Abstract
Essential oil was extracted from Flos Sophorae Immaturus (FSI) by simultaneous distilling-extraction (SDE) and vapor-distillation extraction (VDE), gave a yield of 2 and 1.2 mg/g, based on GC/MS analysis, 44 and 29 components were identified in SDE and VDE extracts, respectively. Essential oil extracted by SDE was active against common foodborne pathogens, however, it did not show any activity against fungus. The minimal lethal concentration (MLC) was 0.39 μL/mL using the spread-plate method. The antibacterial activity might be due to phenols, such as eugenol in the essential oil. For essential oil-treated Staphylococcus aureus, pretreatment with potassium sorbate, sodium benzoate, or the essential oil markedly lowered bacterial count (p < 0.05), suggesting that FSI essential oil might be used in food preservation to reduce the concentration of chemical preservatives.
INTRODUCTION
Flos Sophorae, or “Japanese Pagodatree Flower-bud,” is the flower of the plant Sophora japonica L. of the legume family (Fabaceae). The fresh flowers are edible and have a fresh, elegant, slightly sweet and agreeable taste. The dry flower buds have been used as a medicine in the name of Flos Sophorae Immaturus (FSI).The book, Shennong's Classic of Materia Medica, which was the oldest traditional Chinese medicine written before BC 202 years, has recorded that FSI has the functions of cooling blood, cleansing the liver, stopping bleeding, and relieving inflammation. Its main ethanol- and water-soluble compositions have been reported to be genistin, genistein, rutin, kaempferol, and quercetin.[Citation1] Some researchers in China have analyzed the oil-soluble composition, i.e., essential oil of FSI,[Citation2,Citation3] and found that it is different from the ethanol- and water-soluble compositions. However, so far there are negligible reports about the function of FSI essential oil. Studies have demonstrated that essential oils from certain plants, such as lilac, have an antibacterial activity.[Citation4,Citation5] Some natural plant extracts have been shown to possess antimicrobial activity holding great potential against resistant microorganisms, such as tea, coffee, and herbs.[Citation6,Citation7] Flos Sophorae blossoms in May, a season when the temperature begins to rise and airborne microorganisms accelerate their propagation. So, it was hypothesized that Flos Sophorae blossoming at this time should have an antibacterial function. In this study, the FSI essential oil composition was analyzed and its antimicrobial effects were investigated. Furthermore, taking a practical approach, the feasibility of applying FSI essential oil as a food preservative in combination with routine sterilization measures was studied. The purpose was to explore its potential use as a natural product—FSI essential oil—to replace or to use in combination with certain routine hurdle factors in food processing and preservation to achieve a better antimicrobial effect and improved food safety.
MATERIALS AND METHODS
Materials and Equipment
Flos Sophorae Immaturus was produced in Jiangsu province and was purchased from the local market. Bacterial strains were provided by Wuxi Municipal Center for Disease Control and Prevention. Baird-Parker agar medium and potassium Tellurite Egg-Yolk Emulsion (reagent grade) were from Beijing Luqiao Tech Co., Ltd. (Beijing, China) All the other biochemical reagents used were Reagent Grade (Sinoreagent Group Chemical Reagent Co., Ltd, Shanghai, China). GC-MS instrument was Finnigan Trace (Thermo. Electron Corp., Waltham, MA, USA).
Extraction of Essence Oil from FSI
When the vapor-distillation extraction (VDE) method was used,[Citation8] from 50 g FSI, ∼800 mL distillate was obtained. Twice extraction of the distillate with anhydrous ethyl ether (each 400 mL) yielded 800 mL ethyl ether solution. To this solution 60 g anhydrous sodium sulphate was added and the solution was dried overnight. After removing the desiccant, the ethyl ether solution was concentrated into ∼5 mL and further purged with nitrogen to ∼1 mL, which was a light yellow-green volatile oil and was used directly in GC-MS analysis.
