Abstract
Different antioxidant assays were employed in order to evaluate antioxidant activities of porcine hemoglobin hydrolysate (PHH) prepared with pepsin and compared with natural and synthetic antioxidants. Molecular weight distributions of PHH polypeptide components were determined. PHH exhibited DPPH/superoxide/hydroxyl radicals scavenging (EC50 0.95 ± 0.01 mg/ml, 20.34 ± 2.83 mg/ml, 7.90 ± 0.58 mg/ml, respectively); reducing power (EC50 3.02 ± 0.91 mg/ml); and metal chelating activity (EC50 2.59 ± 0.05 mg/ml) in a dose-dependent manner. Hydroxyl radicals scavenging activity of PHH was equivalent to ascorbic acid. Relatively less active of PHH exhibited strong synergistic antioxidant activity with α-tocopherol (TOH). Tested combinations (2:1 and 1:1 of PHH:TOH) showed significantly higher antioxidant activities (P < 0.05) compared to combinations with low PHH rates (1:2 and 1:4 of PHH:TOH). The main synergistic antioxidant mechanism could be due to the regeneration of TOH by PHH. Radicals scavenging mechanism, more than metal chelation, could play a major role in the antioxidant process of PHH.
INTRODUCTION
Free radical-mediated reactive oxygen species (ROS) and lipid peroxidation (LPO) have attracted considerable attention nowadays.[Citation1–4] ROS are unstable and react readily with other groups or substances in living organisms during metabolism, resulting in several human diseases, including atherosclerosis, cancer, diabetes mellitus, and Alzheimer's diseases.Citation[5] In addition, the autoxidation of lipids occurring in food materials is also kept on by a free radical mechanism,Citation[6] and the LPO contributes to the subsequent development of unpleasant off-flavors, dark colors, poor texture, and may also generate potentially toxic end products.Citation[7] The oxidation process can be prevented or delayed by antioxidants via single or a combination of free radical scavenging mechanism and metal chelating mechanism. Currently, some synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate, are commonly used to act against free radicals in food and biological systems. However, their applications are restricted due to potential health hazards. Therefore, interest in employing antioxidants from natural sources is considerably enhanced by consumer preference for natural products. In recent years, various protein hydrolysates have been reported to exhibit good antioxidant activity.[Citation8–11] However, the antioxidant mechanism of protein hydrolysates is not fully understood.
Porcine blood is an abundant by-product in the slaughtering process in China with an estimated amount of one million tons per year, and the availability is less than 10%. Except some porcine blood utilized as “blood tofu” for human consumption and blood powder for animal feed, quite a few are discharged as sewage, which is causing severe environmental problems. Blood protein is one of most important animal by-products with a high biological value, especially the proportion of hemoglobin in blood protein, which is 60–70%. Over the past several years, hemoglobin has been studied as novel sources that have been shown to produce a variety of hydrolysate with opioid activity,Citation[12] bradykinin-potentiating activity,Citation[14] antibacterial activity,Citation[13] and angiotensin I-converting enzyme inhibition activity.Citation[15] Recently, a study on the antioxidant activity of porcine hemoglobin hydrolysate (PHH) produced using alcalase and flavourzyme has been performed but in limited antioxidant systems.Citation[16] The reports of antioxidant activity and its mechanisms of PHH prepared with other proteases are still scarce. In recent studies, we found that the PHH produced by several kinds of commercial proteases exhibited antioxidant activity, and that the hydrolysate digested with pepsin—a gastrointestinal enzyme—possessed stronger antioxidant potential than the others (data not shown). Elucidation of the antioxidant activities and their mechanisms of PHH will provide a better understanding of the antioxidant role of PHH in functional food and medicine products. The aim of this work was to investigate the antioxidant activities and the related mechanisms of PHH prepared with pepsin using comprehensive measurements, including the ability to inhibit the linoleic acid oxidation, the scavenging effects on DPPH/superoxide/hydroxyl radicals, reducing power and metal chelating activity.
MATERIALS AND METHODS
Materials and Chemicals
Fresh porcine blood was obtained from Shunxin Agricultural Co. Ltd. (Shun-Yi District, Beijing, China). The porcine blood was collected as the animal was bled and an anticoagulant, sodium citrate (3 g/L blood), was quickly added to prevent clotting, and kept below 4°C to avoid bacterial proliferation. The blood was centrifuged at 9000×g and 4°C for 10 min using a high speed refrigerated centrifuge (Model TGL-16A, Changsha PINGFAN Instrument and Meter Co., Ltd., Changsha, China), and the blood cells were obtained after plasma decanting. The blood cells were then added with an equal volume of water, and centrifuged at 4000×g and 4°C for 10 min to remove the stroma and obtain the hemoglobin. The hemoglobin was lyophilized using a FD-1PF freeze dryer (Beijing DETIANYOU Technology Development Co., Ltd., Beijing, China) and the obtained powder was kept at 4°C until use. Pepsin (from porcine stomach mucosa) used for protein hydrolysis with declared activities of 1:3000 U/g, α-tocopherol, acrylamide, and bisacrylamide were purchased from Amresco (Solon, OH, USA). 2-Deoxy-D-ribose (D5899), linoleic acid (L1376), BHA (B1253), 3-(2-pyridyl)-5, 6-bis (4-phenyl-sulfonic acid)-1, 2, 4-triazine (ferrozine) (P9762), DPPH (D9132), and ascorbic acid (A7506) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Low-range molecular weight protein standard markers were purchased from HOU-BIO Tech. Co. Ltd. (Hong Kong, China). All reagents were of analytical grade.
