Abstract
Antioxidant activity of three different extracts (ethanolic, chloroformic, and hexanic) of red flesh pitaya (Hylocereus polyrhizus) seed using free radical scavenging assay, linoleic acid model system, and ferric thiocyanate (FTC) method was determined. Ethanolic extract inhibit 74.76% of free radicals at 1000 μg/mL, while chloroformic extract gave the highest inhibition using linoleic acid model system (98.90% at 100 μg/mL) and FTC (96.34%) method. Total phenolic and ascorbic acid contents of the seed were 13.56 ± 2.04 and 0.36 ± 0.01 mg/g, respectively, while catechin was the major flavonoid detected. In conclusion, the study showed that both polar and non-polar compounds contribute to the antioxidative activity measured.
INTRODUCTION
Cardiovascular diseases, diabetes, cancer, arthritis, and many other diseases suffered by the patients may be caused by genetic or the age factors.[Citation1] However, personal lifestyle that includes dietary intakes and physical activity, along with environmental surroundings, may also contribute to the risk of the diseases. In addition, free radicals produced by our body inducing oxidative stress is related to several other harmful effects, including coronary heart diseases, neodegenerative disorders, and aging.[Citation2] Consumption of fresh fruits and vegetables are inevitably important to help prevent the action of free radicals.[Citation2]
For many years, fruits have become the main subject for researchers to investigate for their bioactive compounds that are beneficial for human health and red pitaya fruit is a new promising one. The red pitaya (Hylocereus polyrhizus) fruit, that is known also as pitahaya, and dragon fruit fall under the Cactaceae family under the genus Hylocereus.[Citation3] This exotic fruit is gaining much interest nowadays due to its unique taste and shape, along with the attractive colour. Currently, researchers are more interested in the red pigment of pitaya fruits, known as betacyanin that falls under the group of betalain, which can be used to replace synthetic dyes. Extensive research had been carried out on the pigment[Citation4,Citation5] including the beneficial effect of betalains as antiradical and antioxidative agent.[Citation6–8 However, the studies conducted on the antioxidative activity of red pitaya fruit[Citation9,Citation10] were mainly on its pulp and peel. Recently, there was a report on the essential fatty acids found in the red and white pitaya seeds, in particular, linoleic acid.[Citation11] Nevertheless, there is negligible report on the antioxidative activity of pitaya seeds, as well as their potential bioactive compounds.
Seeds from other fruits, such as the grape seeds,[Citation12,Citation13] berries,[Citation14] and from a Cactaceae family, Opuntia sp.,[Citation15] are potent antioxidative agent. They are abundant with various compounds ranging from hydrophilic to lipophilic, such as flavonoids, phenolic acids, carotenoids, tocopherols, and essential fatty acids that may prevent the oxidative damage done by the free radicals. The grape seed procyanidin is renowned as a good antioxidative agent,[Citation16] and the grape seed oil were available commercially due to its goodness comparable to vitamin E in reducing lipid oxidation and scavenging free radicals. Hence, this study was conducted to determine the antioxidant activity of the red pitaya seed through extraction using solvents of different polarities and to examine the potential bioactive compounds responsible for the activity.
MATERIALS
Chemicals
Tween 20, 3-t-butyl-4-hydroxyanisole (BHA), ferrous chloride, 1,1-diphenyl-2-picrylhydrazyl (DPPH), disodium hydrogen phosphate, sodium dihydrogen phosphate, and α-tocopherol were obtained from Sigma (St. Louis, MO, USA). Ammonium thiocyanate, Folin-Ciocalteu reagent, sodium carbonate, citric acid, and trifluoroacetic acid (HPLC grade) were purchased from Fisher Scientific (Leicestershire, UK). Both analytical and HPLC grade methanol and 95% ethanol were from BDH (VWR International Ltd., England), while chloroform and n-hexane were from Merck (Darmstadt, Germany). Gallic acid and linoleic acid were from Acros Organic (NJ, USA).
