Abstract
The bioactive compounds of 16 tomato cultivars, grown in the same field for 2 years (2003 and 2004) were investigated. Lycopene (5.7–26.3 mg kg−1) was the predominant carotenoid, while β-carotene (2.1–11.2 mg kg−1) and a small amount of lutein (0.02–0.49 mg kg−1) were found in all tomato cultivars. Kagome 77, the richest source of total carotenoids and L-ascorbic acid, also showed the highest antioxidant activity. β-Carotene and lutein contents of tomatoes differed between 2 years statistically. Antioxidant activities of tomatoes were found between 48 and 118 μmol TEAC 100 g−1. A significant correlation was only observed between L-ascorbic acid (2.2–13.8 mg 100 g−1) and antioxidant activity using TEAC assay.
INTRODUCTION
Fruits and vegetables contain biologically active components, which have an important impact on health and disease reduction. In tomato fruit, the main bioactive compounds are carotenoids, phenolics, and L-ascorbic acid; these compounds act as antioxidants having a protective effect against various forms of cancer and cardiovascular diseases.[Citation1] Tomato is one of the significant parts of the human diet and is also an abundant source of bioactive compounds.[Citation2] Studies have shown that high consumption of tomato is correlated with a reduced risk of some types of cancer[Citation3,Citation4] and heart disease.[Citation5] These defensive roles have been mainly attributed to the carotenoid compounds. Carotenoid compounds are reported to be mainly responsible for the prevention of these diseases. Numerous epidemiological studies have shown that lycopene intake, amount or serum lycopene, had an inverse association with the risk of prostate, pancreas, and possibly stomach cancers.[Citation3,Citation6] Lycopene, responsible for the characteristic red colour of ripe tomato and tomato products, has the ability to function as an antioxidant and to quench singlet oxygen in vitro.[Citation7] Tomatoes also contain the other carotenoids, phytofluen, β-carotene, neurosporene, lutein, and zeaxanthin, which play an important role in the human body's defence system. In addition to this, it is thought that lutein and zeaxanthin mainly prevent age-related macular degenaration and cataracts.[Citation8]
Tomato also contains L-ascorbic acid and phenolic compounds as natural antioxidants. Ascorbic acid is known to be an oxygen scavenger and phenolic antioxidants act as electron or hydrogen donors and quench electron mobility of free radical chain reactions.[Citation9] Significant correlation was also reported between antioxidant capacity and total phenolics and ascorbic acid in date palm fruits.[Citation10] They found a significant correlation between the antioxidant activity and total phenolic content (r = 0.595) and ascorbic acid (r = 0.385) at the unripe stage, but not at the ripe stage. It was reported that the phenolics were the major contributor for the antioxidant activity. In this study, authors wanted to show which bioactive compound contributed to the antioxidant activity of the tomato. The main objective of this study was to determine the carotenoids, total phenolics, and L-ascorbic acid content of 16 tomato varieties grown in the same field for two consecutive years. The antioxidant activity of tomatoes was also assayed to evaluate the relationship between bioactive compounds and antioxidant activity.
MATERIALS AND METHODS
Materials
Tomato cultivars (Alta, H-9888, H-9663, H-9478, H-9780, H-9776, Kagome 932, Kagome 77, Nema Krimson, Primopack, Red Gold, TT 105, UG 902, UG 1802, UG 5602, Unirex) were obtained from a company (Tat Tohumculuk A.Ş., Bursa) in Turkey. In September 2003 and 2004, tomatoes were harvested at the ripe stage. Tomatoes were randomly selected for analyses and all the analyses were performed in three replicates and completed within 15 days.
Chemicals
Lycopene, β-carotene, lutein, ABTS (3-ethylbenzothiazoline-6-sulfonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl), and trolox (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid) were obtained from Sigma Aldrich, Inc. (Saint Louis, MO, USA). Catechin was supplied by Fluka Chemie (Buchs, Switzerland). HPLC grade solvents, butylatedhydroxytoluene (BHT), L-ascorbic acid, Folin-Cioceltau reagent, and the other chemicals were purchased from Merck (Darmstadt, Germany). Ethanol was supplied from Riedel-de Haën (Seelze, Germany).