Preparation of PHH with Pepsin
Five g of dried hemoglobin were dissolved in 100 ml distilled water. The hemoglobin solution was digested by pepsin (enzyme to substrate ratio = 1.6%, w/w) at 40 ± 1°C for 60 min. During hydrolysis, pH was maintained at 1.6 by addition of 1 M HCl. The enzymatic hydrolysis was stopped by heating at 100°C for 10 min. Hydrolysate was centrifuged at 4200×g and 4°C for 10 min to remove the precipitates (low degraded hemoglobin and heme) and lyophilized for 96 h at -60°C, 0.04 mbar, and stored at -20°C. The residual water content was determined by placing approximately 2 g of powder into a pre-weighted aluminium dish. Powder was then dried in an oven at 105°C until a constant weight.Citation[17]
Molecular Weight Distribution
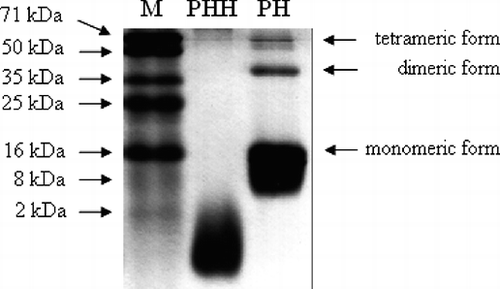
Molecular weight distribution of PHH polypeptide components were determined by SDS-PAGE using 15% Tricine gels on a BG-verMINI system (Baygene Biotech Co., Ltd., Beijing, China) according to the procedure by Laemmli.Citation[18] The electrophoresis was run at 25 mA in 1-mm-thick gels. Gels were stained using Coomassie Brillant Blue R-250 (1 mg/ml) in 25% methanol and 10% acetic acid and destained with the same solvent. Molecular weights of standard markers were 71, 50, 35, 25, 16, 8, and 2 kDa, respectively.
Antioxidant Activity in Linoleic Acid/FTC Model System
The antioxidant activity was determined according to the method of Saha et al.Citation[19] with some modifications. A 2.5-ml aliquot of PHH solutions (0.5–40 mg/ml, prepared in 50 mM phosphate buffer, pH 7.0) was added with 2.5 ml of ethanol (99.5%) and 32.5 μl of linoleic acid (99%), was sealed tightly with a screw cap, and was kept at 40°C in the dark. The same mixture without a sample was used as the control. The degree of oxidation was evaluated by using the ferric thiocyanate (FTC) method.Citation[20] An aliquot (0.1 ml) of the reaction mixture was mixed with 4.7 ml ethanol (75%) followed by the addition of 30% ammonium thiocyanate (0.1 ml) and 20 mM of ferrous chloride solution (0.1 ml) in 3.5% HCl. After 3 min, the degree of color development was measured at 500 nm (Model UV-2600A, UNICO, Shanghai, China). α-Tocopherol (TOH) and BHT were used as positive controls. TOH was diluted to 0.05, 0.1 mg/ml and BHT was diluted to 0.1 mg/ml for further uses.
DPPH Radical Scavenging Activity
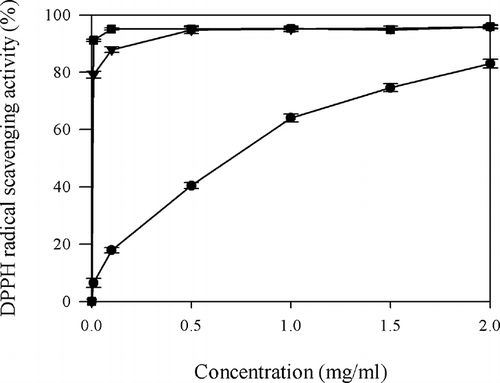
The DPPH radical scavenging activity was measured according to the procedure described by Shimada et al.Citation[21] PHH solutions (1.5 ml, 0.01–2 mg/ml) were prepared in distilled water and mixed with 1.5 ml of 1 mM DPPH ethanol solution. This mixture was shaken and kept at 25°C for 30 min, and then the absorbance of the mixture was measured at 517 nm. The EC50 value is the effective concentration at which DPPH radicals were scavenged by 50% and was obtained by interpolation from linear regression analysis. Ascorbic acid and BHA were used as positive controls and diluted to 0.01, 0.1, 0.5, 1, 1.5, and 2 mg/ml for further uses.