METHODS
Sample Preparation and Extraction
The red pitaya fruits were obtained from a pitaya farm in Sepang, Selangor. Upon arrival at the laboratory, the fruits were peeled and the seeds were separated from the pulp manually and washed with tap water several times to remove mucilages and pulp leftover. The seeds were air-dried, ground, and sieved (<0.85 mm particle sized). Some of the ground seed were kept in a capped-bottle for determination of ascorbic acid and flavonoids content, while the others were extracted with solvents of different polarity, namely 95% ethanol, chloroform, and n-hexane. Extraction was carried out, according to Siddhuraju and Manian,[Citation17] with some modifications. Briefly, 20 g of the ground seed were extracted with 200 mL solvent and extraction were carried out in an Innova 4000 incubator shaker (New Brunswick Scientific, NJ, USA) at 30°C, 150 rpm for 24 h. The extracts were vacuum filtered through Whatman No. 4 filter paper and rotary evaporated (Büchi, Switzerland) at 40°C to remove the solvents. The extracts were stored at −20°C until further analysis.
DPPH Free Radical Scavenging Activity
The antioxidative activities of the different extracts were measured using the DPPH free radical scavenging assay.[Citation18] 100 μL of extracts with different concentrations (200–1000 μg/mL) were mixed with 3.9 mL DPPH• (25 mg/L) in methanolic solution and the reaction mixture were allowed to stand for 30 min in the dark before measuring the optical density at 515 nm. Methanol served as a blank, whereas the control is prepared with 100 μL methanol with DPPH solution. The antioxidant activity is expressed as percentage of inhibition of the free radical calculated using this equation:
A higher percentage of inhibition indicated a better antioxidant. BHA served as a positive control.
Antioxidant Activity in Linoleic Acid Model System
Formation of conjugated diene was determined to measure the antioxidative activity of the extracts.[Citation19] The range of concentration used in this assay was 20–100 μg/mL. BHA served as a positive control. The linoleic acid emulsion was prepared by mixing 10 mM linoleic acid and Tween 20 (with the same amount as linoleic acid) with 0.2 M sodium phosphate buffer, pH 6.5. The antioxidant activity (AOA%) was calculated as follow:
Ferric Thiocyanate Assay
The linoleic acid peroxidation was determined using the ferric thiocyanate (FTC) method.[Citation20] Preparation of linoleic acid emulsion was made by mixing 500 μL of 1 mg/mL of different extracts, α-tocopherol, and BHA with 2.5 mL linoleic acid emulsion and 2.5 mL phosphate buffer pH 7.0 in an amber capped-vial. Reaction mixture without the test sample served as a control. All the test samples were incubated at 40°C and measured every day until the absorbance of the control reached a maximum. The measurement of the antioxidant activity was carried out by withdrawing 100 μL aliquots from the test samples and mixed with 5 mL of 75% ethanol, 100 μL of ammonium thiocyanate, and 100 μL of 20 mM ferrous chloride in 3.5% HCl. The changes in colour of the mixtures were measured at 500 nm exactly after 3 min. Lipid peroxidation inhibition (LPI) was calculated up until day three of measurement as follows:
Determination of Total Phenolic Content
Determination of total phenolic content in the seed was carried out following the Folin-Ciocalteu method by Singleton and Rossi[Citation21] as described by Peschel et al.[Citation22] 100 μL of the ethanolic extract (1 mg/mL) was mixed up with 7.9 mL distilled water and 0.5 mL Folin-Ciocalteu's reagent. After 2 min, 1.5 mL of 7.5% sodium carbonate solution was added in and mixed thoroughly. The sample was measured spectrophotometrically (Shimadzu, Japan) at 765 nm after 2 h of incubation. The amount of total phenolics was determined as gallic acid equivalent (GAE) and expressed as mg GAE/g seed dry weight.
Ascorbic Acid Content
Determination of ascorbic acid content in the seed was carried out using Waters 2487 dual wavelength absorbance detector HPLC system (Waters Associates, Milford, MA). Quantification of the ascorbic acid was performed using the Waters reversed-phase Symmetry C18 column (150 × 3.9 mm i.d., 5 μm) at 25°C. Mobile phase used was acetonitrile:methanol (70:30 v/v) that was run isocratically at flow rate of 1 mL/min by Waters 600 pump. The wavelength was set at 254 nm with 20 μL injection volume. Extraction of ascorbic acid was carried out by homogenizing 1 g dried seed with 20 mL 3% citric acid for 3 min.[Citation23] The extract and ascorbic acid standard were filtered through Waters C18 Sep-Pak cartridge before refiltered with 0.45 μm Whatman nylon membrane filter prior to HPLC analysis. Solvents used were of HPLC grade. Identification was done based on the retention time and absorbance spectra of ascorbic acid standard used while quantification was done using a standard curve (0–100 μg/mL).