Methods
Analysis of carotenoids
One gram of CaCO3 was added to 10 g of tomato puree and homogenized with 25 mL methanol for 2 min at 11,000 rpm. Then, 25 mL of acetone:hexane (1:1) solution containing 0.1% BHT was added and homogenized for another 2 min. The resulting suspension was filtered through a Buchner funnel and the pellet was reextracted two times with the acetone:hexane solution. All filtrates were put in a separatory funnel and water was used for the phase separation.[Citation11] After discarding the water, the hexane phase was evaporated under nitrogen and stored at −26°C.
Determination of carotenoids was performed on a Zorbax ODS column (5 μm, 250 × 4.6 mm i.d.) (Agilent Technologies, Santa Clara, CA, USA) preceded by a Zorbax ODS guard column (5 μm, 12.5 × 4.6 mm i.d.) (Agilent Technologies) according to a modified method of Hart and Scott.[Citation12] Waters 486 UV-VIS detector (Millipore Co., Milford, MA, USA) set at 470 nm and Waters 510 HPLC pump (Millipore Co.) were used. An isocratic mobile phase composed of acetonitril:dichlorometan:methanol (70:20:10) was used at a flow rate of 1 mL min−1. The injection volume was 20 μL. Identification of carotenoids was carried out by comparison of the HPLC retention times with corresponding standards of lycopene, β-carotene, and lutein.
Analysis of tatal phenolics
A modified method was performed for the extraction of phenolics.[Citation13] One g of tomato puree was extracted three times with 10 mL of 70% (v/v) aqueous methanol using a homogenizer at 11,000 rpm for 1 min. After centrifuging the slurry at 4000 rpm for 15 min, combined supernatants were evaporated to dryness at 45°C under vacuum. The extracts were dissolved in 25 mL methanol and then stored at −26°C after filtration until analysis.
The content of total phenolics was determined using a modified Folin-Cioceltau (FC) colorimetric method.[Citation14] 0.5 mL of extract was mixed with 7 mL of distilled water in a test tube followed by the addition of 0.5 mL of FC reagent and allowed to stand for 3 min. After that, 2 mL of 20% sodium carbonate was added and mixed well. Absorbance of the resultant solution was read at 720 nm using UV-VIS spectrophotometer (Unicam, Cambridge, England) after 1 h standing in a water bath (Polyscience, Niles, IL, USA) at 25°C. The total phenolics content was expressed as mg catechin equivalents per 100 g.
Analysis of L-ascorbic acid
Ten g of tomato puree was mixed with 50 mL 2% metaphosphoric acid and transferred to a conical flask. After mechanical shaking for 15 min, the mixture was filtered through a Buchner funnel with Whatman no. 1 paper. The volume was made up to 50 mL with 2% metaphosphoric acid and stored at −26°C until analysis.[Citation2] Identification of L-ascorbic acid was carried out on a Zorbax ODS column preceded by a Zorbax ODS guard column (Agilent Technologies) according to the method of Sanchez-Mata et al.[Citation15] A Waters 510 HPLC pump (Millipore Co.) was used and Waters 486 UV-VIS detector (Millipore Co.) was set at 245 nm. An isocratic mobile phase consisting of 0.05 M KH2PO4 was used at a flow rate of 1 mL min−1. L-ascorbic acid was identified by comparing the retention time with those of authentic standards and also by spiking the samples with the standard.
Extraction of Antioxidants
A modified method of Dewanto et al.[Citation16] was performed for the extraction of antioxidants. Twenty-five grams of tomato puree was blended with 80% acetone (1:2 w/v) for 3 min using a Waring blender (Torrington, CT, USA). The sample was homogenized for another 3 min and the slurry was filtered through Whatman no. 1 paper. The pellet was homogenized with another 25 mL of 80% acetone and filtered. The filtrates were collected and the acetone was evaporated at 45°C. Tomato extracts were made to the volume of 10 mL with 80% acetone and stored at −26°C.
