Abstract
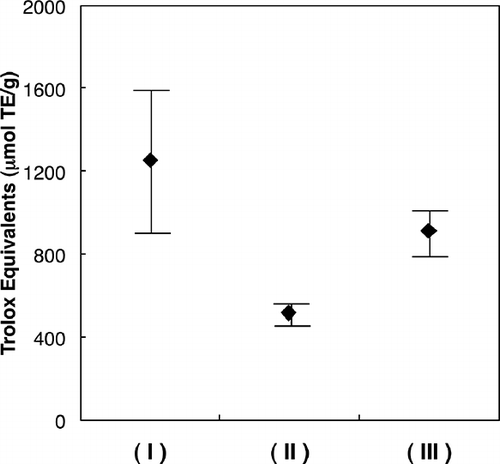
The volatile oils from the Japanese wild edible plants of Diplazium squamigerum, Laportea macrostachya, and Vitis coignetiae were investigated by capillary gas chromatography and gas chromatography-mass spectrometry. The major components of D. squamigerum oil were linalool (28.7%), palmitic acid (13.9%), and α-terpineol (5.5%); of L. macrostachya oil were palmitic acid (14.1%), nonanal (9.2%), and linoleic acid (8.9%); and of V. coignetiae oil were nonanal (13.2%), geraniol (11.6%), and phenylacetaldehyde (8.5%). These oils were assayed to determine their antioxidant activity by the oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. The oxygen radical absorbance capacity values varied from 1255 ± 393 trolox equivalents (μmol TE/g) for D. squamigerum oil, from 514 ± 65 μmol TE/g for L. macrostachya oil, and from 911 ± 118 μmol TE/g for V. coignetiae oil. The difference in the antioxidant activities among D. squamigerum oil, L. macrostachya oil, and V. coignetiae oil were attributed to their different monoterpene alcohol contents and composition in the samples. These data provided evidence that the volatile oil from Japanese edible plants is a good dietary source of antioxidants.
INTRODUCTION
The role of free radicals in many disease conditions has been well established. Several biochemical reactions in the human body generate reactive oxygen species and these are capable of damaging crucial bio-molecules. If they are not effectively scavenged by cellular constituents, they lead to disease conditions. The harmful action of the free radicals can be blocked by antioxidant substances, which scavenge the free radicals and detoxify the organism. Several plant extracts and different classes of phytochemicals have been shown to have antioxidant activity. The search for newer natural antioxidants, especially of plant origin, is increasing recently.Citation[1]
Japan is blessed naturally. The insular country of Japan is a long archipelago stretching from north to south and it is a complex topography and is influenced by ocean currents. In addition, it has four seasons. Because of this, there are a number of edible wild plants that vary widely in odor, figure, color, size, and constituent in Japan. These edible wild plants have been used in a wide range of native ingredients in Japan, for they have a characteristic flavor different from the vegetable. Therefore, many species of the edible wild plants have been used as traditional Japanese food. In addition, the edible wild plants were found to have high antioxidant activities.Citation[2] They have often been cultivated in recent years. Consequently, they are regarded as familiar and healthy food. However, there were no detailed reports on the volatile components. The authors have investigated components and characteristic odors of the volatile oil from the edible wild plants.Citation3–6
The edible wild plant, Diplazium squamigerum (Kiyotakishida, Akakogomi in Japanese), is a perennial of the family Dryopteridaceae. D. squamigerum is native to moist and shadowy areas. In Japan, this plant is found in the northern areas. In particular, this plant stem is red. It has been eaten as tempura and, when boiled, it has a distinctive aroma that is generally described as fresh, floral, and green. Because D. squamigerum is a relatively unique, edible wild plant, little investigation on its chemical composition was performed earlier.
Laportea macrostachya (Miyamairakusa, Aiko in Japanese) belongs to the family of Urticaceae. It is a perennial and grows wild in the mountains and fields of the northern area of Japan. Due to its characteristic and unusual flavor, it is used as an ingredient in Japanese cuisine, for example in the Tenpura and soy soup. In addition, it contains high levels of vitamins, minerals, and amino acid,Citation[7,Citation8] a reason why in recent years cultivation of the species has been started. Consequently, it is regarded as a familiar and healthy food. However, there is no report of the volatile components of L. macrostachya.