The simultaneous distillation extraction (SDE) method was also used to extract FSI essential oil.[Citation9] A microscale simultaneous distillation–extraction apparatus (Chrompack, Middelburg, Netherlands) was used. An amount of 1 g of FSI and 100 mL of internal standard were extracted for 2 h using dichloromethane as solvent, and the extract was concentrated with nitrogen. The obtained essential oil was used in GC-MS analysis and also used for antimicrobial assays after dissolving in acetone.
Analysis of Chemical Composition of FSI Essential Oil
The analysis was performed according to Yang et al.[Citation8] Each sample (10 mL) was put into a 20 mL vessel and sealed. The head space gas from the vessel was auto-injected by a HP7694E Headspace sampler into the HP6890 plus (Agilent Technologies Inc., Santa Clara, CA, USA) gas chromatography. The operating conditions were as follows: GC: Chromatogram column: HP-5, 30 m × 0.25 mm × 0.25 um; Detector temperature: 220°C; Split: 3:1; Flow rate: 0.8 mL/min; Column temperature: 80°C for 1 min and raised to 220°C at a rate of 10°C/min and then kept 10 min; Carrier gas: helium. Warm-up conditions: 60°C, 20 min. MS: ion: EI+; electron energy: 70 eV; joint temperature: 250°C; detector pressure: 350 V; Carrier gas: helium. The total amount of flavor detected by GC was expressed as the total peak area.
Antibacterial Activity of FSI Essential Oil
The agar diffusion test was used to determine the diameter of antibacterial circle.[Citation10] To each hole with a diameter of 2 mm, 8 μL of essential oil was added. The test was performed in triplicate. Acetone (8 μL) and 200,000 units/mL penicillin (8 μL) were used as negative and positive control, respectively. The diameter of the antibacterial circle was measured and the average of the three tests was calculated.
Minimum Lethal Concentration (MLC) of FSI Essential Oil
Single colonies of the selected strains of bacteria were pre-inoculated into 10 ml of sterile nutrient broth (Sinopharm Chemical Reagent Co., Ltd., Shanghai, P. R. China) at 37°C for 8 h, and then 1 mL of culture was added to 100 mL of culture broth at 37°C for 24 h. The culture was diluted to ∼106 cfu/mL and 0.1 mL of this culture was mixed into 0.9 mL nutrient broth with FSI essential oil. The FSI essential oil was two-fold serial diluted by acetone and the final concentration in nutrient broth was from 25.0 to 0.05 μL/mL. Following incubation of the tubes at 37°C for 24 h, 0.1 mL medium from the last tubes in which the microorganism exhibits growth and all tubes showing no growth (no turbidity), were plated on a nutrient agar using the spread-plate method. The plates were prepared in triplicate. MLC is defined as the lowest concentration of essential oil that produces ≥99.9% killing of the test microorganism.[Citation11] Acetone and penicillin were used as negative and positive control, respectively.
Effect of FSI Essential Oil Concentration and Treatment Time on the Rate of Bacterial Inhibition
Each 0.9 mL bacteria (∼105 cfu/mL) mixed with 0.1 mL, 20, 10, or 5 μL/mL FSI essential oil (the cosolvent was acetone) to control the concentration of FSI essential oil in culture medium with 2.0, 1.0, 0.5 μL/mL, and then cultured. After different periods of time, samples were taken and surviving bacteria were counted (by culture for 24 h; all in triplicate). The co-solvent acetone (0.1 mL) was inoculated with 0.9 mL bacteria (∼105 cfu/mL) and run in parallel as the 0% inhibition control. The rate of bacterial inhibition was calculated according to the following formula: Rate of bacterial inhibition (%) = [(control colony number – sample colony number)/control colony number] × 100%.