TEAC assay
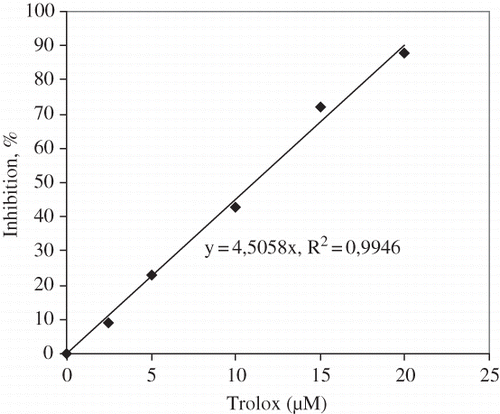
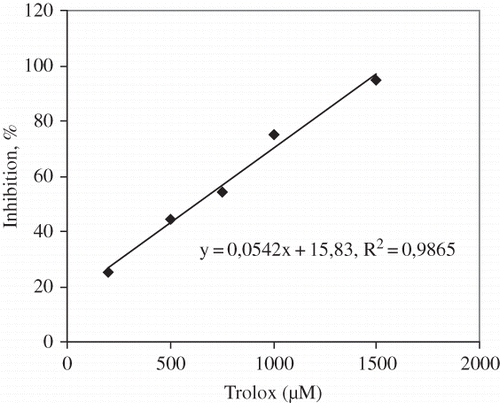
The antioxidant activity was estimated by the trolox equivalent antioxidant activity (TEAC) method.[Citation17] Five to 15 μL of tomato extract was added to 1 mL of diluted ABTS*+ solution, the mixture was vortexed, and the absorbance was read after 1 min. The disappearance of ABTS*+ was determined by measuring the decrease of absorbance at 734 nm for 6 min. The percentage inhibition of absorbance is calculated and plotted as a function of concentration. Results were analyzed by reference to the trolox () and expressed as μM trolox equivalent antioxidant capacity (μM Trolox 100 g−1 fresh weight).
DPPH assay
A modified method was used to detect the DPPH radical scavenging activity.[Citation18] The stock solution of DPPH was prepared by dissolving 25 mg DPPH with 100 mL methanol. Fifty μL of tomato extract was added to 2450 μL of DPPH solution. The decrease in absorbance was measured at 517 nm using a UV-VIS spectrophotometer after 30 min. A control was treated with 50 μL of methanol instead of the extract. The antioxidant activity was determined on the basis of percent quenching of DPPH radical and expressed as the percent decrease in the absorbance of the DPPH radical solution using the following equation:
The results were analyzed by reference to trolox () and antioxidant activities of tomato cultivars were expressed as μM trolox equivalent per 100 g fresh weight (μM TE 100 g−1).
Statistical analysis
All data were subjected to analysis of variance (repeated measurement ANOVA) by using the SPSS (version 9.05, Chicago, IL, USA) software programme. Significant differences between sample means were assessed by Duncan's multiple-comparison test at p < 0.05. Linear correlation coefficients were calculated by using MINITAB (version 14.0, State College, PA, USA).