Superoxide Radical Scavenging Activity (SRSA)
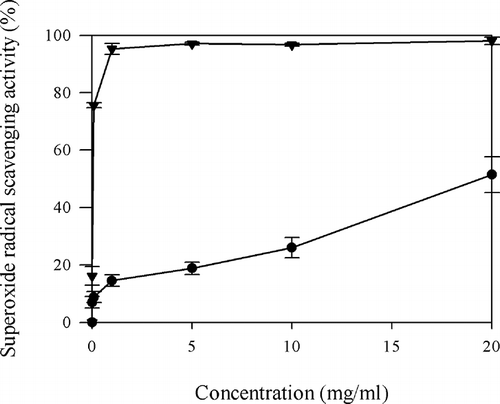
The SRSA was determined using a slightly modified method of Marklund et al.Citation[22] PHH solutions (0.3 ml, 0.01–20 mg/ml) prepared in distilled water and 2.61 ml of 50 mM phosphate buffer (pH 8.2) were added into freshly prepared 90 μl of 3 mM pyrogallol (dissolved in 10 mM HCl). The reaction mixture was added to 100 μl of 0.2 M ascorbic acid immediately after incubation for 4 min and the absorbance was measured at 325 nm. The EC50 value is the effective concentration at which superoxide radicals were scavenged by 50%. Ascorbic acid was used as the positive control compound and diluted to 0.01, 0.1, 1, 5, 10, and 20 mg/ml for further uses.
Hydroxyl Radical Scavenging Activity (HRSA)
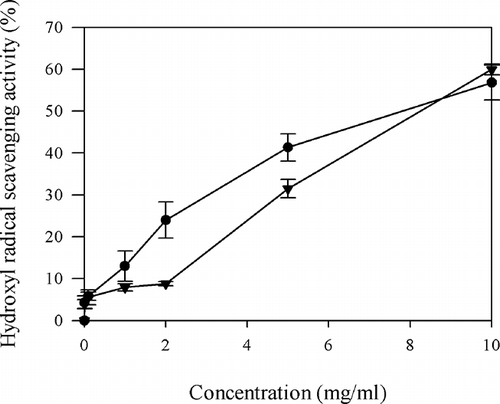
The HRSA was measured by the deoxyribose methodCitation[23] with some modifications. The reactions were performed in 0.1 M, phosphate buffer (pH 7.4), containing 10 mM deoxyribose, 10 mM FeSO4·7H2O, 10 mM EDTA, and PHH at different concentrations (0.01–10 mg/ml). The reaction was activated by adding H2O2 to a concentration of 10 mM and the reaction mixture was incubated for 1 h at 37°C in a water bath. After incubation, the color was developed by adding 1 ml of 1% thiobarbituric acid and 1 ml of 2.8% ice cold trichloroacetic acid (TCA) and heated at 100°C for 10 min. After cooling, the absorbance was measured at 532 nm. The EC50 value is the effective concentration at which hydroxyl radicals were scavenged by 50%. Ascorbic acid was used as the positive control compound and diluted to 0.01, 0.1, 1, 2, 5, and 10 mg/ml for further uses.
Reducing Power (RP)
The RP was measured by the method of OyaizuCitation[24] with some modifications. PHH solutions (1 ml, 0.01–20 mg/ml) were prepared in distilled water and mixed with 2.5 ml of 0.2 M, pH 6.6 sodium phosphate buffer, and potassium ferricyanide. A 1% mixture (2.5 ml) was incubated at 50°C for 20 min, and then the reaction mixture was acidified with 2.5 ml of TCA (10%) and centrifuged at 3000×g for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of ferric chloride (0.1%), and the absorbance was measured at 700 nm. The EC50 value is the effective concentration at which the absorbance was 0.5. Ascorbic acid was used as the positive control compound and diluted to 0.01, 0.1, 1, 5, 10, and 20 mg/ml for further uses.
Metal Chelating Activity (MCA)
The MCA was estimated by the method of Dinis et al.Citation[25] PHH solutions (1 ml, 0.1–5 mg/ml) were prepared in distilled water mixed with 3.7 ml of distilled water and 0.1 ml of 2 mM ferrous chloride. The reaction was initiated by the addition of 0.2 ml of 5 mM ferrozine. Then the mixture was shaken vigorously and left at room temperature for 10 min. The EC50 value is the effective concentration at which ferrous ions were chelated by 50%. The absorbance of the mixture was determined at 562 nm. EDTA was used as positive control and diluted to 0.01, 0.1, 0.5, 1, 2, and 5 mg/ml for further uses.
Synergistic Antioxidative Effects
PHH and TOH (2:1, 1:1, 1:2, and 1:4) were mixed at different weight ratios (total weight 0.5 mg) and applied to linoleic acid/FTC model system. Degree of lipid peroxidation at day 7 was measured according to the FTC method.
