic acids (e.g., cynarin), and chlorogenic acid. These compounds are considered to be responsible for the choleretic, hypocholesterolemic, hepatoprotective, anticarcinogenic, and antioxidative properties traditionally attributed to artichoke.[Citation23, Citation24] Polyphenolic compounds are present mainly in the leaves rather than in the artichoke head, and they have been documented as the active substances of this plant. The results from several clinical investigations showed the efficacy and safety of artichoke leaf extracts in the treatment of hepato-biliary dysfunction and digestive complaints, such as loss of appetite, nausea, and abdominal pain.[Citation25] Gebhardt et al. (1997) reviewed that artichoke leaf extracts may reduce the atherogenic risk in at least a dual fashion: (i) by preventing oxidative modification of blood lipoproteins and (ii) by reducing blood cholesterol levels.[Citation26] Also, artichoke leaf is used as a diuretic in the treatment of diabetes, due to its flavonoidic property.[Citation27]
An important point of this study was that, besides other parts of the plant,[Citation28–32] PPO is also found in artichoke head and a remarkable browning was found. However, the existence of this enzyme in artichoke leaves and its enzymatic properties remains unknown. Artichoke leaf extract is one of the important nutraceuticals widely used in Europe and America, and has been used in order to prevent heart disease and atherosclerosis by reducing LDL cholesterol levels, to enhance detoxification reactions and protect liver cells, and to alleviate digestive disorders and increase biliary excretion. A comparative study of polyphenol oxidases from artichoke head and artichoke leaves-and-stem was carried out to provide information about probable enzymatic browning. It has been thought that this information may be useful to control the results of probable enzymatic browning in the nutraceutical preparation from artichoke leaves as well as to provide useful information for guiding food processing operations. The objective of this study was to determine the properties of PPO of artichoke leaves-and-stem, and to compare the properties of PPO of artichoke leaves-and-stem to those of PPO from artichoke head.
MATERIALS AND METHODS
Materials
Artichokes of marketable quality were obtained from Guzelyurt-Cyprus and stored at 4°C. l-DOPA, ascorbic acid, polyvinylpyrrolidone, Triton X-100, cysteine, β-mercaptoethanol, and ethylene glycol were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Catechol, pyrogallol, ascorbic acid, ammonium sulfate, citric acid, sodium bisulphite, EDTA, potassium cyanide, benzoic acid, thiourea, sodium azide, thioacetamid, urea, sodiumdodecylsulfate, and cupric sulfate were purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade and used without further purifications.
Isolation of Polyphenoloxidase
For the preparation of the crude extracts, 200 g of artichoke head and outer leaves-and-stem were separated and cut quickly into thin slices and homogenised with 200 mL of 0.1 M phosphate buffer, pH 7.0 containing 10 mM ascorbic acid, 0.1% polyvinylpyrrolidone, and 0.5% Triton X-100 in a blender for 3 min. The homogenate was filtered through cheese cloth, and the filtrate was centrifuged at 8500 × g for 30 min at 4°C. The supernatant was brought to 90% (NH4)2SO4 saturation with solid (NH4)2SO4. The precipitated PPO was separated by centrifugation at 8500 × g for 30 min at 4°C. The precipitate was dissolved in a small amount of 0.1 M phosphate buffer, pH 7.0, and dialysed at 4°C in the same buffer changing the buffer every 8 h for three times during dialysis.
Assay of PPO Activity
PPO activity was determined by measuring the increase in absorbance at 420 nm for catechol, at 334 nm for pyrogallol, at 475 for l-DOPA, in the presence of air oxygen. The reaction mixture contained 0.05 mL of enzyme solution and 2.95 mL of 10 mM substrate solution prepared in 50 mM phosphate buffer, pH 7.0, and at 25°C. The blank sample contained only 3.0 mL of substrate solution. Enzyme activity was calculated from the linear portion of the curve.[Citation33] One unit of PPO activity was defined as the amount of enzyme leading to an increase in absorbance of 0.001 per min. Protein concentration was determined by the method of Lowry with bovine serum albumin as standard.[Citation34] All activity and protein assays were expressed as the mean of at least three different samples.