Calculation of Stressed Staphylococcus aureus Cells
When subjected to environmental stress, microorganisms can bear varying degrees of sub-lethal dose of physiological and structural changes; the phenomenon is known as stressed.[Citation12] The most prominent feature of stressed bacterial cells is that they are unable to form colonies on selective medium but are able to recover and grow on non-selective medium.[Citation12,Citation13] In this study, different stresses were applied to Staphylococcus aureus, and then the number of stressed cells was quantified by the difference in colony numbers on Baird-Parker selective medium and nutrient broth non-selective medium. The calculation was based on a modification of formula used by the former researchers which is as follows[Citation14]:
Preparation of Stressed Staphylococcus aureus Cells
A single colony was picked from culture slant and inoculated into 25 mL fresh nutrient broth medium, which was shaker-cultured at 37°C for 24 h to obtain the seed liquid. The seed liquid was inoculated into fresh nutrient broth medium in volume ratio of 1:100 and cultured at 37°C for 8 h to obtain bacterial culture at the log growth phase. The bacterial cells were collected by refrigerated centrifugation (3000 g, 30 min, 4°C), washed with sterile saline and collected again by centrifugation for three times. The final bacteria pellet was partly kept as pellet and partly diluted with sterile saline to ∼1010 cfu/mL. They were used in the following steps as specified.
Heat stress treatment: A 15-mL bacterial suspension of ∼1010 cfu/mL were placed into 60°C water bath for heat treatment of 20, 40, 60, 80, 100, and 120 min.
Acid stress treatment: The pH of a 15-mL bacterial suspension sample of ∼1010 cfu/mL was adjusted with 0.1 mol/L citric acid solution (sterilized) to 2.5 and the sample was then placed into 37°C water bath for 20, 40, 60, 80, 100, and 120 min.
Osmosis stress treatment: A 180 g/L NaCl solution was prepared, sterilized, and then used to dilute bacterial cells in pellet to ∼1010 cfu/mL (15 mL). The bacterial suspension was placed into 37°C water bath for 20, 40, 60, 80, 100, and 120 min.
Sodium benzoate stress treatment: A series of sodium benzoate solution of concentration 50.0, 25.0, 12.5, 6.2, 3.1, and 1.6 g/L (∼pH 5.0) were prepared and used to dilute bacterial cells in pellet to ∼1010 cfu/mL (2 mL each). The bacterial suspensions were placed into 37°C water bath for 2 h.
Potassium sorbate stress treatment: A series of potassium sorbate solution of concentration of 50.0, 25.0, 12.5, 6.2, 3.1, and 1.6 g/L (∼pH 5.0) were prepared and used to dilute bacterial cells in pellet to ∼1010 cfu/mL (2 mL each). The bacterial suspensions were placed into 37°C water bath for 2 h.
Treatment with FSI essential oil stress: A series of FSI essential oil acetone solutions of 8 ×, 4 ×, 2 ×, 1 ×, 0.5 ×, 0.25 × MLC were prepared (2 mL each) and used to dilute bacterial cells in pellet to ∼1010 cfu/mL. The bacterial suspensions were placed into 37°C water bath for 2 h.
After each stress treatment, bacterial suspension was taken and serially diluted to be cultured on both nutrient agar plates and Baird-Parker plates. After 37°C/24 h culture for nutrient agar plates and 37°C/24 h culture for Baird-Parker plates, bacterial colonies were counted and Log Nd value of stressed cells was calculated. The plates were in duplicates.
Further Treatment of Stressed Cells with FSI Essential Oil
For each stress treatment described above, as the bacterial cells were used for counting, 1 mL of bacterial suspensions (∼105 cfu/mL) were taken and into which FSI essential oil was added to reach a final concentration of 2.0 or 4.0 μL/mL (acetone as co-solvent), which corresponding to 1 × MLC, 2 × MLC, respectively. After 1 h treatment, 0.1 mL suspension was taken from each sample and spread onto a nutrient agar plate (done in duplicates). The colonies were counted after 37°C/24 h culture and taken as the number of surviving viable cells (Ns, cfu/mL).