Vitis coignetiae (Yamabudou in Japanese) is a vine of the family Vitaceae. V. coignetiae is one of the well-known edible wild plants, and grows wild in the mountains and fields in sunny areas of Japan. In general, part of the fruit of V. coignetiae is eaten, but the young leaf and stems are eaten as the edible wild plant. This plant has a fresh-green and fruity odor. It has been eaten boiled with salt. In previous studies, the fruit, skin, and seed of V. coignetiae showed antioxidant activity and radical scavenging activities.Citation9–13 However, there is no report of the aerial part of the volatile components of V. coignetiae.
With increasing interest in the function and diversity of antioxidants in foods, several in vitro rapid methods for measuring antioxidant activity of food, beverages, and biological samples have been developed.Citation[14] Among them, the oxygen radical absorbance capacity (ORAC) assay has gained much attention because it deals with peroxyl radicals, the most abundant radicals in biological systems. The ORAC assay considers both the inhibition time and the inhibition degree (as the reaction goes to completion) and directly estimates the chain-breaking antioxidant activity.Citation[15] Recently, the ORAC assay has been improved by using fluorescein (ORAC-FL) instead of β-phycoerythrin as the fluorescent probe.Citation[16,Citation17] This article describes the chemical composition of volatile oils from edible wild plants collected in northern Japan and determines the range of variability of the oxygen radical scavenging activity of edible wild plants by the ORAC-FL.
MATERIALS AND METHODS
Plant Material
Aerial parts of Diplazium squamigerumand Laportea macrostachya were harvested at the Mountain of Yamagata, Japan, on April 2009. The aerial part of Vitis coignetiae was harvested at the Mountain of Hukushima, Japan, on March 2009. A voucher specimen of each plant was deposited at the biotechnology laboratory of Kinki University of Osaka, Japan (Diplazium squamigerum, WP-006; Laportea macrostachya, WP-004; Vitis coignetiae, WP-026).
Chemicals
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2′-azinobis (2-amidinopropane) dihydrochloride (AAPH), fluorescein sodium salt were purchased from Sigma Aldrich (Tokyo, Japan). β-Cyclodextrin was obtained from Cyclochem (Hyogo, Japan).
Extraction of the Volatile Oil
Fresh plant materials (1 kg whole aerial parts) from all individual samples were subjected to steam distillation for 2 h using a Likens-Nickerson-type apparatus (Osaka Rikou, Osaka, Japan). The obtained volatile oils were dried over anhydrous sodium sulphate and diethylether dilution of each one was used for gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS) measurements. The yields of oils were as follows: Diplazium squamigerum, 10 mg/1 kg fresh sample (0.0010%); Laportea macrostachya, 144 mg/kg fresh sample (0.0144%); and Vitis coignetiae, 99 mg/kg fresh sample (0.0099%).
Gas Chromatography (GC)
GC was carried out with an Agilent Technologies 6890 chromatograph (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with flame ionization detector on a capillary column (HP-5, 30 m × 0.25 mm i.d., film thickness of 0.25 mm). The column temperature was programmed from 40°C to 260°C at 4°C/min and held at 260°C for 5 min. The injector and detector temperature were 270°C and 280°C. The flow rate of the carrier gas (helium) was 1.8 ml/min. Peak areas were quantified using a computer integrator.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS was carried out with an Agilent Technologies 6890-Agilent Technologies 5973N. The GC condition was equipped on two capillary columns (HP-5MS, 30 m × 0.25 mm i.d., film thickness of 0.25 mm and DB-WAX, 15 m × 0.25 mm i.d., film thickness of 0.25 mm). On HP-5MS, the column temperature was programmed from 40°C to 260°C at 4°C/min and held at 260°C for 5 min. On DB-WAX, the column temperature was programmed from 40°C to 240°C at 4°C/min and held at 240°C for 5 min. The injector and detector temperature were 270°C and 280°C. The flow rate of the carrier gas (helium) was 1.8 ml/min with the actual temperature in the MS source reaching approximately 230°C and the ionization voltage 70 eV. Acquisition mass range was 39–450 amu.