Substrate Specificity
Substrate specificity was determined by using catechol, pyrogallol, and l-DOPA as substrate. All substrate solutions were prepared as 10 mM in 50 mM phosphate buffer, pH 7.0.
Effects of pH and Temperature on the Activity
The effect of pH on PPO activity was examined with 100 mM citrate/200 mM phosphate, 100 mM phosphate, and 100 mM borate buffers between pH 4 and 9 by using 10 mM active substrates, including catechol, l-DOPA, and pyrogallol at 25°C. To determine optimum temperature of PPO, the enzyme activity was measured in the temperature range from 25 to 90°C by using catechol, l-DOPA, and pyrogallol solutions as a substrate. The thermal stability of artichoke PPO was determined by putting 2.0 mL of the enzyme solution in a test tube in a water bath at the appropriate temperatures, which ranged 40–80°C; 0.05 ml of enzyme solution was withdrawn at appropriate time intervals and rapidly cooled in an ice bath, then the PPO activity was assayed as described above.
Kinetic Parameters
Michaelis constant (Km ) and maximum rates (V max) were determined by using catechol and pyrogallol solution in the range of 2–10 mM concentrations, and by using l-DOPA in the range of 0.5–1.5 mM. The reaction was followed in a spectrophotometer and data were plotted according to Lineweaver-Burk.
Effect of Inhibitors and Activators
PPO activity was measured by using 12 different inhibitors (citric acid, EDTA, ascorbic acid, cysteine, potassium cyanide, thioacetamid, sodium azide, benzoic acid, thiourea, β-mercaptoethanol, ethylene glycol, and sodium bisulphite) at different concentrations with catechol as substrate. Each inhibitor solution was prepared in 100 mM phosphate buffer, pH 7.0. The PPO activity was measured in previous reaction conditions (pH 7.0, 25°C) by using 10 mM catechol prepared in the same buffer as substrate. The graphs of activity percentage versus inhibitor concentrations were drawn, and I50 values were determined from these graphs.
PPO activity was measured at two various concentrations of three different probable activators, including sodiumdodecylsulfate, urea, and cupric sulfate, in order to determine effects of some chemical reagents on activities of artichoke PPOs. The reaction medium contained 0.1 mL of enzyme solution, 2.8 mL of 10 mM catechol solution in 50 mM phosphate buffer, pH 7.0, and 0.1 mL of activator solution (1 mM to 10 mM).
RESULT AND DISCUSSION
Isolation of Polyphenoloxidase
The presence of PPO in the artichoke head was previously reported.[Citation28–32, Citation35] In this study, artichoke PPO extracts (ALSPPO and AHPPO) were prepared using ammonium sulfate precipitation and dialysis from either artichoke leaves-and-stem or artichoke head. The existence of PPO in artichoke leaves may be problematic during preparation of this extract due to enzymatic browning. The data obtained about properties of artichoke leaf PPO may be helpful to control probable enzymatic brownings. Extraction of PPO was carried out in 100 mM phosphate buffer, pH 7.0, containing 1 mM ascorbic acid, 0.1% polyvinylpyrrolidone, and 0.5% Triton X-100, and then precipitated by (NH4)2SO4 method.[Citation17] Several precipitations with solid (NH4)2SO4 between 0–20, 20–40, 40–60, 60–80, and 80–90% were tested to find the best saturation point. As a result, PPO activity of precipitate of 90% (NH4)2SO4 was found to be the highest. This saturation point was used in all the extraction procedures. It is well known that oxidation of phenolics by PPO produces quinones, which would inhibit PPO. Therefore, ascorbic acid was used to reduce quinones to phenolics substrates during extraction. Polyvinylpyrrolidone, which is an insoluble polymer, was added to extraction medium in order to bind to phenolic substrates in the media, thus preventing phenol oxidation and polymerization.[Citation8] Triton X-100 helps release the PPO from the thylakoid lumen, and avoids phenolic oxidation during and after extraction. An increase of enzyme activity by this detergent treatment has already been reported.[Citation36] Ammonium sulfate precipitation and dialysis resulted in the activity increase. The increase in PPO activities of artichoke head and leaves-and-stem are about three-fold and two-fold, respectively (data not shown). The enzyme extracts obtained from ammonium sulfate precipitation and dialysis were used in characterization of PPOs.