For each type of stress pre-treatment, a curve was plotted using logNd value as the X axis and Ns as the Y axis, which presented the relation between the bacterial count after further treatment with FSI essential oil and the degree of stress in various pretreatment. The correlation coefficient r and significance level p were calculated with the software SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Extraction and Composition Analysis of FSI Essential Oil
FSI essential oil was separately obtained by two methods; simultaneous distillation extraction (SDE) and vapor-distillation extraction (VDE), which produced markedly different yields, 2 and 1.2 mg/g, respectively. Essential oil samples obtained by the two methods were both analyzed with GC-MS (GC spectra not shown). The components identified and their contents are presented in .
Table 1 Comparison of the chemical composition of essential oil of Flos Sophorae Immaturus extracted by simultaneous -distilling extraction (SDE) or vapor-distillation extraction (VDE)
As shown in , the main composition of FSI essential oil obtained by both methods were 5-methyl-2-furancarboxaldehyde, hexanoic acid, benzeneacetaldehyde, guaiacol, 4-vinyl-guaiacol, eugenol, trans-caryophyllene, trans-β-ionone, caryophyllene oxide, 8-heptadecene, tetrohydro-4,4,7a-trimethyl-2(4H)-benzofuranone, 6,10,14-trimethyl-2-pentadecanone, and hexadecanoic acid. The number of the compounds obtained by VDE was far fewer than that by SDE, and the two methods also differ greatly in compound composition. VDE appeared to be more suitable for analyzing neutral composition with relatively low boiling point, while SDE obtained a broader range of neutral essential oil composition. The difference might be due to the different solvent, temperature and pressure used by the two methods.
The composition and content of FSI essential oil in this study differed from a report by Chen et al.,[Citation3] where using a microwave-assisted extraction method and ethanol as the extractant the main composition were 1,9,12-trioxy-4,6-diaminocyclotetradecane-5-thion (20.58%), hexadecanoic acid (9.05%), 9,12,15-octadecatrien (7.05%), 9,12-octadecadienoic acid (6.85%), and β-sitosterol (6.11%), etc. The discrepancy could be explained by the differences in extraction method and in polarity of extractant. Jia et al. also used the SDE method to extract FSI produced in Zhengzhou,[Citation2] and found the volatile oil mainly 6,10,14-trimethyl-2-pentadecanone, 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol, 2-aminobenzoic acid methyl ester, which also differed significantly from the current study where FSI produced in Jiangsu province was used. This suggests that geographic origin of the raw material could also result in composition difference in FSI essential oil. The cultivation of Flos Sophorae has not yet involved specific breeding and breed improvement. Wild Flos Sophorae evolved through the times and its essential oil composition diversity could be shaped by local climate, soil, and surroundings. Therefore, the authors suggest that it is necessary to conduct a survey on the composition of FSI essential oil grown in different parts of the country and to provide comprehensive data for developing the national Flos Sophorae resource.
As shown in , FSI essential oil contains eugenol (6–7% for both methods), which has been demonstrated to have antibacterial activity.[Citation4] The MIC of the standard eugenol was very low (0.0976–0.3906 μL/mL) against Salmonella spp. in pork, feces, sewage, pen floor, and water.[Citation15] Therefore, the interest of the authors was to evaluate the antimicrobial activity of FSI essential oil. Since essential oil obtained by the two extraction methods had similar eugenol content but SDE has higher overall yield, simpler procedures and uses less amount of organic solvent than VDE, therefore, considering the potential application of FSI essential oil in industry, the essential oil used in further experiments was extracted with SDE method. There are some compounds with a strong smell, such as 2-heptenal, 2-pentyl-furan, and 2,6-nonadienal, however, the total amount is small; for example, heptenal 0.51%, 2-pentyl-furan 0.59%, 2,6-nonadienal 0.37%, respectively. They could not affect the smell in large degree and the sensory evaluation also verified it.