Identification of Components
Identification of the individual components was based on (1) comparison of their GC-MS retention indices on apolar and polar columns determined relative to the retention times of a series of n-alkanes (C8–C29) with those of authentic compounds or literature date,Citation[3 – Citation6] and (2) computer matching with commercial mass spectral librariesCitation[18] and comparison of spectra with literature date.Citation[19]
ORAC Assay
The ORAC method used with fluorescein (FL) as the “fluorescent probe” was described in the literature method.Citation20–22 The automated ORAC assay was carried out on a MTP-800AFC (Corona Electric Co., Ltd., Hitachinaka, Japan) with fluorescence filters for an excitation wavelength of 480 nm and emission wavelength of 540 nm. The measurements were made in a plate with 96 black flat-bottom wells (Greiner Bio-One, Frickenhausen, Germany). The reaction was performed at 37°C as the reaction was started by thermal decomposition of AAPH in 75 mM phosphate buffer (pH 7.4) because of the sensitivity of FL to pH. The fluorescein stock solution was made in 75 mM phosphate buffer (pH 7.4) and stored under dark conditions at 4°C for four weeks. AAPH and trolox solutions in 75 mM phosphate buffer (pH 7.4) were prepared daily. Samples were dissolved in 100% acetone and then diluted in a solution of 7% β-cyclodextrin and 50% acetone. The mixture should then be incubated for 1 h at room temperature with mixing. Appearance
Briefly, the reaction was performed in 75 mM phosphate buffer (pH 7.4) and the final assay mixture (200 μl) contains fluorescein (160 μl, 63 nM final concentration) as an oxidizable substrate, AAPH (20 μl, 12.8 mM final concentration) as an oxygen radical generator, and antioxidant (20 μl, either trolox [0.78-50 μM, final concentrations] or samples [100 μg/ml, final concentration]). The reaction was performed at 37°C and fluorescence was recorded every minute for 120 min. A blank (control) using phosphate buffer instead of the antioxidant was carried out in each experiment. All reaction mixtures were prepared in duplicate, and at least three independent runs were performed for each sample. Fluorescent measurements were normalized to the curve of the blank (no antioxidant). The ORAC values, expressed as μM trolox equivalents (μM TE/g) were calculated by applying the following formula:
where CTrolox is the concentration (μM) of trolox (5 μM), CSample is the concentration (g/L) of the sample, and AUC is the area below the fluorescence decay curve of the sample, blank, and trolox, respectively, calculated by applying the following formula:
where f0 is the initial fluorescence and fn is the fluorescence at time n.
RESULTS AND DISCUSSION
The volatile oils collected by steam distillation from the aerial parts of Diplazium squamigerum, Laportea macrostachya, and Vitis coignetiae were obtained in yields of 0.0010%, 0.0144%, and 0.0099% (use mg/kg fresh sample), respectively. Distinct qualitative and quantitative differences were observed in the oils studied. Gas chromatograms of these oils showed the presence of 166 compounds (). As a result, 87 compounds of D. squamigerum, 95 compounds of L. macrostachya, and 76 compounds of V. coignetiae, accounting for 99.7%, 99.6%, and 99.8%, were identified, respectively. The classification of the oils on the basis of structure type is summarized in .
Table 1 Chemical Composition of the volatile Oils from D. squamigerum (I), L. macrostachya (II) and V. coignetiae (III)
Table 2 Components of the volatile Oils from D. squamigerum (I), L. macrostachya (II) and V. coignetiae (III)
The volatile oil of D. squamigerum contained 27 monoterpenes, 7 sesquterpenes, 3 diterpenes, 34 aliphatic compounds, 7 aromatic compounds, and 7 miscellaneous compounds. It was determined to be rich in monoterpenes alcohol. The major constituents were linalool (28.7%), palmitic acid (13.9%), α-terpineol (5.5%), phytol (5.5%), and p-vinyl-guaiacol (5.0%). It seems that these components make the green-floral odor.
L. macrostachya volatile oil contained 17 monoterpenes, 13 sesquterpenes, 2 diterpenes, 45 aliphatic compounds, 10 aromatic compounds, and 6 miscellaneous compounds. It was determined to be rich in aliphatic acids and aldehydes. The major constituents were palmitic acid (14.1%), nonanal (9.2%), linoleic acid (8.9%), (2E)-hexenal (8.5%), and linolenic acid (7.0%). It seems that these components make the green-oily odor.
V. coignetiae volatile oil contained 15 monoterpenes, 4 sesquterpenes, 2 diterpenes, 40 aliphatic compounds, 7 aromatic compounds, and 5 miscellaneous compounds. It was determined to be rich in monoterpene alcohols and aliphatic hydrocarbons, alcohols, and aldehydes. The major constituents were palmitic acid (14.1%), nonanal (9.2%), linoleic acid (8.9%), (2E)-hexenal (8.5%), and linolenic acid (7.0%). It seems that these components make the green-oily odor.