RESULTS AND DISCUSSION
The carotenoid contents of 16 tomato cultivars are represented in . Lycopene was the main carotenoid found in tomatoes obtained in 2003 and 2004 averaging 16.4 mg kg−1 and 18.9 mg kg−1, respectively. β-Carotene and a small amount of lutein were also determined in all tomato cultivars. β-Carotene ranged from 2.1 to 7.1 mg kg−1 in 2003, whereas in 2004 it varied between 2.7 and 11.2 mg kg−1. Kaur et al.[Citation19] reported lycopene content in the pulp of the seven tomato cultivars between 0.04 and 6.73 mg 100 g−1 during ripening from green to red ripe stage. The mean lutein content of tomatoes in 2003 and 2004 was 0.13 mg kg−1 and 0.21 mg kg−1, respectively. Contents of lycopene and β-carotene in tomatoes were similar with previous data.[Citation20,Citation21] However, lutein content of tomatoes was lower than the data (0.8–2.1 kg−1) reported in the literature.[Citation10,Citation20,Citation22]
Table 1 Carotenoid profile of 16 tomato cultivars harvested in 2003 and 2004 (mg/kg).Footnote a, Footnote b
In 2003, the tomato cultivar with the highest lycopene (24.4 mg kg−1) and β-carotene (7.1 mg kg−1) contents was Kagome 77. The highest lutein content was found in UG 902 and was different from all the cultivars except for Red Gold and UG 1802 (p < 0.05). In 2004, the highest lycopene and β-carotene contents were determined to be in H 9888 (26.3 mg kg−1) and Kagome 77 (11.2 mg kg−1), respectively. The lutein content of UG 1802 (0.49 mg kg−1) was found to be significantly high compared with the other cultivars except for UG 902 (p < 0.05), while the poorest source of lutein was H 9663 (0.07 mg kg−1). Since the variation in lycopene and total carotenoid contents among tomato cultivars harvested in 2004 was found to be the same as the variation determined in 2003, the differences in those values of tomato cultivars between 2 years did not evaluate statistically. On the other hand, in the year 2004, the decrease in the β-carotene content of Alta and the increase in β-carotene contents of the other cultivars were found statistically important (p < 0.05) when compared to the results obtained in 2003. It was also observed that the lutein contents of most of the cultivars significantly increased in 2004 (p < 0.05) when compared to the results obtained in the year 2003.
The content of L-ascorbic acid varied between 2.2 and 7.4 mg 100 g−1 in tomatoes in the year 2003 (). The highest L-ascorbic acid content was found in Kagome 77 and was statistically significant (p < 0.05) from that of all the other cultivars except for Alta and UG 902. In the year 2004, L-ascorbic acid ranged from 2.7 to 13.8 mg 100 g−1. The richest source of L-ascorbic acid was Kagome 77, followed by Nema Krimson and H 9888. The L-ascorbic acid content of Kagome 77 was significantly different from that of all the cultivars except for Nema Krimson (p < 0.05). H 9478 and TT 105 with the lowest L-ascorbic acid contents did differ from all the cultivars apart from H 9780 (p < 0.05). No significant variation was observed in L-ascorbic acid contents of Alta, H 9780, H 9478, Red Gold, TT 105, and UG 902 between 2 years, whereas the amounts of L-ascorbic acid of the other cultivars increased significantly in 2004 (p < 0.05) (). The data of the present study for L-ascorbic acid content of tomatoes were lower than the range of 8.8–25 mg 100 g−1 reported in previous studies.[Citation23,Citation24]
Table 2 L-ascorbic acid, total phenolics content, and antioxidant activity of 16 tomato cultivars harvested in 2003 and 2004.Footnote a, Footnote b
The average concentrations of total phenolics in tomatoes (as mg catechin 100 g−1) harvested in 2003 and 2004 were 49.4 and 35.3, respectively (). The lowest total phenolics content was found in H 9478, followed by TT 105 and H 9663 in 2003. The total phenolics content of Unirex (81.9 mg catechin 100 g−1) was remarkably high compared to that of other cultivars in 2004. The total phenolics determined in 2004 for H 9663, H 9478, Red Gold, and Unirex were significantly higher than those of 2003. In this study, total phenolics content of tomato cultivars harvested in both years varied within a wide range (18.8–81.9 mg catechin 100 g−1). In the literature, total phenolics content of the tomato was found to be 404 mg gallic acid 100 g−1 DW,[Citation24] 34–38 mg gallic acid 100 g−1,[Citation25] and 355–439 mg gallic acid 100 g−1 DW.[Citation23]