Antimicrobial Activity of FSI Essential Oil
As shown in , FSI essential oil exhibited strong inhibition against most of the bacteria tested except Aspergillus niger ATCC 16404 and Candida albicans ATCC 10231, which are fungus, indicating that FSI essential oil had antibacterial activity. From the MLC data, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes were most sensitive to FSI essential oil.
Table 2 Antibacterial activity of essential oil of Flos Sophorae Immaturus
The MLC of essential oil from FSI was 0.39–1.56 μL/mL against Escherichia coli ATCC 8099, Staphylococcus aureus ATCC 6538, Listeria monocytogenes ATCC 19116, Listeria welshimeri ATCC 35897, Salmonella Typhimurium CMCC 50013, and Shigella dysenteriae CMCC 51334. The concentration of 2 μL/mL of essential oils from lemongrass, cinnamon, or geraniol was enough to inactivate Salmonella enteritidis, E. coli, and L. innocua in apple and pear juices.[Citation16] Our result showed the MLC of essential oil from FSI was at the same level with the tested pathogens. And cinnamon has been used as a preservative in the food industry. So, based on the same level of MLC, it showed the potential application of FSI essential oil as a preservative. It was found to be valuable to further study the characteristic of antibacterial activity.
Influence of FSI Essential Oil Concentration and Treatment Time on Antibacterial Activity
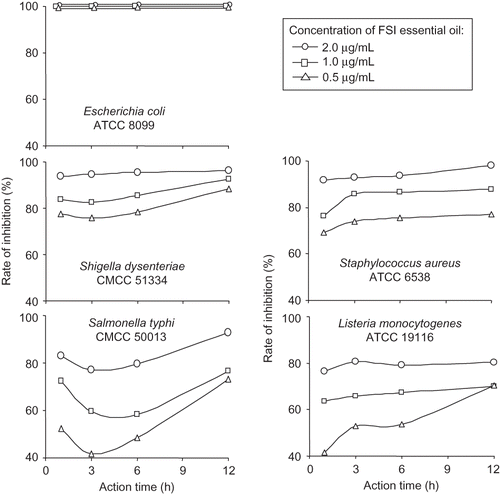
Based on the above data, several sensitive bacterial strains from were selected as target strains to evaluate the influence of FSI essential oil concentration and treatment time on the rate of bacterial inhibition (). At the concentration of 2.0, 1.0, and 0.5 μL/mL (∼4 ×, 2 ×, and 1 × MLC), the inhibition for E. coli was always near 100%; for other bacteria, the growth inhibition deepened as FSI essential oil concentration and treatment time increased, but at even the highest concentration (2.0 μL/mL), after 12 h treatment, 100% inhibition had not been achieved. Therefore, in practical application of FSI essential oil, its concentration should be chosen according to the target pathogen and treatment time has to be ensured to achieve bacterial inhibition. For example, to inhibit the E. coli, 0.5 μL/mL of FSI essential oil is enough; but to inhibit Listeria monocytogenes would require >2.0 μL/mL of FSI essential oil.
Synergetic Effect with Other Antibacterial Factors
According to the Methods sections of Preparation of Stressed Staphylococcus aureus Cells and Further Treatment of Stressed Cells with FSI Essential Oil, Staphylococcus aureus cells were first treated by varies stresses and then further treated with FSI essential oil, and the correlation between the number of surviving cells and stress pretreatment was summarized in . Pretreatment with heat or acid stress correlated poorly with the number of surviving cells. In contrast, sodium benzoate and potassium sorbate stress pretreatment had strong negative correlation with surviving cell number; in particular, the correlation was very strong for the combination of potassium sorbate pretreatment and 2 MLC essential oil further treatment (r = −0.941, p < 0.01). The same level of correlation (p < 0.01) was found for pretreatment and further treatment both with FSI essential oil. The good negative correlation in antibacterial activity between FSI essential oil and potassium sorbate and sodium benzoate indicated that FSI essential oil might replace or be used in combination with potassium sorbate and sodium benzoate to effectively reduce the dose of chemical preservatives.