Flavonoids Content
Several flavonoids in the seed (catechin, epicatechin, quercetin, myricetin, kaempferol, and rutin) were detected and quantified according to the modified method[Citation24] of Hertog et al.[Citation25] using the HPLC system and column mentioned before. The flavonoids content were compared with a known standard and expressed as mg flavonoid/g seed dry weight. Two mobile phases were used. Solvent A consisted of triflouroacetic acid in deionized water, pH 2.5 whereas solvent B is made up of 100% methanol and runs using a linear gradient: at 0 min, 100% solvent A; at 20 min, 50% solvent A and 50% solvent B; at 30 min, 100% solvent B; and at 35–40 min, 100% solvent A. The wavelength was set at 280 nm with 20 μL injection volume. Extraction of the compounds was done by hydrolyzing 1 g of seed with 12 mL methanol, 8 mL deionized water, and 5 mL 6 M HCl at 95°C for 2 h. The test sample was allowed to cool down to room temperature before being filtered with 0.45 μm Whatman nylon membrane filter. Solvents used were of HPLC grade.
Statistical Analysis
Statistical analysis of the data were subjected to a one way analysis of variance and the significant difference was determined by Tukey's multiple range test (P < 0.05) using the Minitab Release 14 (Minitab Inc., PA, USA). All analyses were done in triplicate and data were expressed as means ± SD.
RESULTS AND DISCUSSION
Extraction Yield
Three solvents with different polarities were chosen for extraction of the red pitaya seed. Extraction using ethanol attained both solid and oil fraction since the seed was not defatted before analysis, whereas for non-polar solvents, chloroform and hexane, only the oil fractions were extracted out. Defatting of sample may reduce the bioactive compounds in the seed since some of the compounds were probably eliminated out and lessen the total phenolic content as reported by Yilmaz and Toledo[Citation16] against the de-oiling of Muscadine seed. Result obtained in show the extraction yield; in decreasing order with chloroform > hexane > ethanol. From the result, around 27–35% of oil in the seed was extracted through extraction using chloroform and hexane. Chloroformic extract gained the highest (p < 0.05) yield with 34.9% and 2.27-fold to that of ethanolic extract (15.4%). Hexanic oil extract was 26.9% and even higher than the caneberry seed oil (11–19% oil) extracted using the same solvent.[Citation14] Nevertheless, high yield does not necessarily imply that it will also give high antioxidative activity as proven by several researchers,[Citation12,Citation13,Citation26] since the antioxidative activity depends on the potent antioxidant present in the extract.
Table 1 Extraction and percent yield of the red pitaya seed extracts using different solvents
DPPH Free Radical Scavenging Activity of the Extracts
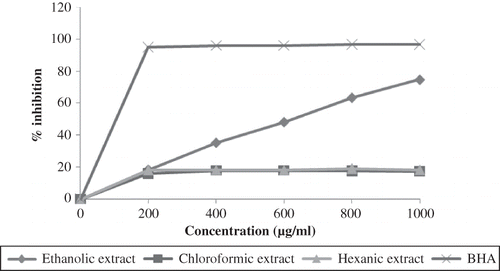
DPPH assay is one of the widely used methods to test the antioxidative activity of the sample due to its stability, simplicity, and the short time required for analysis. Basically, the antioxidant capacity of the samples are measured through their ability to reduce the DPPH• by donating the hydrogen atom and can be determined through the discoloration of the mixture using the spectrophotometer.[Citation18] It can be seen in that only the ethanolic extract showed the inhibitory effect towards the free radicals with high antioxidant activity in a dose-dependent behaviour as the chloroformic and hexanic extracts showed a very limited activity. At 1000 μg/mL, the ethanolic extract scavenged 74.76% of DPPH free radicals and is significantly (p < 0.05) different as compared to the chloroformic and hexanic extract with only 17.53% and 18.28%, respectively. However, the reducing ability of the ethanolic extract at that concentration is still not equivalent to BHA.