The antioxidant activities of tomato cultivars are shown in . The TEAC values of tomatoes were in the range of 48–118 μmol TEAC 100 g−1. The sample with the highest antioxidant activity was Kagome 77 followed by Unirex and H 9780. Apart from H 9780 and Unirex, Kagome 77 antioxidant activity was significantly different from the other cultivars (p < 0.05). H 9478 was determined as the cultivar having the lowest TEAC value. The TEAC values of tomato cultivars in 2004 varied within a range of 57–118 μmol TEAC 100 g−1. The tomato cultivar with the highest antioxidant activity was Kagome 932, followed by H 9888. These two tomato cultivars did differ from the other tomatoes (p < 0.05). In 2004, the antioxidant activities of Alta, H 9780, Kagome 77, Primopack, and Unirex showed a significant decrease, whereas the antioxidant activities of H 9478, and Kagome 932 significantly increased (p < 0.05). In this study, the antioxidant activities of tomato cultivars were in agreement with the reported values of 1400–2730 μmol 100 g−1 DW,[Citation22,Citation24] but were higher than the data (5.4–20.9 μmol TEAC 100 g−1) determined by Zhou and Yu.[Citation26]
The mean value of antioxidant activities of tomatoes determined by DPPH assay was 52 μmol TE 100 g−1 in 2003 (). UG 1802 and UG 5602 had the lowest antioxidant activity and they were significantly different from the other samples except for Alta and Primopack (p < 0.05). In 2003, Kagome 77 and UG 902 showed the highest antioxidant activities. In 2004, the antioxidant activities of tomatoes were found in the range of 42–57 μmol TE 100 g−1. The antioxidant activity of UG 1802 was extremely low compared to the other cultivars and was significantly different from all the cultivars except for Kagome 77, TT 105, and UG 902 (p < 0.05). In 2004, the antioxidant activities of Kagome 77, TT 105, and UG 902 significantly decreased, whereas Alta and UG 5602 antioxidant activities showed a significant increase (p < 0.05). The variation in the antioxidant activity of the other cultivars between 2 years was not significant (p > 0.05). The data in the literature related to DPPH radical scavenging activity of tomatoes are limited; however, Chang et al.[Citation25] reported the DPPH radical scavenging activity of tomato cultivars between 97 and 98%. In this study, the inhibition effect of tomatoes on DPPH radical was found to be in the range of 72–94%, which was expressed as 42–58 μmol TE per 100 g.
CONCLUSION
The observed differences of bioactive compounds of tomato cultivars between 2 years are probably caused by seasonal and growing conditions. In order to observe the relationship between bioactive compounds and the antioxidant activity of tomatoes, correlation coefficients were calculated. No significant correlation between antioxidant activity and carotenoids or total phenolics was observed. On the other hand, L-ascorbic acid showed low but significant correlation with the antioxidant activity determined by only TEAC assay (r = 0.50–0.56, p < 0.05). Moreover, the correlation between two radical scavenging assays was found to be extremely low. This result can be attributed to having different radical scavenging capacities of bioactive compounds at different conditions.
ACKNOWLEDGMENTS
This work was supported by research Grant 2003-0711077 from Scientific Research Projects at Ankara University. The authors also wish to thank Tat Tohumculuk A.Ş. for providing the tomato cultivars and Dr. Özgur Koşkan for his assistance with statistical analysis.
REFERENCES
- Kopsell , D.A. and Kopsell , DE. 2006 . Accumulation and bioavailability of dietary carotenoids in vegetable crops . Trends in Plant Science , 11 : 499 – 507 .
- Abushita , A.A. , Hebshi , E.A. , Dadood , H.G. and Biacs , P.A. 1997 . Determination of antioxidant vitamins in tomatoes . Food Chemistry , 60 : 207 – 212 .
- Chan , J.M. , Gann , P.H. and Giovannucci , E.L. 2005 . Role of diet in prostate cancer development and progression . Journal of Clinical Oncology , 23 : 8152 – 8160 .
- Rao , A.V. and Ali , A. 2007 . Biologically active phytochemicals in human health: Lycopene . International Journal of Food Properties , 10 : 279 – 288 .
- Rao , A.V. and Agarwal , S. 1999 . Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases . Nutrition Research. , 19 : 305 – 323 .
- Perera , C.O. and Yen , G.M. 2007 . Functional properties of carotenoids in human health . International Journal of Food Properties 2007 , 10 : 201 – 230 .