Table 3 Correlation between different stresses pretreatment before treatment with essential oil of Flos Sophorae Immaturus (FSI) and the number of surviving Staphylococcus aureus.
Huang[Citation17] systematically studied the relationship between molecular structure and antibacterial activity for dozens of substances with antibacterial activity, and found that the α,β-unsaturated carbonyl group of sorbic acid and benzoic acid and their potassium salts is the effective functional structure responsible for the antibacterial activity. The same structure was found in eugenol (6.71%) and guaiacol (0.636%) in FSI essential oil. Therefore, they might be contributing to the antibacterial activity of FSI essential oil.
It is worth noting that osmosis stress pretreatment before FSI essential oil treatment correlated positively with surviving bacterial cell number, in particular for the 2 MLC FSI essential oil group (r = 0.814, p < 0.05). This data indicated that the more bacterial cells stressed by osmosis, the more difficult it is for FSI essential oil to influence the cells. In this experiment, the osmosis stress and the following FSI essential oil treatment were both controlled in the range of stressing but not killing bacteria. High concentration of salt mainly causes osmotic pressure and water migration out of the cells, leading to swelling of cell wall and loosening of the network of cell wall interconnections, but these changes do not cause complete inactivation of the cells. The phenols in FSI essential oil such as eugenol (6.71%) are known to inhibit bacteria through interaction with membranes of the cell wall,[Citation5] therefore, it is possible that when cell wall has been loosened, some effective compositions of FSI essential oil go directly through the big openings in cell wall and do not have time to interact with cell wall membrane. When FSI essential oil and other extracellular substances go into the cells, they buffer the osmotic pressure and help to maintain the intracellular balance, and in a degree corrected cell injury due to osmotic pressure. Therefore, in food processing, FSI essential oil might have the function of repairing osmotic pressure caused cell injury.
CONCLUSIONS
For the first time, this study demonstrated that Flos Sophorae Immaturus essential oil has good antibacterial activity. Its composition is relatively complex and its content of effective antibacterial composition is not very high, such as eugenol (6.71%) and other phenols. Our results indicate that in food processing FSI essential oil might be adopted to replace potassium sorbate or sodium benzoate or be used together with them as hurdle factor to control food spoilage or prolong the shelf life of food. In practical application, other accessory measures, such as adjusting CO2 concentration and package design, can be used in combination to enhance the potential use of FSI essential oil. Most important, FSI essential oil smelled fresh, elegant, and slightly sweet, not like the other plant essential oils with a strong smell, such as cinnamon and lilac. So, it has more potential application because it could not affect the origin flavor of applied food.
ACKNOWLEDGMENTS
The authors wish to thank Wuxi Centre for Disease Prevention and Control and Zhangjiagang Entry-Exit Inspection and Quarantine Bureau for supplying the foodborne pathogens. This article is supported by 111 project-B07029 and PCSIRT0627, the Fundamental Research Funds for the Central Universities No. JUSRP 21124, 30908 and 31106, National Key Technology R & D Program in the 11th Five Year Plan of China, No. 2009BADB9B04.
REFERENCES
- Chu , Q. , Wu , T. , Fu , L. and Ye , J. 2004 . Determination and comparison of phytoestrogens in both crude and parched Flos Sophorae Immaturus by capillary electrophoresis with electrochemical detection . Microchimica Acta , 148 : 311 – 315 .
- Jia , C.X. , Sun , X.L. , Mao , D.B. and Niu , J.P. 2004 . Analysis of chemical constituents in essential oil of Sophora japonica L. flower in Zhengzhou city area . Journal of Zhengzhou Institute of Light Industry (Natural Science) , 19 ( 2 ) : 15 – 18 .
- Chen , D.W. , Li , J.H. and Wang , F. 2006 . Determination of volatile components in Flos Sophorae immaturus by GC-MS . West China Journal of Pharmaceutical Sciences , 21 ( 5 ) : 450 – 451 .