Substrate Specificity
The activities of artichoke PPOs for various substrates are shown in . The substrate specificity of ALSPPO was similar to that of AHPPO. As seen in , the both artichoke PPOs oxidised catechol, l-DOPA, and pyrogallol. Similar substrate specificity was also found in dog-rose PPO,[Citation11] Jerusalem artichoke PPO,[Citation14] cocoa bean PPO,[Citation37] artichoke head PPO,[Citation32] and Malatya apricot PPO.[Citation38]
Table 1 Substrate specificity of artichoke PPO
For each substrate, the kinetic data were plotted as 1/Activity versus 1/Substrate concentration, according to the method of Lineweaver-Burk. Km and V max values calculated from the Lineweaver-Burk graphs were shown in . Km values for ALSPPO were 5.4, 2.7, and 8 mM with catechol, pyrogallol, and l-DOPA, respectively. For AHPPO, Km s were 7.7, 1.7, and 2.1 mM with the same substrates, respectively. Our findings showed that the both artichoke PPOs have the greatest reactivity towards pyrogallol having the lowest Km value among the substrates used. The data for Km exhibits that pyrogallol binds the enzyme most strongly. But based on the enzyme efficiency, catechol, which has the highest V max/Km value, is the best substrate for both artichoke PPOs under the mentioned conditions. Km of ALSPPO was similar to those of Jerusalem artichoke PPO (5.09 mM) and medlar fruit PPO (5.7 mM).[Citation14, Citation39] Km of AHPPO is approximate to that of Malatya apricot PPO (6.6 mM).[Citation38] In general, PPO has low affinity for its substrates and the reported Km values are often in the 1–10 mM range.[Citation40] The reported Km values for catechol as substrate are 10.2, 0.11, 0.49, and 71 mM for artichoke head, cocoa bean, tea leaf, and bean sprouts, respectively.[Citation16, Citation32, Citation37, Citation41] Using pyrogallol as the substrate, Km value from mandarin orange and edible burdock were 7.1 and 1.8 mM, respectively.
Table 2 K m and V max values of artichoke head and artichoke leaves-and-stem PPO
Table 3 The I50 values of different inhibitors for both artichoke PPOs
Effect of pH and Temperature
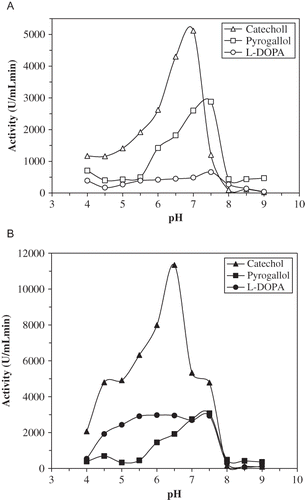
The pH activity profiles for artichoke PPOs are shown . As seen, the pH optimum for ALSPPO were found to be 7.0, 7.5, and 7.5 for catechol, pyrogallol, and l-DOPA, respectively. For AHPPO, these values were 6.5, 7.5, and 7.5 for the same substrates, respectively. It was reported that the pH optimum for PPO is observed in the wide range (4.0–7.0) depending on extraction method, substrate, location of enzyme in the cell and enzyme source.