Abstract
Vegetable soybean differs from the conventional soybean in its distinct taste. Genetic variability has been scarcely investigated in vegetable soybean for taste-related compounds viz. sucrose, aspartic acid, glutamic acid, glycine, alanine, and isoflavones. In the present study, analysis of green seeds of 12 vegetable-type genotypes, between reproductive stages of R6 and R7 (i.e., when the pods were completely filled but the seeds and pods shell not yet turned yellow), revealed ranges of 1.28–7.12, 0.37–1.51, 0.64–2.82, 0.17–0.72, 0.11–0.51 g/100 g for sucrose, aspartic acid, glutamic acid, glycine, alanine content, respectively, while a range of 8.64–33.19 mg/100 g was observed for total isoflavones content. Genotypes with high levels of sucrose, aspartic acid, glutamic acid and alanine scored high for taste. Results did not indicate any significant relationship between isoflavones content and the taste score.
INTRODUCTION
Vegetable soybean can be a potential mode for harnessing health components of soybean in countries like India, United states, Brazil, and Argentina, where the traditional soy products viz. soy milk, tofu, miso, natto from the South-East Asian countries could not become popular due to the off-flavour associated with them and the time consuming process involved in their preparation. For consumption, the immature pods of vegetable soybean are picked as the plants reach approximately 80% of maturity (between reproductive stages of R6 and R7) and still retain about 65% moisture content. The green seeds shelled from the immature pods are quick-to-cook, free from off-flavour, and more importantly, their appearance as well as cooking method being similar to that of other immature beans viz. green pea, chick pea, French bean, makes it convenient for the common household to include vegetable soybean in the daily cuisine. In Japan, China, and Korea, where vegetable soybean is known by different nomenclature viz. edamame (Japan), mao dou (China), and poot kong (Korea), green pods as a whole are boiled with a pinch of salt for 5 min and the tender seeds are directly popped into the mouth. Also, green seeds of vegetable soybean can serve as a nutritious snack once they are stir-fried or added into soups and salads after boiling. Furthermore, in India, the usage of vegetable soybean like other common legumes would be in the interest of the economy especially in the backdrop of the prevailing crunch scenario in legumes and pulses sector with wide gap between demand and supply.
In general, vegetable-type soybean genotypes bear comparatively large pods and large seed size than the grain-type soybean; however, it is the sweet taste of the green seeds of the former type that distinguishes it from the latter. Apart from the sucrose content, four amino acids, namely aspartic acid, glutamic acid, glycine, and alanine, were suggested to contribute positively to the taste of immature seeds of soybean.[Citation1] On the contrary, isoflavones in soybean seeds, which have been well documented for their estrogenic and antioxidant activities, are thought to impart astringency to soy milk and cooked whole soybean grain.[Citation2] Therefore, levels of isoflavones in immature seeds may affect the taste of vegetable soybean as well. Extensive plant breeding programs are underway in many Asian countries for the selection and development of suitable genotypes to use as vegetable entity with good flavour and taste.[Citation3] For this purpose, information on the levels of sucrose, taste-related amino acids, and isoflavones in immature seeds is essential and found limiting in the literature.[Citation4,Citation5] In the present study, the contents of sucrose, 4 free amino acids viz. aspartic acid, glutamic acid, glycine, alanine, and isoflavones in the green seeds of 12 vegetable-type genotypes were determined with an aim to assess their genotypic variability and to investigate their relationships with the taste of green seeds.
MATERIALS AND METHODS
Seeds of 12 vegetable-type soybean genotypes namely ‘AGS435’, ‘AGS436’, ‘AGS437’, ‘AGS439’, ‘AGS440’, ‘GC98017-7-196-1-2’, ‘GC99010-35-1-2-2’, ‘Table Variety’, ‘Dada-cha 2000’, ‘Dada-cha ma-me’, ‘ASG328 Kohine’ and ‘ASG328 Sricanan’ were sown in the experimental field of National Research Centre for Soybean, Indore, M.P. in cropping season 2008 in three replications in the three-row plot in randomized complete block design. Each plot was 3 m long while the row-to-row distance maintained was 0.45 m. The most suitable picking stage for vegetable purpose is between R6–R7 stage of the reproductive phase of soybean plant, i.e., when the pods are completely filled with seeds but the colour of the seeds as well as pod shell is still green.[Citation6] The pods picked at R6–R7 stage were stored in a deep freezer at −30°C until analyzed. All the analyses in green seeds were completed with in 30 days. Green seeds shelled from these pods were freeze-dried using lyophilizer (Labconco) and ground to obtain fine flour (100-mesh). Soy flour was defatted using petroleum ether (bp 40°C) and the defatted flour so obtained was used for extraction of sucrose, taste-related amino acids viz. aspartic acid, glutamic acid, glycine, and alanine and individual forms of isoflavones.