Statistical Analysis
Experimental results were mean ± standard deviation (SD) of three measurements. Analysis of variance was tested by the ANOVA procedure. The correlation was calculated by linear regression. A significant difference was considered at the level of P < 0.05 (SAS Institute, 1999).
RESULTS AND DISCUSSION
Molecular Weight Distribution
The SDS-PAGE pattern of porcine hemoglobin (PH) and PHH are presented in . With regard to the molecular weight standards, the three bands in the pattern of PH corresponded to three globin forms: tetra-, di-, and monomeric (67 kDa, 37 kDa, and 16 kDa, respectively). It is well documented that dietary protein rich in low molecular weight peptides will have a high nutritional value.[Citation26, Citation27] As shown in , the molecular weight of most peptides of PHH was lower than 2 kDa, implying that PHH could be of high nutritive value and can also be absorbed effectively by the organism. Further on, the peptic hydrolysates are able to cross the digestive epithelial barrier and reach the blood vessels, which allow them to reach peripheral organs and have beneficial effects for the organism.Citation[28]
Antioxidant Activity in Linoleic Acid/FTC Model System
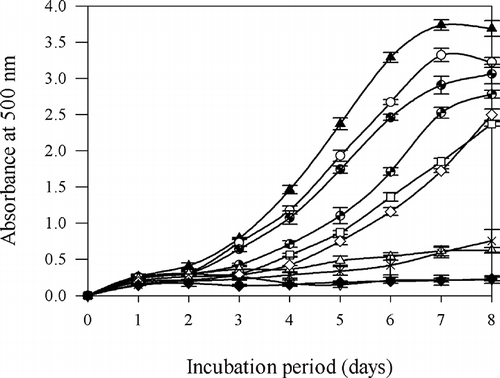
Several toxic by-products of LPO can damage biomolecules including DNA, although these biomolecules are distant to the site of their generation.Citation[29] The auto-oxidation of linoleic acid of control was accompanied by a rapid increase of peroxide value at day 1 of testing, reached maximum levels on day 7, and dropped on day 8. PHH (10–40 mg/ml) showed a significant concentration-dependent inhibition of LPO and strong inhibitory effects compared to the control (P < 0.05), and significantly prolonged the induction period of auto-oxidation of linoleic acid (). From the results, significant differences (P < 0.05) in inhibitory effects were found among PHH with a different concentration at day 7 of testing. The inhibition of linoleic acid peroxidation by PHH with a different concentration was significantly (P < 0.05) higher than that exhibited by 5 mg/ml of hemoglobin (11.16 ± 1.07%). The inhibitory effects of PHH at 10 and 20 mg/ml were equivalent to the TOH (0.05, 0.1 mg/ml) (P > 0.05), respectively. The inhibition of LPO by the addition of 40 mg/ml of PHH is equivalent to 0.05 mg/ml of BHT (P > 0.05) and significantly higher than the effect of 0.1 mg/ml of TOH (P < 0.01). Pepsin preferably digests peptide bonds by cleaving after the N-terminal of aromatic amino acids, such as phenylalanine and tryptophan, resulting in increased numbers of small protein chains containing independent aromatic amino acids at the N-terminal. The phenyl groups of the residues at peptide ends can be easily adsorbed or loosely localized at oil/water interface where oxidation takes places and thereby reduce radical-mediated LPO. Peptides from the hydrolysate of hoki skin gelatinCitation[30] also showed a strong inhibitory effect on LPO.
Figure 2 Antioxidative activity measured in a linoleic acid/FTC model system in the presence of PHH. Higher UV absorbance at 500 nm represents higher lipid peroxidation: (▴) control, (○) 5 mg/ml porcine hemoglobin, (![]()
DPPH Radical Scavenging Activity
The dose-dependent manner for DPPH scavenging activity of PHH was found in , which is similar to egg yolk protein hydrolysates.Citation[9] The dose-dependent fashion of the PHH suggested the presence of the same scavenging mechanism at different concentrations. From linear regression, the scavenging effect correlated well with PHH concentration (r = 0.96). The scavenging activity of PHH was 83.0 ± 1.52% at 2 mg/ml, which was nearly equal to that of 0.01 mg/ml ascorbic acid (91.1 ± 0.36%) and 0.1 mg/ml BHA (87.8 ± 0.94%). The PHH at 0.1 mg/ml exhibited 17.8 ± 0.93% scavenging activity, which was significantly lower than that of the same dose of ascorbic acid (95.1 ± 0.32%) and BHA (87.8 ± 0.94%) (P < 0.01). From the EC50 of PHH, it was seen that 0.95 ± 0.01 mg/ml, which was significantly higher (P < 0.05) than that of BHA (6.3 μg/ml) and ascorbic acid (5.5 μg/ml). This result demonstrated that PHH did show the hydrogen donating ability to act as antioxidants, thereby terminating the oxidation chain reaction of fat.