[Citation4] Aydemir (2004) reported that pH optimum of artichoke head PPO was observed in the range of pH 5.0–7.0 for catechol.[Citation32] The pH optimum of PPO from some other sources also is observed in the range of pH 4.0–8.0. The maximum activity was found as pH 7.0 for d'Anjou pears,[Citation7] edible burdock,[Citation42] cocoa bean,[Citation37] oil bean seeds,[Citation43] and Amasya apple.[Citation33] Also, optimum pHs were determined as 8.5 for dog-rose fruit and Malatya apricot,[Citation11, Citation38] as 6–7 for kiwi fruit,[Citation5] as 7.5 for Allium sp.,[Citation44] and as 6.6 for potato.[Citation12]
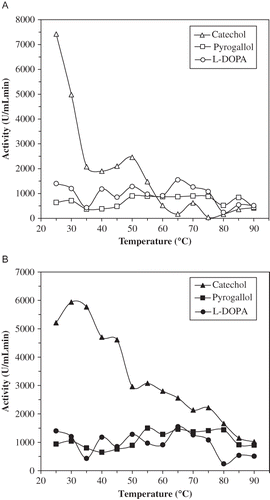
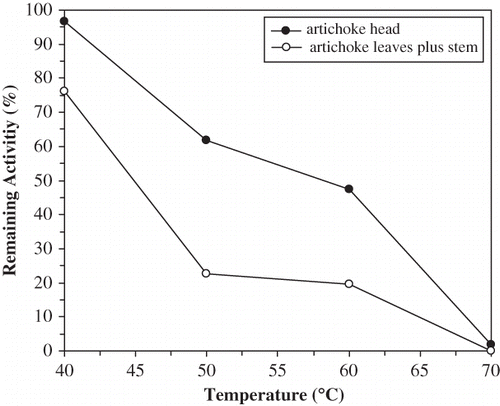
The optimum temperatures for ALSPPO were around 25, 50, and 65°C with catechol, pyrogallol, and l-DOPA, respectively, whereas 30, 55, and 65°C for AHPPO, respectively (). Optimum temperature for artichoke head was 25°C.[Citation32] This parameter for cocoa bean and edible burdock PPO were 45 and 60°C, respectively.[Citation37, Citation42] At 50°C, the ALSPPO lost about 75% of its activity, whereas the AHPPO lost about 40% of its activity under previously mentioned conditions after 30 min. Thermal characteristics of both artichoke PPOs at 60°C were similar to those of 50°C. It was seen in and that AHPPO was more thermostable than ALSPPO at 50 and 60°C. At a temperature >60°C, as expected, the rate of inactivation increased depending on temperature. At these temperatures, ALSPPO and AHPPO lost nearly the whole activities within 10 min.
Figure 3 Effect of temperature on stability of ALSPPO activity (A) and AHPPO activity (B). The enzyme solution was incubated for various time intervals (5–60 min) at the specified temperature (40–80°C) and rapidly cooled. The activity was measured at 25°C, was taken as 100% and activities measured (40–80°C) were compared with the activity measured at 25°C.
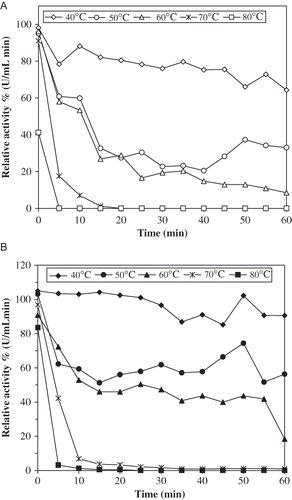
In addition, it was determined that t 1/2 values for ALSPPO were 39.4 and 17.3 min at 50 and 60°C, respectively, whereas these values for AHPPO were 68.6, 26, and 9.2 min at 50, 60, and 70°C, respectively. The midpoint of thermal inactivation (Tm ), where the activity is diminished by 50%, is calculated from the plot of percent residual activity versus temperature.[Citation45] For this purpose, both ALSPPO and AHPPO were incubated for 30 min at various temperatures between 40–70°C, and remaining activities were assayed. Tm values were found to be 45 and 58°C, respectively (). This difference in Tm has been emphasized that AHPPO has higher thermal stability than ALSPPO.