High phenolic content were usually correlated with high radical scavenging activity.[Citation9] Moreover, phenolic compounds were more extractable using polar solvents as stated elsewhere.[Citation17,Citation26] Hence, antioxidant activity of the pitaya seed ethanolic extract was most probably due to the presence of polyphenols, such as flavonoids and phenolic acids in the extract, which have the hydrogen-donor ability to scavenge the free radicals. It has been suggested that flavonoids, such as procyanidin B2, epicatechin, and epigallocatechin gallate, which were found mainly in the ethanolic extract of loquat seed, contributed towards its high antioxidative activity against the free radicals.[Citation27] Reports by Brand-Williams et al.[Citation18] stated that the free radical scavenging activity of phenolics is dependable on their structure and the antioxidant activity was proportional with the number of hydroxyl group present.
Even though this method might not affected by substrate polarity,[Citation28] the less polar compounds extracted in the chloroformic and hexanic extracts may not have the compounds that can easily donate the hydrogen atom/electron to reduce the DPPH•. Their complex mechanism of action may also be the contributor of low antioxidant activity, as proven by Xu et al.[Citation29] They observed that there was a slow reaction in the kinetic behaviour of sesame seed n-hexane extract in contrast to other polar solvents' extracts. Hence, it is necessary to conduct more than one antioxidative test to evaluate the antioxidant activity of the samples due to the differences in reaction behaviour.
Antioxidant Activity of the Extracts in Linoleic Acid Model System
Formation of conjugated diene is an indication of lipid oxidation during the initiation phase and can be measured spectrophotometrically at 234 nm. A good antioxidant should have the ability to act as a chain breaker during that phase and inhibiting the oxidation process.[Citation30] All the extracts possessed good inhibitory effect towards the oxidation process, as shown in At 20 μg/mL, all extracts showed inhibition of 60–80% and were not significantly different among each other except with BHA (97.25%). At 100 μg/mL, all the samples tested showed relatively the same level of inhibitory effect with 95.60, 98.90, and 88.46% for ethanolic, chloroformic, and hexanic extract, respectively, and were not significantly different with that of BHA.
Figure 2 The antioxidant activity of the red pitaya seed extracts as measured by linoleic acid model system.
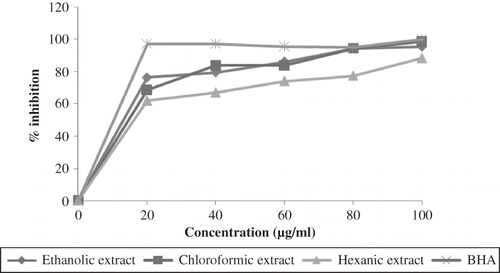
Using this method, the trend in the antioxidant activity of the extracts were not similar with that of the DPPH• assay. All the extracts possessed nearly the same inhibitory effect towards the formation of conjugated diene, while in the previous assay only ethanolic extract exhibited strong inhibitory activity. Furthermore, the concentrations used were much smaller as compared to DPPH• method indicating the effectiveness of the extracts in inhibiting the oxidation process in lipid model system. Emulsified media was able to reveal the extracts antioxidative ability due to their potential interactions with the emulsion system. According to Koleva et al.,[Citation31] lipophilic antioxidants were concentrated in the oil phase that gives powerful protection of the emulsions against oxidation that showed the occurrences of polar paradox.
