Abstract
Nine kinds of oligopeptide were obtained from the yak bone-marrow protein via the hydrolysis of some single or mixed proteases, and the functions of various yak bone peptides were compared. The results indicated that the peptide from yak bone hydrolyzed by Alcalase plus Flavourzyme at 50°C for 4 h showed the strongest antioxidant activity in a H2O2 system and chelating activity to Cu2+; Yak bone peptide derived from the hydrolysis by papain PSM500 plus Peptidase R presented the most efficient radical-scavenging activity to 2,2-diphenyl-1-picrylhydrazyl free radicals; Yak bone peptide hydrolyzed by Protamex single had the best calcium binding activity and the peptide from the hydrolysis of yak bone by trypsin displayed the most ideal chelating activity on iron (II). Taken together, yak bone peptide hydrolyzed by Alcalase plus Flavourzyme at 50°C for 4 h had the best integration function. Therefore, it might be useful for the application in functional foods.
INTRODUCTION
Yak is the only breed of cattle in the world that can survive at an altitude of more than 3000 m.[Citation1] There are about 14 million yaks in the world and the number of yak in China accounts for about 92% of the world, so China is the main yak-reproducing country. The yaks mainly live in China's Qinghai, Gansu, Tibet, Sichuan, Yunnan, and other areas.
The peptide-regulating physiological functions of the organism are called functional peptide.[Citation2] Many functional peptides are derived from the degradation of protein-compound and have a variety of human metabolic and physiological adjustment functions. With the improvement of people's living standards, functional peptide will have a good prospect. The purpose of this study is to investigate the functional properties of peptides derived from enzymatic hydrolysis of yak marrow protein. The six commercial enzyme preparations used were Protamex, Alcalase, trypsin, Papain PSM500, Peptidase R, and Flavourzyme. The hydrolysis was carried out by a single enzyme or by different combinations of enzymes in a total of nine groups. Then, the functions, such as antioxidant activity, radical-scavenging activity, metal-chelating activity, and calcium binging, of the nine kinds of different yak bone peptide were compared. Moreover, the optimal conditions for producing best yak bone peptide were also studied.
MATERIALS AND METHODS
Materials
Protamex, Flavourzyme, and Alcalase were obtained from Novozymes North America Inc. (Franklinton, NC). Papain PSM500, Peptidase R, and trypsin were obtained from Belgium Enzyme Preparation Company (Deurne, Belgium), Japan Mano Company (Nagoya, Japan), and Beijing Chemical Reagent Company (Beijing, China), respectively. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), pyrocatechol violet, and vitamin C were purchased from Sigma Chemical Co. (St. Louis, MO, USA). H2SO4, H2O2, KI, Na2S2O3, ethanol, and starch were purchased from Beijing Chemical Engineering Factory (Beijing, China). Acetonitrile (chromatographic grade) was purchased from Fisher Scientific Co., Ltd. (Pittsburgh, PA, USA), formic acid (chromatographic grade) was from Acos Company (USA), and water used was prepared by Milli-Q purifier (Millipore, USA).
Preparation of Yak Hydrolysate
One kg yak bone was cleaned and broken up into small pieces (about 5 cm3), and then put into a 2000 ml beaker. With the addition of 1.2 L water, the beaker was put into an autoclave and boiled at 120°C for 4 h. After cooling, the upper fat was removed. The yak bone-marrow protein was hydrolyzed by enzymes [Protamex (P; 1.5 AU/g), Flavourzyme (F; 500 LAPU/g), Alcalase (A; 2.4 AU/g), trypsin (1:250), papain PSM 500 (1:500), and Peptidase R (Pep; 1:250)] by a single enzyme or by different combinations. The detailed enzymatic hydrolysis conditions and degree of hydrolysis were shown in . Finally, yak bone peptides were derived from the hydolysates.
Table 1 Conditions of enzymatic hydrolysis and degree of hydrolysis (DH)
Antioxidant Activity
The antioxidant activity in an H2O2 system was assayed by a modified procedure of Fu et al.[Citation3] DPPH radical-scavenging activity was measured by the method of Xu et al. with some slight modifications.[Citation4–5] The procedure of metal-chelating, quoted from Kong et al.,[Citation6] was followed to determine Cu2+ and Fe2+ chelating activities of yak bone peptide. The calcium binding assay was performed basically according to the method of Jung et al.[Citation7]
Purification of Peptides from Bone Hydrolysate and Identification
Taking antioxidant activity, DPPH radical-scavenging ability, metal-chelating activity, and calcium binding in all, the scores of all functions mentioned for each group were summed up. The peptide with the highest score was considered the most suitable for functional foods. The peptides separation and characterization were made by the RP-HPLC-QTOF-MS methods. RP-HPLC and QTOF-MS were carried out by Agilent 1100 LC (Agilent, USA) and Nanoelectrospray Ionization Quadrupole QTOF-MS (Micromass, UK), respectively.