Effect of Inhibitors and Activators
The control of enzymatic browning has always been a challenge to the fruit processing. Besides halide salts and aromatic carboxylic acids known to inhibit PPO, numerous chemicals exhibiting reducing properties, such as sulfites, ascorbic acid, and its derivatives, thiol compounds like cysteine, β-mercaptoethanol were proposed.[Citation46] In this study, it was used 12 different inhibitors (citric acid, EDTA, ascorbic acid, cysteine, potassium cyanide, thioacetamid, sodium azide, benzoic acid, thiourea, β-mercaptoethanol, ethylene glycol, and sodium bisulphate) as PPO inhibitor. The I50 values of different inhibitors for both artichoke PPOs are listed in . Both artichoke PPOs were markedly inhibited by potassium cyanide, ascorbic acid, and l-cysteine. Catecholase activity was also inhibited by sodiummetabisulphite, thiourea, and β–mercaptoethanol, but their inhibitory effects were lesser. shows that potassium cyanide, ascorbic acid, and L-cysteine have very low I50 values, thus they are strong inhibitors of the enzyme. Potassium cyanide might interact with the cofactor of the enzyme as chelating agent. Dehydroascorbic acid, the oxidation product of ascorbic acid, can react with amino groups in close proximitiy to the active site(s) of the enzyme through Strecker degradation. It was reported that ascorbic acid was a good inhibitor of PPOs isolated from the other sources.[Citation16, Citation28, Citation44, Citation47] Also Aydemir (2004) reported that ascorbic acid was competitive inhibitor of artichoke head PPO, and it can be used to prolong its shelf-life of artichoke.[Citation32] Cysteine and sodium bisulphite, on the other hand, may react directly with sulfhydryl groups with the reduction of o-quinone.[Citation37, Citation48] L-cysteine, which is a naturally occurring amino acid and non-toxic, can be used in the food technology, like ascorbic acid in order to prevent enzymatic browning of artichoke products.
Table 4 The effect of different activators on catecholase activity for both artichoke PPOs
Polyphenol oxidase activity has been activated by a variety of treatments or agents such as proteases, urea, fatty acids, polyanmines, divalent cations, acid and basic shock, and anionic detergents such as SDS.[Citation49] The activator effect of different activators on catecholase activity for both artichoke PPOs was investigated by using sodiumdodecylsulfate, urea, and cupric sulfate, and their results were given in . It was reported that PPO from some plant sources was activated by unusual treatments, such as acid shock, exposure urea, or SDS or other anionic detergents.[Citation41] In our study, the use of SDS as activator has no effect on the activity of ALSPPO whereas it increased about 30% of the activity of AHPPO. The results of the use of urea as activator are similar to that of SDS, the increase of activity is about 20% in the artichoke head PPO. Halder et al.[Citation41] (1998) couldn't detect any activation of enzyme in the presence of SDS (0.1 to 5 mM) and urea (0.5 to 2 M). Sanchez-Ferrer et al.[Citation50] (1993), and Sojo et al. [Citation51] (1998) reported that activation is generally observed at low concentrations of SDS, and higher concentrations may inhibit activity. But coffee PPO was slightly activated by SDS only between 0.35 and 1.75 mM and higher concentrations did not have a major inhibitory effect.[Citation42] Cu++ ion activated the oxidation of catechol, and increased the activity about 30%. The similar PPO activation by Cu++ ion was recorded by others.[Citation28, Citation52]
CONCLUSION
It was our objective to determine the properties of artichoke leaves PPO and to compare those of artichoke head PPO. The obtained data may be used to prevent probable problems due to PPO when preparing artichoke extract as nutracetical from artichoke leaves. The PPO content of ALSPPO was comparable to that of AHPPO. The obtained results showed that some properties such as substrate specificity, optimum pH, optimum temperature, kinetic values of both enzymes, were similar to each other whereas thermal stability of AHPPO was better than ALSPPO. The most effective inhibitors were potassium cyanide, ascorbic acid, l-cysteine, and thiourea for both enzymes. Sodiumdodecylsulfate, urea, and cupric sulfate caused an increase about 20–30% in the PPO activity.
ACKNOWLEDGMENT
This research was funded by Research Fund of Trakya University (TUBAP-621).
REFERENCES
- Kingsly , A.R.P. , Balasubramaniam , V.M. and Rastogi , N.K. 2009 . Influence of high-pressure blanching on polyphenoloxidase activity of peach fruits and its drying behavior . International Journal of Food Properties , 12 : 671 – 680 .
- Lydakis , D. , Fysarakis , I. , Papadimitriou , M. and Kolioradakis , G. 2003 . Optimization study of sulfur dioxide application in processing of sultana raisins . International Journal of Food Properties , 6 : 393 – 403 .