- Nychas , G.J.E. , Skandamis , P.N. and Tassou , C.C. 2003 . “ Antimicrobials from herbs and spices ” . In Natural Antimicrobials for the Minimal Processing of Food , Edited by: Roller , Sibel . 176 – 200 . Cambridge : Woodhead Publishing Limited .
- Vigil , A.L.M. , Palou , E. and Alzamora , S.M. 2005 . “ Naturally occurring compounds-plant source ” . In Antimicrobials in Food , Edited by: Davidson , P.M. , Sofos , J.N. and Branen , A.L. 429 – 452 . Boca Raton : Taylor & Francis Group LLC .
- Arora , D.S. , Kaur , G.J. and Kaur , H. 2009 . Antibacterial activity of tea and coffee: Their extracts and preparations . International Journal of Food Properties , 12 ( 2 ) : 286 – 294 .
- Sagun , E , Durmaz , H. , Tarakci , Z. and Sagdic , O. 2006 . Antibacterial activities of the extracts of some herbs used in Turkish herby cheese against Listeria monocytogenes serovars . International Journal of Food Properties , 9 ( 2 ) : 255 – 260 .
- Yang , Z.P. , Yao , W.R. and Qian , H. 2006 . Studies on vapor phase extraction of rose oil enhanced by β-glucosidase . Flavour and Fragrance Journal , 21 ( 5 ) : 776 – 782 .
- Consuelo, Díaz-Maroto , M. , Soledad , Pérez-Coello M. and Dolores , C.M. 2002 . Supercritical carbon dioxide extraction of volatiles from spices: Comparison with simultaneous distillation-extraction . Journal of Chromatography A , 947 : 23 – 29 .
- Rauha , J.P. , Remes , S. , Heinonen , M. , Hopia , A. , Kähkönen , M. , Kujala , T. , Pihlaja , K. , Vuorela , H. and Vourela , P. 2000 . Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds . International Journal of Food Microbiology , 56 : 3 – 12 .
- NCCLS . 2002 . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard , 6th , Wayne, PA : National Committee for Clinical Laboratory Standards .
- McFeters , G.A. 1990 . “ Enumeration, occurrence, and significance of injured indicator bacteria in drinking water ” . In Drinking Water Microbiology: Progress and Recent Developments , Edited by: Gordon , A. 479 – 492 . New York : Springer-Verlag New York, Inc .
- Jay , J.M. , Loessner , M.J. and Golden , D.A. 2005 . Modern Food Microbiology , 7th , 215 New York : Springer Science Business Media, Inc .
- Agranovski , V. , Ristovski , Z. , Hargreaves , M. , Blackall , P.J. and Morawska , L. 2003 . Performance evaluation of the UVAPS: Influence of physiological age of airborne bacteria and bacterial stress . Journal of Aerosol Science , 34 : 1711 – 1727 .
- Simasatikul , N. , Boonruangphisan , P. , Pichpol , D. , Gatphayak , K. , Padungtod , P. , Pirintra , P. , Yamsakul , P. , Vearasilp , T. and Meulen , U.T. October 7–9 2008 . Antibacterial activity of standard Eugenol against Salmonella spp. Tropentag 2008: Competition for Resources in a Changing World: New Drive for Rural Development October 7–9 , Hohenheim, , Germany
- Raybaudi-Massilia , R.M. , Mosqueda-Melgar , J. and MartÃn-Belloso , O. 2006 . Antimicrobial activity of essential oils onSalmonella enteritidis, Escherichia coli, and Listeria innocua in fruit juices . Journal of Food Protection , 69 ( 7 ) : 1579 – 1586 .
- Huang , Z.L. 2002 . Studies on the synthesis and anti-microbial activity of sorbic acid derivatives , Ph.D Thesis, South China University of Technology . DOI: CNKI: CDMD:1.2005.107493