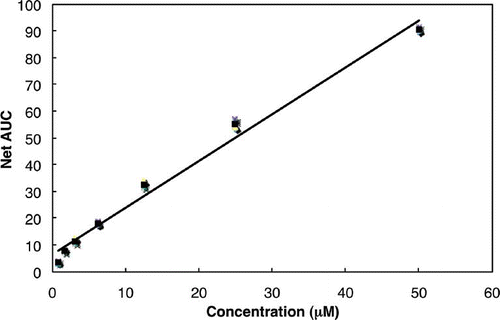
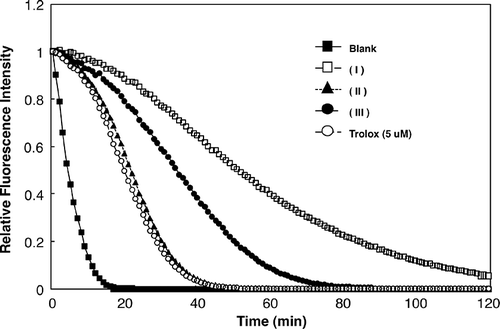
During the ORAC assay, the decrease in fluorescence intensity was followed to monitor the decay of the fluorescence curve. A calibration curve was obtained by plotting the area under the curve (AUC) against trolox concentrations in the 0–50 μM range (). The equation of the calibration curve was y = 1.7495x + 6.5541 with a good correlation coefficient (r 2 = 0.987). shows the FL decay curve for the three samples. The results obtained appear in , which shows the sum of the ORAC values in the volatile oil from D. squamigerum, L. macrostachya, and V. coignetiae. All compounds showed moderate to high activities of 514 to 1255 trolox equivalents in a concentration of 100 μg/ml. For the most active volatile oil, D. squamigerum oil (1255 ± 393 μmol TE/g), this is comparable to other oils. The antioxidant capacity of the volatile oils measured by the ORAC methods followed the order: D. squamigerum> L. macrostachya> V. coignetiae. It is of interest that volatile oils possessing a monoterpenes alcohol level followed the order: D. squamigerum > L. macrostachya > V. coignetiae. The authors believe that difference in the antioxidant activities among D. squamigerum oil, L. macrostachya oil, and V. coignetiae oil were attributed to their different monoterpene alcohol contents and composition in the samples. These data confirm the volatile oil from Japanese edible plants as good dietary sources of antioxidants. There are only a few reports of the antioxidant capacity of the volatile oils measured by the ORAC methods;Citation[22] therefore, further studies are needed on the antioxidant capacity of the volatile oils measured by the ORAC methods.
CONCLUSION
The major components of volatile oil from D. squamigerum oil were linalool (28.7%), palmitic acid (13.9%), and α-terpineol (5.5%); of L. macrostachya oil were palmitic acid (14.1%), nonanal (9.2%), and linoleic acid (8.9%); and of V. coignetiae oil were nonanal (13.2%), geraniol (11.6%), and phenylacetaldehyde (8.5%). These oils were assayed to determine their antioxidant activity by the oxygen radical absorbance capacity (ORAC) assay using fluorescein (FL) as the fluorescent probe. The ORAC values varied from 1255 ± 393 trolox equivalents (μmol TE/g) for D. squamigerum oil, from 514 ± 65 μmol TE/g for L. macrostachya oil, and from 911 ± 118 μmol TE/g for V. coignetiae oil. The differences in the antioxidant activities among D. squamigerum oil, L. macrostachya oil, and V. coignetiae oil were attributed to their different monoterpene alcohol contents and composition in the samples. These data provided evidence that the volatile oils from Japanese edible plants are good dietary sources of antioxidants.
REFERENCES
- Ebrahimzadeh , M.A. , Nabavi , S.F. and Nabavi , S.M. 2009 . Essential oil composition and antioxidant activity of Pterocarya fraxinifolia Pak . Journal of Biological Science , 12 ( 13 ) : 957 – 963 .
- Umegaki , K. and Kajiki , I. 2007 . Component analysis of cultivated Shi-O-de (an edible wild plant) . Eiyogaku Zasshi , 65 ( 2 ) : 81 – 83 .
- Miyazawa , M. , Horiuchi , E. and Kawata , J. 2007 . Components of the essential oil from Matteuccia struthiopteris . Journal of Oleo Science , 56 : 457 – 461 .
- Miyazawa , M. , Horiuchi , E. and Kawata , J. 2007 . Components of the essential oil from Adenophora triphylla var. japonica . Journal of Essential Oil Research , 20 ( 2 ) : 125 – 127 .