- Shi , C. , Dai , Y. , Xu , X. , Xie , Y. and Liu , Q. 2002 . The purification of polyphenol oxidase from tobacco . Protein Expression and Purification , 24 : 51 – 55 .
- Beena , P. and Gowda , L.R. 2000 . Purification and characterization of a polyphenol oxidase from the seeds of field bean (Dolichos Lablab) . Journal of Agricultural and Food Chemistry , 48 : 3839 – 3846 .
- Park , E.Y. and Luh , B.S. 1985 . Polyphenol oxidase of kiwifruit . Journal of Food Science , 50 : 678 – 684 .
- Wisseman , K.W. and Montgomery , M.W. 1985 . Purification of d'Anjou pear (Pyrus Communis L.) polyphenol oxidase . Plant Physiology , 78 : 256 – 262 .
- Halim , D.H. and Montgomery , M.W. 1987 . Polyphenol oxidase of d'Anjou pears (Pyrus Communis L.) . Journal of Food Science , 43 : 603 – 608 .
- Galeazzi , M.A.M. and Sgarbieri , V.C. 1981 . Substrate specificity and inhibition of polyphenol oxidase (PPO) from a dwarf variety of banana (Musa Cavendishii L.) . Journal of Food Science , 46 ( 5 ) : 1404 – 1406 .
- Yang , C.P. , Fujita , S. , Ashrafuzzaman , M.D. , Nakamura , N. and Hayashi , N. 2000 . Purification and characterization of polyphenol oxidase from banana (Musa Sapientum L.) pulp . Journal of Agricultural and Food Chemistry , 48 : 2732 – 2735 .
- Fujita , S. , Tono , T. and Kawahara , H. 1991 . Purification and properties of polyphenol oxidase in head lettuca (Lactuca Sativa) . Journal of the Science of Food and Agriculture , 55 : 643 – 651 .
- Şakiroğlu , H. , Küfrevioğlu , O. , Kocaçalişkan , I. , Oktay , M. and Onganer , Y. 1996 . Purification and characterization of dog-rose (Rosa Dumalis Rechst.) polyphenol oxidase . Journal of Agricultural and Food Chemistry , 44 : 2982 – 2986 .
- Cho , Y.K. and Ahn , H.K. 1998 . Purification and characterization of polyphenol oxidase from potato: i purification and properties . Journal of Food Biochemistry , 23 : 577 – 592 .
- Gilabert , M.P. and Carmona , F.G. 2000 . Characterization of catecholase and cresolase activities of eggplant polyphenol oxidase . Journal of Agricultural and Food Chemistry , 48 : 695 – 700 .
- Ziyan , E. and Pekyardimci , S. 2001 . Characterization of polyphenol oxidase from Jerusalem artichoke (Helianthus Tuberosus) . Turkish Journal of Chemistry , 27 : 217 – 225 .
- Yagar , H. and Sagiroglu , A. 2002 . Partially purification and characterization of polyphenol oxidase of quince . Turkish Journal of Chemistry , 26 : 97 – 103 .
- Nagai , T. and Suzuki , N. 2003 . Polyphenol oxidase from bean sprouts (Glycine Max L.) . Journal of Food Science , 68 : 16 – 20 .
- Yagar , H. 2004 . Some biochemical properties of polyphenol oxidase from celery . Preparative Biochemistry & Biotechnology , 34 : 387 – 397 .
- Aydemir , T. and Akkanli , G. 2006 . Partial purification and characterization of polyphenol oxidase from celery root (Apium Graveolens L.) and the investigation of the effects on the enzyme activity of some inhibitors . International Journal of Food Science & Technology , 41 : 1090 – 1098 .
- Gawlik-Dziki , U. , Szymanowska , U. and Baraniak , B. 2007 . Characterization of polyphenol oxidase from broccoli (Brassica Oleracae Var. Botrytis Italica) florets . Food Chemistry , 105 : 1047 – 1053 .
- Unal , U. , Sener , A. and Sen , K. 2007 . Characterization Sultaniye grape (Vitis Vinifera L. Cy. Sultana) polyphenol oxidase . International Journal of Food Science & Technology , 42 : 1123 – 1127 .