- DiMascio , P. , Murphy , M.C. and Sies , H. 1991 . Antioxidant defense systems, the role of caroteoid, tocopherol and thiols . American Journal of Clinical Nutrition , 53 : 194 – 200 .
- Mares-Perlman , J.A. , Fisher , A.I. , Klein , R. , Patla , M. , Block , G. , Millen , A.E. and Wright , J.D. 2001 . Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey . American Journal of Epidemiology , 153 : 424 – 432 .
- Blokhina , O. , Virolainen , V. and Fagerstedt , K.V. 2003 . Antioxidants, oxidative damage and oxygen deprivation stress: A review . Annals of Botany , 91 : 179 – 194 .
- Allaith , A.A.A. 2008 . Antioxidant activity of Bahraini date palm (Phoenix dactylifera L.) . fruit of various cultivars. International Journal of Food Science and Technology , 43 : 1033 – 1040 .
- Ferruzzi , M.G. , Sander , L.C. , Rock , C.L. and Schwartz , S.J. 1998 . Carotenoid determination in biological microsamples using liquid chromatography with a coulometric electrochemical array detector . Analytical Biochemistry , 256 : 74 – 81 .
- Hart , D.J. and Scott , K.J. 1995 . Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK . Food Chemistry , 54 : 101 – 110 .
- Shahidi , F. , Chavan , U.D. , Naczk , M. and Amarowicz , R. 2001 . Nutrient distribution and phenolic antioxidants in air-classified fractions of beach pea (Lathyrus maritimu L.) . Journal of Agricultural Food Chemistry , 49 : 926 – 933 .
- Tanner , H. and Brunner , H.R. 1979 . Gentranke-Analytik , 206 Germany : Verlag Heller Chemie und Verwaltungesellschaft mbH .
- Sanchez-Mata , M.C. , Camara-Hurtado , M. , Diez-Marques , C. and Torija-Isasa , M.E. 2000 . Comparison of high-performance liquid chromatography and spectrofluorimetry for vitamin C analysis of green beans (Phaseolus vulgaris L.) . European Food Research and Technology , 210 : 220 – 225 .
- Dewanto , V. , Wu , X. , Adom , K.K. and Liu , R.H. 2002 . Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity . Journal of Agricultural Food Chemistry , 50 : 3010 – 3014 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Zhang , D. and Hamauzu , Y. 2004 . Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking . Food Chemistry , 88 : 503 – 509 .
- Kaur , D. , Sharma , R. , Wani , A.A. , Gill , B.S. and Sogi , D.S. 2006 . Physicochemical changes in seven tomato (Lycopersicon esculentum) cultivars during ripening . International Journal of Food Properties , 9 : 747 – 757 .
- Müller , H. 1997 . Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection . European Food Research and Technology , 204 : 88 – 94 .
- Abushita , A.A. , Dadood , H.G. and Biacs , P.A. 2000 . Change in carotenoids and antioxidant vitamins in tomato as a fuction of varietial and technological factors . Journal of Agriculture and Food Chemistry , 48 : 2075 – 2081 .
- Khachik , F. , Goli , M.B. , Beecher , G.R. , Holden , J. , Lusby , W.R. , Tenorio , M.D. and Barrera , M.R. 1992 . Effect of food preparation on qualitative and quantitative distribuition of major carotenoid constituents of tomatoes and seceral green vegetables . Journal of Agriculture and Food Chemistry , 40 : 390 – 398 .
- Sahlin , E. , Savage , G.P. and Lister , C.E. 2004 . Investigation of the antioxidant properties of tomatoes after processing . Journal of Food Composition and Analysis , 17 : 635 – 647 .
- Toor , R. and Savage , G.P. 2005 . Effect of semi-drying on the antioxidant components of tomatoes . Food Chemistry , 94 : 90 – 97 .
- Chang , C.-H. , Lin , H.-Y. , Chang , C.-Y. and Liu , Y.-C. 2006 . Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes . Journal of Food Engineering , 77 : 478 – 485 .
- Zhou , K. and Yu , L. 2006 . Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado . Swiss Society of Food Science and Technology , 39 : 1155 – 1162 .