RESULTS AND DISCUSSION
Antioxidant Activity
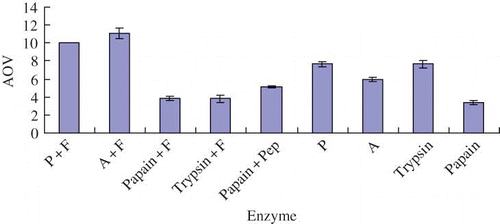
The antioxidant activities of different yak bone peptides were shown in . As we could see, the No. 2 yak bone peptide, hydrolyzed by Alcalase plus Flavourzyme at 50°C for 4 h, had the strongest antioxidant activity in a H2O2 system and the antioxidant activity value (AOV) was 11.1 mg/g. The No. 1 yak bone peptide, derived from the hydrolysis by Protamex plus Flavourzyme, showed the second strongest antioxidant activity in a H2O2 system and the AOV was 10.03 mg/g. Different yak bone peptides were derived from the hydrolysis of marrow protein by different enzymes, indicating that the efficient antioxidant activities ability of the yak bone peptides might be mainly attributable to the peptide structure.
Effect of Yak Bone Peptide on DPPH Radical-Scavenging
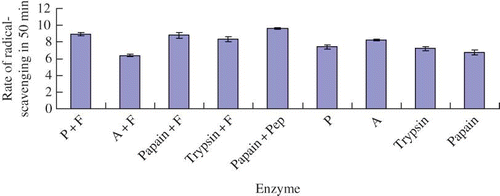
The DPPH radical-scavenging ability of different yak bone peptides were shown in . All nine groups of the yak bone peptide had DPPH radical-scavenging abilities, especially No. 5 yak bone peptide, which was hydrolyzed by Papain PSM500 plus Peptidase R and had the most efficient radical-scavenging activity on DPPH free radicals. Also, No. 1 and No. 3 yak bone peptides had relative high efficient radical-scavenging activity.
Metal-Chelating Activity
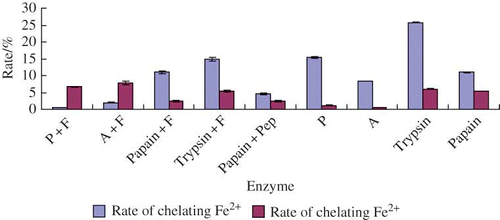
The Fe2+ and Cu2+ chelating abilities of different yak bone peptides were shown in . From , the No. 8 yak bone peptide exhibited the best Fe2+ chelating ability of 25.8%, while the No. 2 yak bone peptide displayed the highest Cu2+ chelating ability of 7.831%. Meanwhile, the ability of Fe2+ chelating was much stronger than Cu2+ chelating. Considering both Fe2+ and Cu2+ chelating activities, the No. 8 yak bone peptide was the best. Also, the No. 3 and No. 6 yak bone peptides exhibited strong rates of Fe2+ chelating activities and weak rates of Cu2+ chelating activities. While the No. 4 and No. 8 yak bone peptides showed strong rates of both Fe2+ and Cu2+ chelating activities, yak bone peptides, which were derived from different enzymatic hydrolysis under different conditions, had different metal-chelating rates to metal ions. Trypsin was involved in both the No. 4 and No. 8 enzyme groups, so trypsin might be the enzyme, which could induce strong metal chelating abilities of yak bone peptide.
Calcium Binding Assay
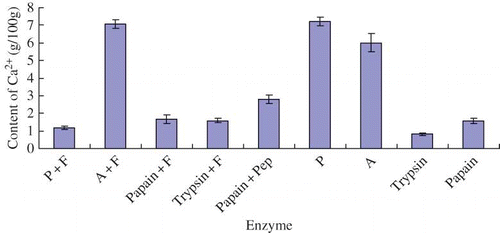
The ability of calcium binding each group of yak bone peptide was shown in . From , the No. 6 yak bone peptide, hydrolyzed by Protamex, displayed the best calcium binding ability of 7.194 g/100 g, and the No. 2 yak bone peptide was second. Zhen et al.[Citation8] reported that the maximum calcium content of sheep bone peptide derived from hydrolysis of Neutrase was about 1.97 g/100 g (dry basis). Jung et al.[Citation7] worked on the enzymatic hydrolysis of hoki bone, and the obtained oligopeptide had a high affinity of calcium of 6.55 g/100 g. By comparing with the above results, it could be concluded that yak bone peptide had an excellent ability of calcium binding.