- Fang , C. , Wang , C.Z. , Xiong , Y.L.L. and Pomper , K.W. 2007 . Extraction and characterization of polyphenol oxidase in pawpaw (Asimina Triloba) fruit . Journal of Food Biochemistry , 31 : 603 – 620 .
- Mulinacci , N. , Prucher , D. , Peruzzi , M. , Romani , A. , Pinelli , P. , Giaccherini , C. and Vincieri , F.F. 2004 . Commercial and laboratory extracts from artichoke leaves: Estimation of caffeoyl esters and flavonoidic compounds content . Journal of Pharmaceutical and Biomedical Analysis , 34 : 349 – 357 .
- Pierluigi , M. and Piergiorgio , P. 2000 . Electrospray characterization of selected medicinal plant extracts . Journal of Pharmaceutical and Biomedical Analysis , 23 : 61 – 68 .
- Schutz , K. , Persike , M. , Carle , R. and Schieber , A. 2006 . Characterization and quantification of anthocyanins in selected artichoke (Cynara Scolymus L.) cultivars by HPLC–DAD–ESI–Msn . Analytical and Bioanalytical Chemistry , 384 : 1511 – 1517 .
- Wegener , T. and Fintelmann , V. 1999 . Pharmacological properties and therapeutic profile of artichoke . Wiener Medizinische Wochenschrift , 149 : 241 – 247 .
- Gebhardt , R. 1997 . Antioxidative and protective properties of extracts from leaves of the artichoke (Cynara Scolymus L.) against hydroperoxide induced oxidative stress in cultured rat hepatocytes . Toxicology and Applied Pharmacology , 144 : 279 – 286 .
- Koseki , P.M. , Villavicencioa , A.L.C.H. , Brito , M.S. , Nahme , L.C. , Sebastiao , K.I. , Rela , P.R. , Almeida-Muradian , L.B. , Mancini-Filho , J. and Freitas , P.C.D. 2002 . Effects of irradiation in medicinal and eatable herbs . Radiation Physics and Chemistry , 63 : 681 – 684 .
- Leoni , O. , Palmieri , S. , Lattanzio , V. and Sumere , C.F.V. 1990 . Polyphenol oxidase from artichoke (Cynara Scolymus L.) . Food Chemistry , 38 : 27 – 39 .
- Lattanzio , V. , Cardinali , A. , Divenere , D. , Linsalata , V. and Palmieri , S. 1994 . Browning phenomena in stored artichoke (Cynara Scolymus L.) heads-enzymatic or chemical reactions . Food Chemistry , 50 ( 1 ) : 1 – 7 .
- Espin , J.C. , Tudela , J. and Canovas , F.G. 1997 . Monophenolase activity of polyphenol oxidase from artichoke (Cynara Scolymus L.) heads . Lebensmittel-Wissenschraft und-Technologie , 30 : 819 – 825 .
- López-Molina , D. , Hiner , A.N.P. , Tudela , J. , García-Cánovas , F. and Rodríguez-López , J.N. 2003 . Enzymatic removal of phenols from aqueous solution by artichoke (Cynara Scolymus L.) extracts . Enzyme and Microbial Technology , 33 ( 5 ) : 738 – 742 .
- Aydemir , T. 2004 . Partial purification and characterization of polyphenol oxidase from artichoke (Cynara Scolymus L.) heads . Food Chemistry , 87 ( 1 ) : 59 – 67 .
- Oktay , M. , Küfrevioğlu , I. and Kocaçalişkan , I. 1995 . Şakiroğlu, H. Polyphenol oxidase from amasya apple . Journal of Food Science , 60 : 18 – 20 .
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.L. and Randal , R.J. 1951 . Protein measurement with the phenol reagent . Journal of Biological Chemistry , 193 : 265 – 275 .
- Doğan , S. , Turan , Y. , Ertürk , H. and Arslan , O. 2005 . Characterization and purification of polyphenol oxidase from artichoke (Cynara Scolymus L.) . Journal of Agricultural and Food Chemistry , 53 ( 3 ) : 776 – 785 .