SRSA
Superoxide radical is known to be very harmful to cellular components as a precursor of the more reactive oxygen species, contributing to tissue damage and various diseases.Citation[31] It was found that the SRSA of the PHH increased with the increase of its concentrations (). The PHH at 1 mg/ml exhibited 14.5 ± 2.00% scavenging activity. This value was lower than that of the same dose of ascorbic acid (98.1 ± 1.28%). However, there is no significant difference between PHH at 1 mg/ml (14.5 ± 2.00%) and ascorbic acid at 0.01 mg/ml (16.1 ± 3.22%) (P > 0.05). EC50 value of PHH (20.34 ± 2.83 mg/ml) was significantly higher (P < 0.05) than the EC50 value obtained for ascorbic acid (0.06 mg/ml). This result implied that PHH is a superoxide scavenger and its capacity of superoxide scavenging may contribute to its antioxidant activity.
HRSA
Hydroxyl radical is the most reactive free radical and induces severe damage in DNA leading to carcinogenesis, mutagenesis, and cytotoxicity.[6] The PHH had excellent HRSA in a dose-dependent manner (). The HRSA of PHH increased from 0.01 to 10 mg/ml and was 24.0 ± 4.31% at the concentration of 2 mg/ml, which was significantly higher than that of the same dose of ascorbic acid (8.8 ± 0.48%) (P < 0.01). At 5 mg/ml of PHH, a HRSA of 41.3 ± 3.28% was obtained, which was significantly higher than that of the same dose of ascorbic acid (31.5 ± 2.20%) (P < 0.05). At the dose of 10 mg/ml, ascorbic acid showed higher HRSA than PHH, but no significant difference was found between ascorbic acid and PHH (P > 0.05). The EC50 value obtained for the PHH (7.90 ± 0.58 mg/ml) was not significantly different (P > 0.05) from that of ascorbic acid (8.36 ± 0.25 mg/ml). Therefore, the HRSA of PHH was equivalent to ascorbic acid. Hydrolysates from various protein sources, such as Alaska pollack frame[8] and conger eel muscleCitation[32] have also been reported to have strong HRSA.
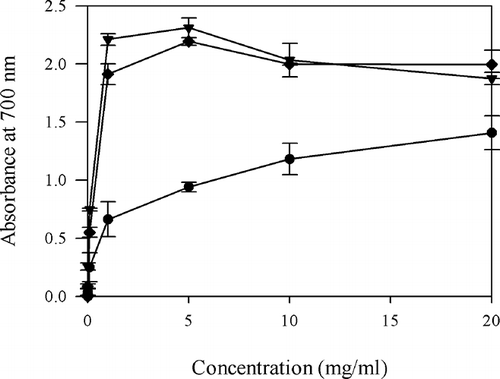
RP
Reducing power assay is often used to evaluate the ability of natural antioxidant to donate electron or hydrogen. shows the dose-response curves for the RP of PHH. Similar dose-dependent manner of RP has been reported for brown alga hydrolysates.[1] The RP of PHH increased from 0.04 ± 0.03 at 0.01 mg/ml to 1.41 ± 0.15 at 20 mg/ml. At a dosage of 20 mg/ml, the RP of ascorbic acid (1.87 ± 0.05) and BHT (2.00 ± 0.12) were significantly higher than PHH (P < 0.05). PHH showed high reducing values of 0.66 ± 0.15 at 1 mg/ml, almost equal to that of ascorbic acid (0.75 ± 0.01) and BHT (0.55 ± 0.04) at 0.1 mg/ml (P > 0.05). This indicated that the PHH could serve as electron donor and react with free radicals, converting them to more stable products and terminating the radical chain reaction, as described by Shimada et al.[21] The EC50 value for the PHH (3.02 ± 0.91 mg/ml), was significantly higher (P < 0.05) than the BHT and ascorbic acid, of which the EC50 values were 0.20 and 0.12 mg/ml.
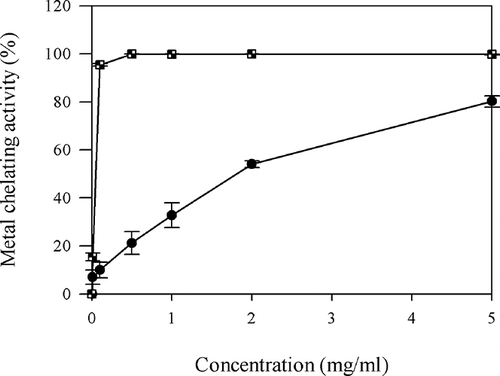
MCA
PHH demonstrated a strong MCA in a dose-dependent manner (). PHH chelated ferrous ions by 80.2 ± 2.33% at 5 mg/ml, whereas EDTA showed the excellent chelating ability of 95.4 ± 0.51% at 0.1 mg/ml. The MCA of PHH was 10.0 ± 3.25% at 0.1 mg/ml, which was very close to that of EDTA (15.4 ± 1.63%) at 0.01 mg/ml (P > 0.05). At the dosage of 5 mg/ml, EDTA showed significantly higher MCA (99.8 ± 0.15%) than PHH (P < 0.05). EC50 value of PHH (2.59 ± 0.05 mg/ml) was significantly higher than that of EDTA (0.05 mg/ml) (P < 0.05). Histidine-containing peptides have been reported to act as metal ion chelators.Citation[33] In fact, high content of histidine exists in PH.Citation[34] Therefore, the high MCA of PHH could be due to its high percentage of histidine residues. Saiga et al.Citation[35] also found that the porcine myofibrillar protein hydrolysates produced by actinase E showed strong MCA. It could be concluded that PHH possessed a similar metal chelating mechanism with porcine myofibrillar protein hydrolysates.