- Miyazawa , M. , Utsumi , Y. and Kawata , J. 2008 . Comparison of the essential oils from wild type and cultivar type of Hosta sieboldiana . Journal of Essential Oil Bearing Plants , 11 ( 4 ) : 413
- Miyazawa , M. , Utsumi , Y. and Kawata , J. 2009 . Aroma-active compounds of Elatostema laetevirens and Elatostema umbellatum var. majus . Journal of Oleo Science , 58 ( 4 ) : 163 – 169 .
- Sugahara , T. , Yamaguchi , H. , Sasaki , H. and Aoyagi , Y. 1988 . Mineral contents of newly commercialized vegetables and herbs . Joshi Eiyo Daigaku Kiyo , 19 : 131 – 138 .
- Sugahara , T. , Yamaguchi , F. , Sasaki , H. and Aoyagi , Y. 1989 . Contents of free amino acids in newly commercialized vegetables and herbus . Joshi Eiyo Daigaku Kiyo , 20 : 77 – 85 .
- Poudel , P. , Tamura , R. , Kataoka , H. and Mochioka , I.R. 2008 . Phenolic compounds and antioxidant activities of skins and seeds of five wild grapes and two hybrids native to Japan . Journal of Food Composition and Analysis , 21 ( 8 ) : 622 – 625 .
- Jeong , H.J. , Park , S.B. , Kim , S. and Kim , H.K. 2007 . Total polyphenol content and antioxidative activity of wild grape (Vitis coignetiae) extracts depending on ethanol concentrations . Han'guk Sikp'um Yongyang Kwahak Hoech , 36 ( 12 ) : 1491 – 1496 .
- Min , K.R. , Kim , Y. , Kang , S.H. , Mar , W. , Lee , K.S. , Ro , J.S. , Lee , S.H. and Kim , Y. 1996 . Inhibitory effects of herbal extracts on cyclooxygenase activity of prostaglandin H2 synthase from sheep seminal vesicle . Natural Product Sciences , 2 ( 1 ) : 56 – 74 .
- Igarashi , K. , Takanashi , K. , Makino , M. and Yasui , T. 1989 . Antioxidative activity of the major anthocyanin isolated from wild grapes (Vitis coignetiae) . Nippon Shokuhin Kogyo Gakkaishi , 36 ( 10 ) : 852 – 856 .
- Kohama , K. , Kishi , A. , Yonekura , Y. , Ohsawa , J. , Sawai , H. and Nagasawa , T. 2003 . Estimation of radical scavenging activities using various methods and antioxidant activity of wild grapes (Vitis coignetiae) . Iwate-ken Kogyo Gijutsu Senta Kenkyu Hokoku , 10 : 77 – 80 .
- Prior , R.L. and Cao , G. 1999 . In vivo total antioxidant capacity: Comparison of different analytical methods . Free Radical Biology and Medicine , 133 : 2812 – 2819 .
- Ou , B. , Huang , D. , Hampsch-Woodill , M. , Flanagan , J. and Deemer , E. 2002 . Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study . Journal of Agricultural and Food Chemistry , 50 : 3122 – 3128 .
- Ou , B. , Hampsch-Woodill , M. and Prior , R.L. 2001 . Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe . Journal of Agricultural and Food Chemistry , 49 : 4619 – 4626 .
- Davalos , A. , Bartolome , B. and Gomez-Cordoves , C. 2005 . Antioxidant properties of commercial grape juices and vinegars . Food Chemistry , 93 : 325 – 330 .
- Adams , R.P. 2001 . Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy , Allured, Illinois : Carol Stream .
- NIST/EPANIH . 1999 . National Institute of Standards and Technology. PC Version 17 of the Mass Spectral Library , Saint Quentin, , France : Perkin-Elmer Corporation .
- Zulueta , A. , Esteve , M.J. and Frigola , A. 2009 . ORAC and TEAC assays comparision to measure the antioxidant capacity of food products . Food Chemistry , 114 : 310 – 316 .
- Liu , H. , Li , J. , Zhao , W. , Bao , L. , Song , X. , Xia , Y. , Wang , X. , Zhang , C. , Wang , X. , Yao , X. and Li , M. 2009 . Fatty acid synthase inhibitors from Geum Japonicum THUNB. var. chinense . Chemistry and Biodiversity , 6 : 402 – 410 .
- Wang , C.Y. , Wang , S.Y. and Chen , C. 2008 . Increasing antioxidant activity and reducing decay of blueberries by essential oils . Journal of Agricultural and Food Chemistry , 56 : 3587 – 3592 .