- Rocha , A.M.C.N. and Morais , A.M.M.B. 2001 . Characterization of polyphenol oxidase (PPO) extracted from “Jonagored apple” . Food Control , 12 : 85 – 90 .
- Lee , P.M. , Lee , K.H. and Karim , M.I.A. 1991 . Biochemical studies of cocoa bean polyphenol oxidase . Journal of the Science of Food and Agriculture , 55 : 251 – 260 .
- Arslan , O. , Temur , A. and Tozlu , I. 1998 . Polyphenol oxidase from Malatya apricot (Prunus Armeniaca L.) . Journal of Agricultural and Food Chemistry , 46 : 1239 – 1241 .
- Dinçer , B. , Çolak , A. , Aydın , N. , Kadıoğlu , A. and Güner , S. 2002 . Characterization of polyphenol oxidase from medlar fruits . Food Chemistry , 77 : 1 – 7 .
- Mazzafera , P. and Robinson , S.P. 2000 . Characterization of polyphenol oxidase in coffee . Phytochemistry , 55 : 285 – 296 .
- Halder , J. , Tamuli , P. and Bhaduri , A.N. 1998 . Isolation and characterization of polyphenol oxidase from Indian tea leaf (Camellia Sinnesis) . Nutrition Biochemistry , 9 : 75 – 80 .
- Murao , S. , Oyama , H. , Nomura , Y. , Tono , T. and Shin , T. 1993 . Purification and characterization of Arctium Lappa L. (edible Burdock) polyphenol oxidase . Bioscience, Biotechnology, and Biochemistry , 57 ( 2 ) : 177 – 180 .
- Chilaka , F.C. , Anosike , E.O. and Egbuna , P.C. 1993 . Purification and properties of polyphenol oxidase from oil bean (Pentaclethra Macrophylla Benth) seeds . Journal of the Science of Food and Agriculture , 61 : 125 – 127 .
- Arslan , O. , Temur , A. and Tozlu , I. 1997 . Polyphenol oxidase from Allium Sp . Journal of Agriculture and Food Chemistry , 45 : 2861 – 2863 .
- Kumar , R.S.S. , Vishwanath , K.S. , Singh , S.A. and Appu Rao , A.G. 2006 . Entrapment of α-amylase in alginate beads: Single step protocol for purification and thermal stabilization . Process Biochemistry , 41 ( 11 ) : 2282 – 2288 .
- Richard , F.C. , Groupy , P.M. , Nicolas , J.J. , Lacombe , J.M. and Pavia , A.A. 1991 . Cysteine as an ınhibitor of enzymatic browning. 1. Isolation and characterization of addition compounds formed during oxidation of phenolics by apple polyphenol oxidase . Journal of Agricultural and Food Chemistry , 39 : 841 – 847 .
- Kavrayan , D. and Aydemir , T. 2001 . Partial purification an characterization of polyphenol oxidase from peppermint . Food Chemistry , 74 : 147 – 154 .
- Vamos-Vigyazo , L. 1981 . Polyphenol oxidise and peroxidase in fruits and vegetables . CRC Critical Reviews in Food Science & Nutrition , 14 : 49 – 129 .
- Jimenez , M. and Garcia-Carmona , F. 1996 . The effect of sodium dodecyl sulphate on polyphenol oxidase . Phytochemistry , 42 : 1503 – 1509 .
- Sanchez-Ferrer , A. , Laveda , F. and Garcia-Carmona , F. 1993 . Substrate-dependent activation of latent potato leaf polyphenol oxidase by anionic surfactant . Journal of Agricultural and Food Chemistry , 41 : 1219 – 1223 .
- Sojo , M.M. , Nunez-Delicado , E. , Garcia-Carmona , F. and Sanchez-Ferrer , A. 1998 . Partial purification of a banana polyphenol oxidase using Triton X-114 and PEG 800 for removal of polyphenols . Journal of Agricultural and Food Chemistry , 46 : 4924 – 4930 .
- Yue-Ming , J. , Zauberman , G. and Fuchs , Y. 1997 . Partial purification and some properties of polyphenol oxidase extracted from Litchi Pericarp . Postharvest Biology and Technology , 10 : 221 – 228 .