Synergistic Antioxidative Effect of PHH
The antioxidant synergism involving TOH and polypeptides has scarcely been studied. Synergistic antioxidant effect of PHH was assessed with natural antioxidant, TOH. As shown in , there was a positive antioxidant interaction between PHH and TOH in linoleic acid/FTC model system. Antioxidant activities were significantly (P < 0.05) higher in mixed weight ratios than that of TOH and PHH alone. It can be concluded that PHH exert synergistic antioxidant properties when mixed with non-peptidic antioxidant TOH.
Figure 8 Synergistic antioxidant effects of PHH. Total weight of PHH and TOH used in the linoleic acid/FTC system was maintained at 0.5 mg and different weight combinations were tested for the antioxidant activity. TOH and PHH were used as positive controls (0.2 mg/ml). Values with different letters are significantly different (P < 0.05).
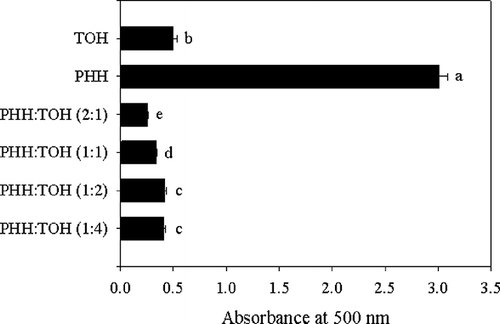
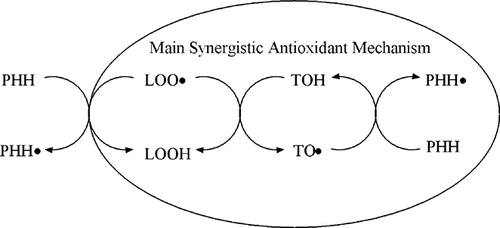
Although PHH (0.2 mg/ml) had very little antioxidant activity, the tested combinations showed strong synergistic effect with TOH. Among the four combinations, 2:1 weight ratio of PHH:TOH possessed the highest significant antioxidant activity (P < 0.05) compared to other tested weight ratios. Conversely, application of PHH at lower rates (1:2 and 1:4 of PHH:TOH) showed significantly lower antioxidant activities (P < 0.05) compared to combinations with high PHH rates (2:1 and 1:1 of PHH:TOH). Therefore, the antioxidant activity of PHH can be improved by introducing a lesser amount of TOH. Chen et al.Citation[36] also found that the relatively less active soybean hydrolysates showed strong synergistic antioxidant activity with different nonpeptidic antioxidants. The antioxidant synergism of PHH and TOH can be rationalized by the mechanism outlined in . PHH can react directly with LOO in the system. However, TOH, being the lipophilic antioxidant in this system, acting as the main antioxidant shall react first with the lipid peroxyl radical (LOO) and form a tocopheroloxyl radical (TO) in oxidant reaction chain. The latter can be reduced by the secondary antioxidant PHH to regenerate TOH, which was the main synergistic antioxidant mechanism, hence prolonging the inhibition period.