Determination of Moisture Content and 100-Green Seed Weight
Twenty green seeds (in triplicate) shelled from the freshly picked pods were kept in a hot air oven at 75°C till their weight became constant. The moisture content of the green seeds was determined gravimetrically. Twenty green seeds (in triplicate) were selected randomly and weighed to compute the weight of 100 green seeds.
HPLC Analysis for Sucrose Content
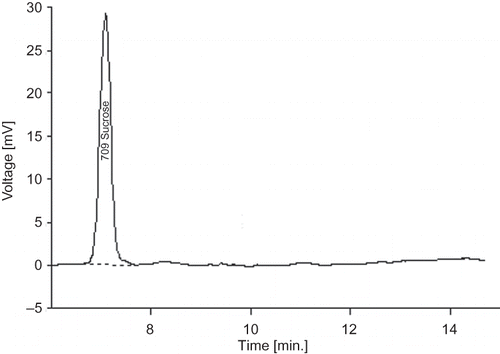
Defatted soy flour (1 g) was extracted with 10 ml of 80% ethanol in a water bath for 4 h at 80°C with occasional shaking. The extract was cleaned by adding 1 ml of 10% lead acetate solution followed by centrifugation at 10,000 rpm for 10 min. The step was repeated again. The supernatant so obtained was filtered through a syringe membrane filter (0.22 μm, 13 mm diameter). A 20 μl of sample was injected into Shimadzu high performance liquid chromatograph (LC10AT vp) (Shimadzu, Japan). The separation of sucrose was achieved using a NH2 column (Phenomenex Luna 5u, Phenomenex, USA, dimension 250 mm × 15 mm), preceded by a guard column, maintained at 40°C in Shimadzu CTO 10AT vp oven (Shimadzu, Japan). The mobile phase, acetonitrile/water (75/25 v/v), was run isocratically at a flow rate of 1.0 ml/min and elution was monitored by means of a refractive index detector (Shimadzu, RID10A, Shimadzu, Japan). The peak of sucrose was detected at 7.09 min (). Quantification of sucrose was done by comparing the area of the peak of sucrose in the sample chromatogram with that of the standard chromatogram.
HPLC Analysis for Taste-Related Amino Acids
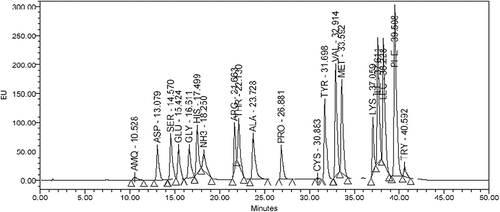
The amino acid composition of immature seeds was determined as the standards used contained mixture of amino acids. The data of only taste-related amino acids viz. aspartic acid, glutamic acid, glycine, and alanine is presented and discussed. The amino acid composition was determined using HPLC-based amino acid analyzer fitted with Waters Model 515 pump and Water 717 plus Autosampler (Waters Corp., Milford, MA, USA). Defatted soy flour (50 mg) was hydrolyzed with 5 ml of 6 N HCl at 120°C for 24 h. The hydrolysate was passed through Whatman No. 1 and an aliquot of filtrate was derivatized using AccQ-tag kit procured from Waters (India) Pvt. Ltd (Waters Corp., Milford, MA, USA). A volume of 20 μl of derivatized sample so obtained was injected into HPLC system fitted with Waters AccQ-tag column. A gradient elution was carried out at a flow rate of 1 ml/min using 10% AccQ fluor-tag eluent as eluent A and 60% aqueous solution of acetonitrile as eluent B. The gradient conditions were: concentration of A was decreased from 100 to 70% over 0 to 38 min, increased to 100% at 40 min and maintained at 100% for next 10 min. The elution was monitored by means of Waters 2457 multi λ fluorescent detector (Waters Corp., Milford, MA, USA) at λex 250 and λem 395. The peaks of aspartic acid, glutamic acid, glycine, and alanine were detected at 13.07, 15.42, 16.61, and 23.72 min, respectively (). These four amino acids were identified by comparing the retention times with a standard mixture of 20 amino acids and quantified by measuring the peak area using Empower 2 chromatography software (Waters Corp., Milford, MA, USA).