Ferric Thiocyanate Method
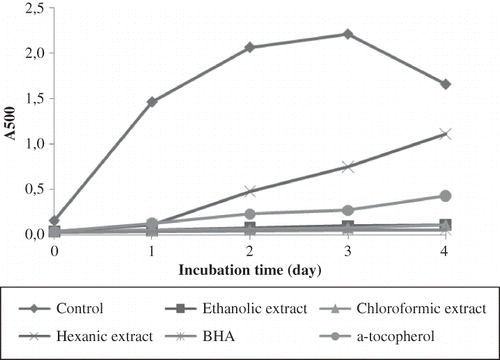
Peroxides formed during the initial stage of linoleic acid oxidation can also be measured using the FTC assay. Peroxides will oxidize Fe2+ to Fe3+ and reaction with SCN- will give the red colour due to formation of thiocyanate.[Citation22] In , the control gave maximum absorbance up to day 3 and started to decrease since the linoleic acid was already used up and no more hydroperoxides were available to oxidize Fe2+ until this stage.[Citation12] Chloroformic extract (96.34%) was slightly higher than ethanolic extract (95.52%) in terms of their lipid peroxidation inhibition using this assay. The ability of ethanolic and chloroformic extracts to slow down the oxidation of linoleic acid is comparable to BHA and α-tocopherol and even better than the latter. Even though hexanic extract displayed the lowest antioxidative activity with 66.02% inhibition, the differences are not significant between all the samples tested. Xu et al.[Citation29] also reported a similar trend referring to the n-hexane extract of sesame seed using the same assay. Inhibition of lipid peroxidation may be due to the seed coat, as proposed by Siddhuraju and Manian,[Citation17] that protects the seed internally by inducing endogenous antioxidants.
These results revealed that the extracts exhibited their efficacy as an antioxidant more in the linoleic acid or lipid emulsion. This may be due to the emulsion properties itself as linoleic acid is likely to control the level of dissociation of the extracts, especially the non-polar compounds that exhibited an interaction between the extract and emulsion.[Citation31] Apart from the hydrophilic compounds present in the ethanolic extract, the lipophilic compounds, such as essential fatty acids, phospholipids, carotenoids, and tocopherols, that may be present in all the extract also possessed a good antioxidant activity.
Total Phenolic, Ascorbic Acid, and Flavonoid Content
Phenolic compounds are widely known for their beneficial effects, such as preventing hormone-related cancers, potent antioxidant, and antibacterial agents.[Citation32] Results obtained by Chang et al.[Citation15] also suggested that phenolic acids and flavonoids were the main contributors in antioxidant activity and anti-LDL peroxidation. The total phenolic content of the pitaya seed, as shown in , is 13.56 ± 2.04 mg GAE/g dry weight. This amount of total phenolic was much lower if compared to the grape seed, which were around 27.35–46.69 mg GAE/g dry weight depending on the varieties.[Citation16] Drying or heating of the seed affect the total phenolic content. Heating at certain degrees of temperature increase the extractable phenolic compounds, but at higher temperature it will start to reduce the compounds, as reported by Soong and Barlow.[Citation33]
Table 2 Total phenolic and ascorbic acid contents of the red pitaya seed
shows that the ascorbic acid content of the seed is 0.36 ± 0.01 mg/g dry weight. This is interesting as the ascorbic acid content of the pitaya pulp itself is 7–11 mg/100 g fresh weight [Citation34] and considered low in comparison to other cactus family, such as Opuntia sp. Nonetheless, there is no ascorbic acid detected in the seed of the Opuntia dillenii Haw fruit.[Citation15] Selected flavonoids in the seed were measured and compared with external standards using HPLC system (). Results obtained are shown in and it was expressed as mg/g seed dry weight. Catechin was the major flavonoid detected in the red pitaya seed with 3.60 ± 2.33 mg/g dry weight. This was followed by quercetin, myricetin, and epicatechin with 1.31 ± 0.45, 0.63 ± 0.30, and 0.60 ± 0.11 mg/g dry weight, respectively. On the other hand, the amount of rutin found in the red pitaya seed was the lowest with 0.53 ± 0.02 mg/g dry weight. The amount of kaempferol in the seed was below detection limit. Catechin, epicatechin, and quercetin were several major flavonoids found in the Opuntia dillenii Haw seed.[Citation15]
Figure 4 HPLC chromatograms of (a) a mixture of flavonoid standard; (b) red pitaya seed [absorbance at 280 nm vs. time (min)]: catechin (1), epicatechin (2), rutin (3), quercetin (4), myricetin (5), and kaempferol (6).