Evaluation on Mechanism of Antioxidant Action of PHH
The antioxidant activity of PHH may not be attributed to a single antioxidant mechanism. There are significant positive correlations between every two assays (). The highest correlation was observed between SRSA and FTC, which indicated that the ability of PHH to quench free radicals by abstracting hydrogen atoms seems to be directly related to the prevention of propagation of LPO. As similarly observed in many studies, antioxidants are chain-breakers of free radical-mediated LPO.[Citation37, Citation38] In addition, the high coefficients among MCA and the other four assays (FTC, DPPH, HRSA, and SRSA) showed that PHH was capable of chelating transition metals to suppress the initiation of radical formation. The antioxidant process of PHH seems to take place via combination of free radical scavenging and metal ions chelating. The coefficients among FTC and the other four assays (DPPH, HRSA, SRSA, and MCA) showed that free radical scavenging could be the main antioxidant mechanism of PHH. The antioxidant synergism of PHH also showed that PHH exert strong radicals scavenging activity by hydrogen donation ability. This mechanism of antioxidant action was similar to that of quercetin.Citation39 A high correlation between RP and other assays indicated that the single electron transfer mechanism is also important when involved in the antioxidant activity of PHH, which was in accordance with other reports.[Citation40, Citation41]
Table 1 Correlation coefficientsa among the different methods for quantifying antioxidant activity
CONCLUSIONS
This study has demonstrated that PHH was capable of inhibiting LPO, directly quenching free radicals to terminate the radical chain reaction, acting as reducing agents, and chelating transition metals to suppress the initiation of radical formation. DPPH, HRSA, SRSA, RP, and MCA were in a dose-dependent manner. PHH showed equivalent scavenging activity on hydroxyl radicals to ascorbic acid based on the EC50 values of antioxidant activities. Relatively less active PHH exhibited strong synergistic antioxidant activity with TOH. A 2:1 weight ratio of PHH:TOH possessed the highest significant antioxidant activity (P < 0.05) compared to other tested weight ratios. The main synergistic antioxidant mechanism could be due to the regeneration of TOH by PHH. The correlation analysis among the different methods for quantifying antioxidant activity and antioxidant synergism indicated that radicals scavenging mechanism, more than metal chelation, could play a major role in the antioxidant process of PHH. PHH may have undergone gastric digestion and possessed potential antioxidant activity in vivo when orally administered. Therefore, PHH would be expected to protect against oxidative damage in living systems in relation to aging and carcinogenesisin and could be used as a promising antioxidant for application in functional foods and medicine industries.
ACKNOWLEDGMENTS
This work was supported financially by the National Science Technology Minister of China (award number: 2006BAD05A16) and Department of Science and Technology of Zhejiang Province of China (award number: 2006C12082).
REFERENCES
- Athukorala , Y. , Kim , K.N. and Jeon , Y.J. 2006 . Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava . Food and Chemical Toxicology , 44 : 1065 – 1074 .
- Manso , M.A. , Miguel , M. , Even , J. , Hernández , R , Aleixandre , A and López-Fandińo , R. 2008 . Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats . Food Chemistry , 109 : 361 – 367 .
- Herken , E.N. , Erel , O. , Guzel , S. , Celik , H. and Ibanoglu , S. 2009 . Total antioxidant, phenolic compounds, and total oxidant status of certified and uncertified Turkey's honeys . International Journal of Food Properties , 12 : 461 – 468 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum Melongena L.) cultivars . International Journal of Food Properties , 12 : 616 – 624 .
- ButterField , D.A. , Castegna , A. , Pocernich , C.B. , Drake , J. , Scapagnini , G. and Calabrese , V. 2002 . Nutritional approaches to combat oxidative stress in Alzheimer's diseases . Journal of Nutritional Biochemistry , 13 : 444 – 461 .
- Je , J.Y. , Qian , Z.J. , Byun , H.G. and Kim , S.K. 2007 . Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis . Process Biochemistry , 42 : 840 – 846 .
- Thiansilakul , Y. , Benjakul , S. and Shahidi , F. 2007 . Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme . Journal of Food Biochemistry , 31 : 266 – 287 .
- Je , J.Y. , Park , P.J. and Kim , S.K. 2005 . Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate . Food Research International , 38 : 45 – 50 .
- Sakanaka , S. and Tachibana , Y. 2006 . Active oxygen scavenging activity of egg-yolk protein hydrolysates and their effects on lipid oxidation in beef and tuna homogenates . Food Chemistry , 95 : 243 – 249 .
- Jung , W.K. , Rajapakse , N. and Kim , S.K. 2005 . Antioxidative activity of low molecular peptide derived from the sauce of fermented blue mussel, Mytilus edulis . European Food Research and Technology , 220 : 535 – 539 .
- Klompong , V. , Benjakul , S. , Kantachote , D. and Shahidi , F. 2007 . Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type . Food Chemistry , 102 : 1317 – 1327 .
- Nyberg , F. , Sanderson , K. and Glämsta , E. 1997 . The hemorphins: A new class of opioid peptides derived from the blood protein haemoglobin . Biopolymers , 43 : 147 – 156 .
- Lignot , B. , Froidevaux , R. , Nedjar-Arroume , N. and Guillochon , D. 1999 . Solvent effect on kinetics of appearance of neokyotorphin VVh4 and a bradykinin-potentiating peptide in the course of peptic hydrolysis of bovine haemoglobin . Biotechnology and Applied Biochemistry , 30 : 201 – 207 .
- Nedjar-Arroume , N. , Dubois-Delval , V. , Miloudi , K. , Daoud , R. , Krier , F. , Kouach , M. , Briand , G. and Guillochon , D. 2006 . Isolation and characterization of four antibacterial peptides from bovine hemoglobin . Peptides , 27 : 2082 – 2089 .