HPLC Analysis for Isoflavones
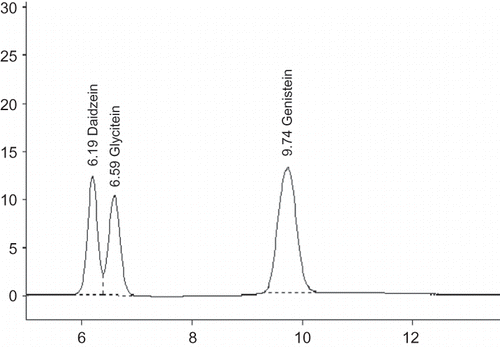
Concentration of individual and total isoflavones in ground flour of lyophilized green seeds was determined in the form of aglycones following the method given by Vyn et al.[Citation7] In brief, 0.125 g of finely ground flour from each genotype was digested with 1 ml of conc. HCl and extracted with 5 ml of 80% ethyl alcohol at 80–90°C for 2 h with occasional shaking followed by centrifugation at 10,000 rpm for 10 min. The supernatant so obtained was filtered through a syringe membrane filter (0.22 μm, 13 mm diameter). A volume of 20 μl of extract from each sample was injected into Shimadzu high performance liquid chromatograph (LC10AT vp) using Shimadzu Auto Sampler (SIL-20A). Separation of individual isoflavone was achieved by using Phenomenex C18 column maintained at 45°C in Shimadzu CTO 10AT vp oven. A gradient of 10% (A) acetonitrile and 38% (B) acetonitrile was run at a flow rate of 0.8 ml/min to separate the individual forms of isoflavone. The concentration of solvent B was decreased from 100% to 90% at 5 min and again increased to 100% at 20 min. The concentration of individual isoflavone was calculated by comparing the peak area of samples with that of respective standards procured from Sigma Aldrich (Bangalore, India). A representative chromatogram depicting the separation of standards is given in .
Consumer Preference
A 5-member panel comprising of the scientists, technical staff and research fellows, engaged in soya food processing research programmes of National Research Centre for Soybean, Indore, India was requested to evaluate the green seed samples (in triplicate) for taste following the procedure of Larmond.[Citation8] The green seeds of each genotype were shelled from the immature pods picked from three replicates in the field between 8.00–9.00 A.M. Green seeds were served to panelists on a glass trays covered with lid and pre-labeled with randomly selected three-digit codes. The temperature of the evaluation desk was 28 ± 0.5°C while the humidity was 60 ± 1.5%. Sunlight was sufficiently available through the large unshaded glass windows. Panelists rinsed their mouth thoroughly with Bislery bottled water to cleanse their palate between the samples. The panelists scored their liking and disliking for the taste using nine-point hedonic scale (1 = dislike extremely; 2 = dislike very much; 3 = dislike moderately; 4 = dislike slightly; 5 = neither like nor dislike; 6 = like slightly; 7 = like moderately; 8 = like very much; 9 = like extremely).
Statistical Analysis
All the experimental procedures were repeated in triplicate and the results were subjected to a completely randomized analysis of variance (ANOVA) using MSTAT-C programme. A pair-wise comparison of means by least significant difference was carried out at p = 0.05.
RESULTS AND DISCUSSION
Moisture content of the green seeds and weight of 100 green seeds of each of 12 vegetable-type genotypes are given in . Moisture content ranged from 65.74 to 76.00 with average value of 70.8g/100g green seeds. Barring ‘Table Variety’, all the vegetable-types were large seeded with the weight of 100 green seeds exceeding 47.0g. ‘AGS440’ exhibited the largest seed size (59.79 g). The ranges of moisture content and 100 green seeds weight observed are near to the values reported for the immature green seeds of 12 Japanese soybean cultivars in an earlier study.[Citation9] Genotypes exhibited significant differences on hedonic scale for the taste. ‘Dada-cha ma-me’ was given the highest (8.8) while ‘Table Variety’ and ‘AGS439’ the lowest taste score (6.0).