![Figure 4 HPLC chromatograms of (a) a mixture of flavonoid standard; (b) red pitaya seed [absorbance at 280 nm vs. time (min)]: catechin (1), epicatechin (2), rutin (3), quercetin (4), myricetin (5), and kaempferol (6).](/cms/asset/8629f772-e487-4cbe-9c64-71c4ce3522e7/ljfp_a_459787_o_f0004g.gif)
Table 3 Flavonoids content of the red pitaya seed
CONCLUSION
Result of the study showed that total phenolic compound and ascorbic acid content of red pitaya seed were found to be 13.56 ± 2.04 and 0.36 ± 0.01 mg/g, respectively. Catechin (3.60 ± 2.33 mg/g) was found to be the major flavonoid detected. The extracts showed different trends of antioxidant activity when determined using different methods, reflecting the different mechanisms of action involved. Chloroformic and hexanic extracts only showed potent antioxidant activity using the linoleic acid model system and FTC method. Ethanolic extract exhibited high radical scavenging activity at 1000 μg/mL with 74.76% that was significantly (p < 0.05) different than that of chloroformic (17.53%) and hexanic extract (18.28%). Overall, the study revealed the health benefits and potential use of red pitaya seed as a source of natural antioxidant.
ACKNOWLEDGMENTS
The authors would like to thank the Universiti Putra Malaysia for the financial support under Research University Grant Scheme (RUGS) (Project No. 02/01/07/0015RU).
REFERENCES
- Ignarro , L.J. , Balestrieri , M.L. and Napoli , C. 2007 . Nutrition, physical activity, and cardiovascular disease: An update . Cardiovascular Research , 73 : 326 – 340 .
- Ferrari , C.K.B. 2007 . Functional foods and physical activities in health promotion of aging people . Maturitas , 58 : 327 – 339 .
- Mizrahi , Y. , Nerd , A. and Nobel , P.S. 1997 . Cacti as crops . Horticultural Reviews , 18 : 291 – 320 .
- Esquivel , P. , Stintzing , F.C. and Carle , R. 2007 . Pigment pattern and expression of colour in fruits from different Hylocereus sp. genotypes . Innovative Food Science and Emerging Technologies , 8 : 451 – 457 .
- Stintzing , F.C. , Schieber , A. and Carle , R. 2002 . Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton and Rose . Food Chemistry , 77 : 101 – 106 .
- Pavlov , A. , Kovatcheva , P. , Tuneva , D. , Ilieva , M. and Bley , T. 2005 . Radical scavenging activity and stability of betalains from Beta vulgaris hairy root culture in simulated condition of human gastrointestinal tract . Plant Foods for Human Nutrition , 60 : 43 – 47 .
- Cai , Y.-Z. , Sun , M. and Corke , H. 2005 . Characterization and application of betalain pigments from plants of the Amaranthaceae . Trends in Food Science and Technology , 16 : 370 – 376 .
- Stintzing , F.C. and Carle , R. 2004 . Functional properties of anthocyanins and betalains in plants, food, and in human nutrition . Trends in Food Science and Technology , 15 : 19 – 38 .
- Wu , L.-C. , Hsu , H.-W. , Chen , Y.-C. , Chiu , C.-C. , Lin , Y.-I. and Ho , J.-A. 2006 . Antioxidant and antiproliferative activities of red pitaya . Food Chemistry , 95 : 319 – 327 .
- Mahattanatawee , K. , Manthey , J.A. , Luzio , G. , Talcott , S.T. , Goodner , K. and Baldwin , E.A. 2006 . Total antioxidant activity and fiber content of select Florida-grown tropical fruits . Journal of Agricultural and Food Chemistry , 54 : 7355 – 7363 .
- Ariffin , A.A. , Bakar , J. , Tan , C.P. , Abdul Rahman , R. , Karim , R. and Loi , C.C. 2009 . Essential fatty acids of pitaya (dragon fruit) seed oil . Food Chemistry , 114 : 561 – 564 .
- Jayaprakasha , G.K. , Singh , R.P. and Sakariah , K.K. 2001 . Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro . Food Chemistry , 73 : 285 – 290 .