- Yu , Y.K. , Hu , J.E. , Miyaguchi , Y. , Bai , X.F. , Du , Y.G. and Lin , B.C. 2006 . Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides derived from porcine hemoglobin . Peptides , 27 : 2950 – 2956 .
- Chang , C.Y. , Wu , K.C. and Chiang , S.H. 2007 . Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates . Food Chemistry , 100 : 1537 – 1543 .
- Association of Official Analytical Chemists (AOAC) . 2005 . Official Methods of Analysis , 16th , Washington, DC : AOAC .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Saha , K. , Lajis , N.H. , Israf , D.A. , Hamzah , A.S. , Khozirah , S. , Khamis , S. and Syahida , A. 2004 . Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants . Journal of Ethnopharmacology , 92 : 263 – 267 .
- Mitsuda , H. , Yasumoto , K. and Iwami , K. 1996 . Antioxidative action of indole compounds during the autoxidation of linoleic acid . Journal of Japanese Society of Nutrition and Food Science , 19 : 210 – 214 .
- Shimada , K. , Fujikawa , K. , Yahara , K. and Nakamura , T. 1992 . Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion . Journal of Agricultural and Food Chemistry , 40 : 945 – 948 .
- Marklund , S. and Marklund , G. 1974 . Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase . European Journal of Biochemistry , 47 : 469 – 474 .
- Siddhuraju , P. and Becker , K. 2007 . The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts . Food Chemistry , 101 : 10 – 19 .
- Oyaizu , M. 1986 . Studies on product of browning reaction prepared from glucose amine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Dinis , T.C.P. , Madeira , V.M.C. and Almeida , L.M. 1994 . Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Archives of Biochemistry and Biophysics , 315 : 161 – 169 .
- Surówka , K. , Surówka , J. and Surówka , J. 2004 . Enzymatic modification of extruded soy protein concentrate as a method of obtaining new functional food components . Trends in Food Science and Technology , 15 : 153 – 160 .
- Lee , K.A. and Kim , S.H. 2005 . SSGE and DEE, new peptides isolated from a soy protein hydrolysate that inhibit platelet aggregation . Food Chemistry , 90 : 389 – 393 .
- Megías , C. , Pedroche , J. , Yust , M.M. , Girón-Calle , J. , Alaiz , M. , Millán , F. and Vioque , J. 2008 . Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin . LWT—Food Science and Technology , 41 : 1973 – 1977 .
- Box , H.C. and Maccubbin , A.E. 1997 . Lipid peroxidation and DNA damage . Nutrition , 13 : 920 – 921 .
- Mendis , E. , Rajapakse , N. and Kim , S.K. 2005 . Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate . Journal of Agricultural and Food Chemistry , 53 : 581 – 587 .
- Halliwell , B. and Gutteridge , J.M.C. 1999 . Free Radicals in Biology and Medicine , Oxford University Press : Oxford .
- Ranathunga , S. , Rajapakse , N. and Kim , S.K. 2006 . Purification and characterization of antioxidative peptide derived from muscle of Conger eel (Conger myriaster) . European Food Research and Technology , 222 : 310 – 315 .
- Chen , H.M. , Muramoto , K. , Yamauchi , F. , Fujimoto , K. and Nokihara , K. 1998 . Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein . Journal of Agricultural and Food Chemistry , 46 : 49 – 53 .
- Piot , J.M. , Guillochon , D. , Leconte , D. and Thomas , D. 1988 . Application of ultrafiltration to the preparation of defined hydrolysates of bovine haemoglobin . Journal of Chemical Technology and Biotechnology , 42 : 147 – 156 .
- Saiga , A. , Tanabe , S. and Nishimura , T. 2003 . Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment . Journal of Agricultural and Food Chemistry , 51 : 366 – 367 .
- Chen , H.M. , Muramoto , K. , Yamauchi , F. and Nokihara , K. 1996 . Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein . Journal of Agricultural and Food Chemistry , 44 : 2619 – 2623 .
- Rajapakse , N. , Mendis , E. , Jung , W.K. , Je , J.Y. and Kim , S.K. 2005 . Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties . Food Research International , 38 : 175 – 182 .
- Sharififar , F. , Dehghn-Nudeh , G. and Mirtajaldini , M. 2009 . Major flavonoids with antioxidant activity from Teucrium polium L . Food Chemistry , 112 : 885 – 888 .
- Lebeau , J. , Furman , C. , Bernier , J.L. , Duriez , P. , Teissier , E. and Cotelle , N. 2000 . Antioxidant properties of di-tert-butylhydroxylated flavonoids . Free Radical Biology and Medicine , 29 : 900 – 912 .
- Chung , Y.C. , Chen , S.J. , Hsu , C.K. , Chang , C.T. and Chou , S.T. 2005 . Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther . Food Chemistry , 91 : 419 – 424 .
- Kanatt , S.R. , Chander , R. and Sharma , A. 2007 . Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat . Food Chemistry , 100 : 451 – 458 .