Table 1 Moisture (%), 100-green seed weight (g) and sensory score (nine point hedonic scale) of vegetable soybean genotypes (between reproductive stages of R6 and R7)
presents the contents of sucrose and taste-related amino acids viz. aspartic acid, glutamic acid, glycine, and alanine of immature seeds of 12 vegetable-type genotypes. Significant genotypic variation (p < 0.05) was observed for sucrose content, which ranged from 1.28 (‘Table Variety’) to 7.12 (‘Dada-cha ma-me’) with a mean value of 5.02 g/100g dry seeds. Seven genotypes namely ‘AGS435’, ‘AGS436’, ‘AGS440’, ‘Dada-cha ma-me’, ‘Dada-cha 2000’, ‘ASG328 Kohine’, and ‘ASG328 Sricanan’ exhibited higher sucrose content than the average value. These seven genotypes exhibited higher sucrose content than the value (5.5 g/100 g dry seeds) reported for vegetable-type ‘Tanbaguro’ in a previous study.[Citation10] Song et al.[Citation11] reported value of 8.2 g/100 g dry seeds for sucrose content for one vegetable-type genotype ‘Seokryang’, which is higher than all the vegetable soybean genotypes investigated in our study. The sucrose content of some of the vegetable-types (‘AGS437’, ‘GC98017-7-196-1-2’, ‘GC99010-35-1-2-2’, ‘Table Variety’) was not significantly different from that of immature seeds of grain-type genotypes reported in a separate study carried out in our laboratory.[Citation12] Significant genotypic differences (p < 0.05) were also observed for four taste-related amino acids viz. aspartic acid, glutamic acid, glycine, and alanine. Ranges for aspartic acid, glutamic acid, glycine, and alanine were: 0.37–1.51, 0.64–2.82, 0.17–0.72, and 0.11–0.51, respectively, with 0.98, 1.64, 0.43, and 0.32 g/100 g dry seeds as the respective average values. Among all the genotypes, glutamic acid was found in the highest concentration, which is in consonance with the results of Abe et al.[Citation5]. ‘ASG328 Sricanan’ exhibited the highest concentration for aspartic acid, glutamic acid, and glycine. Though, for aspartic acid content, the genotypic differences between ‘AGS440’ and ‘ASG328 Sricanan’ were not significant. For alanine content, ‘AGS440’ showed maximum value, though the genotypic differences between ‘AGS440’, ‘Dadacha ma-me’ and ‘ASG328 Sricanan’ were not significant. To compare the levels of amino acids in vegetable-type soybean genotypes observed in the present investigation with other studies, the authors found scarce information in literature. Yanagisawa et al.[Citation4] analysed 20 soybean genotypes, 10 from edamame-type and 10 from grain-type, harvested at 30–40 days after flowering. The authors reported a concentration of more than 15 mM/kg (dry weight basis) for glutamic acid and alanine in case of edamame cultivars and about 5 mM/kg for grain-type cultivars. They reported a concentration of 2 mM/kg (dry weight basis) for aspartic acid in both vegetable and grain-type of soybean. These values (expressed in mM/kg) were converted into g/100 g for comparison with our results, and higher average levels for glutamic acid, alanine and aspartic acid in the present investigation were observed compared to the values reported for the respective amino acid in the study of Yanagisawa et al.[Citation4] Lisiewska et al.[Citation13] reported in raw broad bean seeds (Vicia faba var. major) values of 3.82, 5.32, 1.12, and 1.41 g/100 dry seeds for aspartic acid, glutamic acid, glycine, and alanine, respectively, which are higher than the average values for the respective amino acids in our study.