Purification of Peptides from Bone Hydrolysate and Identification
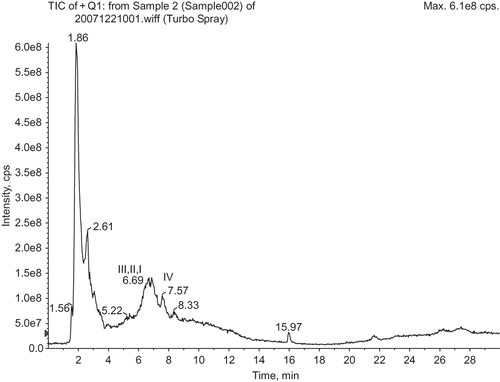
Four Peptides (marked in ) named Peptide I, II, III, and IV were isolated from the No. 1 group of hydrolysate, and their primary structures were determined by Nanoelectrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry. They were Gly-Pro-Ser-Gly-Pro-Lys-Gly-Pro-Ser-Gly-Pro-Leu-Gly-Pro-Lys (Mr = 1331.7), Gly-Leu-Gln-Val-Ala-Val-Arg-Pro-Asp-Pro-Asp-Pro-Ala-Pro-Leu (Mr = 1338.7), Gly-Ser-Leu-Gly-Ser-Leu-Gly-Pro-Asp-Lys-Gly-Thr-Gly-Pro-Val-Ala-Ala-Pro (Mr = 1579.8), and Leu-Thr-Gly-Leu-Tyr-Leu-Cys-Gly-Leu (Mr = 951.5). The structure of peptide I and III had repeated sequences of “gly-pro-X” and “gly-X-Y” (X, Y represented any other amino acid), which was consistent with the characters of collagen structures.[Citation9] According to literature, the size of functional peptides was mostly between 2–15 amino acid residues, pentadeca-peptide was better than dipeptide and tripeptide compared with their functional activities.
CONCLUSIONS
In the present study, all of the yak bone peptide displayed activities of antioxidant, DPPH radical-scavenging, metal-chelating, and calcium binding. The results of Nanoelectrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry indicated that the peptides were derived from animal bone by comparing with the structures of animal bone collagen protein. Considering of the four functions, the No. 2 yak bone peptide was the best, so it was the most suitable for the application in functional foods. Anymore, different yak bone peptides, which were derived from yak bone marrow protein by different enzymatic hydrolysis conditions, had different functions, indicating that the functions of the yak bone peptides depended mainly on their peptide structures.
ACKNOWLEDGMENT
We acknowledge the partial financial support of Academic Innovation Personnel Program of Universities Affiliated to Beijing Municipal Government (PHR200906110).
REFERENCES
- Li , F. , Jia , D. , Yao , K. and Hu , Y. 2006 . The research of the best enzymatic condition of yak bone protein . Amino Acid and Biotic Resources (China) , 28 : 7 – 10 .
- Li , J. and Feng , P. 2004 . The study of functional peptide . Food Sciences (China) , 25 : 415 – 419 .
- Fu , G. , Li , C. , Ma , C. and Li , X. 2006 . The research of enzyme hydrolyze preparation of the pork bone antioxidant peptide . Modern Food Science and Technology (China) , 22 : 136 – 138 .
- Xu , X. , Katayama , S. and Mine , Y. 2007 . Antioxidant activity of tryptic digests of hen egg yolk phosvitin . Journal of the Science of Food and Agriculture , 87 : 2604 – 2608 .
- Saiga , A. , Tanabe , S. and Nishimura , T. 2003 . Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment . Journal of Agricultural and Food Chemistry , 51 : 3661 – 3667 .
- Kong , B. and Xiong , Y.L. 2006 . Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action . Journal of Agricultural and Food Chemistry , 54 : 6059 – 6068 .
- Jung , W.K. , Park , P.J. , Byun , H.G. , Moon , S.H. and Kim , S.K. 2005 . Preparation of hoki (Johnius belengerii) bone oligophosphopeptide with a high affinity to calcium by carnivorous intestine crude proteinase . Food Chemistry , 91 : 333 – 340 .
- Zhen , R. , Ma , L. , Li , J. and Zhang , T. 2007 . Study on preparing calcium-binding peptide from enzymatically hydrolyzed sheep bone . Food Science & Technology (China) , 4 : 226 – 230 .
- Zhao , L. , Su , W. , Hu , H. , Dai , Y. and Yang , Y. 2005 . Research Progress of Collagen Peptides . Food Science (China) , 9 : 578 – 582 .