Table 2 Percent sucrose and taste-related amino acids contents of vegetable soybean genotypes (between reproductive stages of R6 and R7)
Concentration of individual forms of isoflavones in immature seeds of vegetable-type soybean was determined in the form of their respective aglycones. The data (mg/100 g dry seeds) presented in showed significant genotypic variation for individual as well as total isoflavone content. Ranges for daidzein, glycitein, genistein, and total isoflavones content were: 3.78 (‘AGS437’)–17.17 mg/100 g (‘Dada-cha 2000’), 0.75 (‘Table Variety’)–8.55 mg/100 g (‘Dada-cha 2000’), 2.64 (‘AGS439’)–7.61 μmg/100 g (‘AGS437’), and 8.64 (‘AGS440’) to 33.19 mg/100 g (‘Dada-cha 2000’) with 5-, 12-, 2.88-, and 3.84-fold genetic variation, respectively. Average values for daidzein, glycitein, genistein, and total isoflavones content were: 8.77, 3.45, 4.66, 16.87 mg/100 g dry seeds, respectively. Mebrahtu et al.[Citation13] analysed 31 vegetable-type genotypes from different maturity groups for individual forms of isoflavones and reported daidzein, glycitein, genistein, and total isoflavones content in ranges of 5.93–8.89, 2.77–3.44, 2.55–3.66, and 11.25–15.98 mg/g, respectively. The authors reported mean values (mg/100 g dry seeds) of 7.11 (daidzein), 2.98 (glycitein), 3.35 (genistein), and 13.46 (total isoflavones), which are within the ranges of the average values of the respective isoflavones in our study. Our results also showed that ‘Dada-cha 2000’, ‘Dada-cha ma-me’, ‘ASG328 Kohine’, ‘ASG328 Sricanan’ exhibited comparatively higher total isoflavones content than the average value for total isoflavones content for 12 genotypes. It is also noted here that total isoflavones content of the 12 vegetable-type genotypes in our study as well as in the investigation carried out by Mebrahtu et al.[Citation14] for 31 vegetable-type genotypes are significantly lower than that of immature seeds of grain-type soybean genotypes reported in the literature.[Citation15,Citation16] Furthermore, our results as well as that of the Mebrahtu et al.[Citation14] showed daidzein as the predominant form of isoflavones which in contrast to the observation of Kim et al.[Citation15] who reported higher levels of genistein isomers at R6 as well as R7 stage in grain type of soybean.
Table 3 Isoflavone composition (mg/100 g dry seeds) of immature seeds of vegetable-type soybean genotypes (between reproductive stages of R6 and R7)
These studies indicated significant positive relationship of taste score with the sucrose, aspartic acid, glutamic acid and alanine content (). Correlation of taste score with sucrose (r = 0.750; p < 0.05) was stronger than the aspartic acid, glutamic acid and alanine. Results also showed that panelists preferred (with hedonic score 8.0 or above) those vegetable genotypes (‘AGS436’,’AGS440’,’Dada-cha 2000’,’Dada-cha ma-me’), which showed sucrose content to the magnitude of 6.00 g/100 g dry seeds or more. ‘Table Variety’, the genotype which was rated the least (6.0) for taste by the panel, was found to contain the lowest sucrose content (1.28 g/100 g dry seeds), though the genotype has moderate levels of aspartic acid, glutamic acid, glycine, and alanine. This indicated that the sucrose content was the major contributor to the taste of the green seeds. Furthermore, ‘AGS437’ and ‘AGS439’ the genotypes with sucrose content to the magnitude of 6% or more, scored poor (6.2 and 6.0, respectively) on hedonic scale. This may be ascribed to the presence of low levels of amino acids (aspartic acid, glutamic acid) in these two genotypes. This is in consonance with earlier report[Citation1] which suggested the positive relationship of aspartic acid and glutamic acid with taste in vegetable-type soybean. Results did not indicate any significant correlation between glycine content and the panelist's preference which coincides with the earlier study.[Citation1] also shows that correlation coefficients between taste score of the green seeds and the levels of individual forms of isoflavones were positive but not significant .This is an aberration from the studies of Carrao-Panizzi et al.[Citation2] who suggested an association between astringency of soymilk and their isoflavones content. In our study, ‘Dada-cha ma-me’ is among high-ranking genotypes for isoflavones content (27.02 mg/100 g) but still this genotype was rated the highest (8.8) for taste. This may be attributed to the high concentration of sucrose (7.12 g/100 g dry seeds) and glutamic acid (2.21 g/100 g dry seeds) observed in this genotype, which might have offset the astringent effect of high isoflavones content. Similarly, genotype ‘ASG328 Sricanan’ despite having the highest concentration of isoflavones, scored high (8.1) for taste, which may be attributed to its appreciate levels of sucrose content (5.86 g/100 g dry seeds) and high concentration of glutamic acid (2.82 g/100 g dry seeds).
Table 4 Correlation co-efficient of taste score with contents of sucrose, aspartic acid, glutamic acid alanine, daidzein, glycitein, genistein, and total isoflavones
CONCLUSION
Over all, the studies showed genotypic variability for the sucrose content and sweetness imparting amino acids viz. aspartic acid, glutamic acid, glycine. and alanine, which can be exploited in the plant breeding program focusing on the development of vegetable-type genotypes. The results did not indicate the negative impact of isoflavones on the taste of green seeds of vegetable soybean. ‘Dada-cha ma-me’, ‘AGS440’, ‘AGS436’, the genotypes identified with high sucrose as well as aspartic and glutamic acid contents, were found to be the most promising genotypes for vegetable purpose.
REFERENCES
- Wang, Z.; Wang, D. Studies on the correlation between the quality traits of vegetable soybean http://www.avrdc.org/pdf/soybean (http://www.avrdc.org/pdf/soybean) (Accessed: 18 July 2011 ).
- Carrao-Panizzi , M.C. , Beleia , A.D.P. , Prudencio-Ferreira , S.H. , Oliveira , M.C.N. and Kitamura , K. 1999 . Effect of isoflavones on beany flavour and astringency of soymilk and cooked whole soybean grains . Agronomy Series Brazilian Agricultural Research Corporation, Brasilia , 34 ( 6 ) : 1045 – 1052 .
- Shanmughamsudaram , S. and Marr , M.R. Global expansion of high value vegetable soybean . Proceedings of the IV International Soybean Processing and Utilization Conference, Brazil . pp. 915 – 920 .
- Yanagisawa , Y. , Akazawa , T. , Abe , T. and Sasahara , T. 1997 . Changes in free amino acid and Kjeldahl N concentrations in seeds from vegetable-type and grain-type soybean cultivars during the cropping season . Journal of Agricultural Food Chemistry , 45 ( 5 ) : 1720 – 1724 .
- Abe , T. , Ujiie , T. and Sasahara , T. 2004 . Varietal differences in free amino acid and sugar concentrations in immature seeds of soybean under raw and boiling treatment . Journal of the Japanese Society for Food Science and Technology , 51 ( 3 ) : 172 – 176 .
- Fehr , W.R. , Caviness , C.E. , Burmood , D.T. and Penington , J.S. 1971 . Development description of soybean, Glycine max (L) Mer . Crop Science , 11 : 929 – 931 .
- Vyn , T.J. , Yin , X. , Bruulsema , T.W. , Jackson , J.C. , Rajcan , I. and Bruder , S.M. 2002 . Potassium fertilization effects on isoflavones concentrations in soybean . Journal of Agricultural Food Chemistry , 50 : 3501 – 3506 .
- Larmond , E. 1987 . Laboratry Methods of Sensory Evaluation of Foods , Otawa, , Canada : Canada Department of Agriculture Publication 1637 .
- Kumar , V. , Rani , A. , Billore , S.D. and Chauhan , G.S. 2006 . Physico-chemical properties of immature pods of Japanese soybean cultivars . International Journal of Food Properties , 9 : 51 – 59 .
- Tomoko , H. , Konoshin , T. , Akira , F. , Yoshinobu , I. and Katuya , N. 2003 . Effect of holding condition before freezing on quality in immature black soybean ‘Tanbaguro’ as a vegetable . Food Preservation Science , 29 ( 1 ) : 11 – 16 .
- Song , J.Y. , An , G.H. and Kim , C.J. 2003 . Color, texture, nutrient content and sensory values of vegetable soybean [Glycine max (L.) Merrill] as affected by blanching . Food Chemistry , 83 : 69 – 74 .
- Kumar , V. , Rani , A. and Chauhan , G.S. 2007 . A comparative study of oligosaccharides and sucrose content in immature and mature seeds of soybean genotypes . Journal of Food Science and Technology , 44 ( 1 ) : 49 – 51 .
- Lisiewska , Z. , Kmiecik , W. and Slupski , J. 2007 . Content of amino acids in raw and frozen broad beans (Vicia faba var. major) seeds at milk maturity stage, depending on the processing method . Food Chemistry , 105 : 1468 – 1473 .
- Mebrahtu , T. , Mohamed , A. , Wang , C.Y. and Andebrhan , T. 2004 . Analysis of isoflavone contents in vegetable soybean . Plant Food for Human Nutrition , 59 : 55 – 61 .
- Kim , S.L. , Berhow , M.A. , Kim , J.T. , Chi , H.Y. , Lee , S.J. and Chung , I.M. 2006 . Evaluation of soyasaponin, isoflavones, protein, lipid and free sugar accumulation in developing soybean seeds . Journal of Agricultural Food Chemistry , 54 : 10003 – 10010 .
- Kumar , V. , Rani , A. , Dixit , A.K. , Bhatnagar , D. and Chauhan , G.S. 2009 . Relative changes in tocopherols, isoflavones, total phenolic content and antioxidative activity in soybean seeds at different reproductive stages . Journal of Agricultural and Food Chemistry , 57 : 2705 – 2710 .