- Mandic , A.I. , Ðilas , S.M. , Ćetković , G.S. , Ćanadanović-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 : 713 – 726 .
- Bushman , B.S. , Phillips , B. , Isbell , T. , Ou , B. , Crane , J.M. and Knapp , S.J. 2004 . Chemical composition of caneberry (Rubus spp.) seeds and oils and their antioxidant potential . Journal of Agricultural and Food Chemistry , 52 : 7982 – 7987 .
- Chang , S.-F. , Hsieh , C.-L. and Yen , G.-C. 2008 . The protective effect of Opuntia dillenii Haw fruit against low-density lipoprotein peroxidation and its active compounds . Food Chemistry , 106 : 569 – 575 .
- Yilmaz , Y. and Toledo , R.T. 2006 . Oxygen radical absorbance capacities of grape/wine industry byproducts and effect on solvent type on extraction of grape seed polyphenols . Journal of Food Composition and Analysis , 19 : 41 – 48 .
- Siddhuraju , P. and Manian , S. 2007 . The antioxidant activity and free-radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds . Food Chemistry , 105 : 950 – 958 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft und–Technologie , 28 : 25 – 30 .
- Lingnert , H. , Vallentin , K. and Eriksson , C.E. 1979 . Measurement of antioxidative effect in model system . Journal of Food Processing and Preservation , 3 : 87 – 103 .
- Yen , G. and Hsieh , C. 1998 . Antioxidant activity of extracts from Du-zhong (Eucommia ulmoides) towards various lipid peroxidation models in vitro . Journal of Agricultural and Food Chemistry , 46 : 3952 – 3957 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Peschel , W. , Sánchez-Rabaneda , F. , Diekman , W. , Plescher , A. , Gartzía , I. , Jiménez , D. , Lamuela-Raventós , R. , Buxaderas , S. and Codina , C. 2006 . An industrial approach in the search of natural antioxidants from vegetables and fruit wastes . Food Chemistry , 97 : 137 – 150 .
- Wimalasiri , P. and Wills , R.B.H. 1983 . Simultaneous analysis of ascorbic acid and dehydroascorbic acid in fruit and vegetables by high-performance liquid chromatography . Journal of Chromatography , 256 : 368 – 371 .
- Pak-Dek , M.S. , Abdul-Hamid , A. , Osman , A. and Soh , C.S. 2008 . Inhibitory effect of Morinda citrifolia L. on lipoprotein lipase activity . Journal of Food Science , 78 : C595 – C598 .
- Hertog , M.G.L. , Hollman , P.C.H. and Venema , D.P. 1992 . Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits . Journal of Agricultural and Food Chemistry , 40 : 1591 – 1598 .
- Liu , Q. and Yao , H. 2007 . Antioxidant activities of barley seed extracts . Food Chemistry , 107 : 732 – 737 .
- Koba , K. , Matsuoka , A. , Osada , K. and Huang , Y.-S. 2007 . Effect of loquat (Eriobotyra japonica) extracts on LDL oxidation . Food Chemistry , 104 : 308 – 316 .
- Kulisic , T. , Radonic , A. , Katalinic , V. and Milos , M. 2004 . Use of different methods for testing antioxidative activity of oregano essential oil . Food Chemistry , 85 : 633 – 640 .
- Xu , J. , Chen , S. and Hu , Q. 2005 . Antioxidant activity of brown pigment and extracts from black sesame seed (Sesamum indicum L.) . Food Chemistry , 91 : 79 – 83 .
- Yuan , Y.V. , Bone , D.E. and Carrington , M.F. 2005 . Antioxidant activity of dulse (Palmaria palmate) extract evaluated in vitro . Food Chemistry , 91 : 485 – 494 .
- Koleva , I.I. , van Beek , T.A. , Linssen , J.P.H. , de Groot , A. and Evstatieva , L.N. 2002 . Screening of plant extracts for antioxidant activity: a comparative study on three testing methods . Phytochemical Analysis , 13 : 8 – 17 .
- Sidhu , J.S. , Kabir , Y. and Huffman , F.G. 2007 . Functional foods from cereal grains . International Journal of Food Properties , 10 : 231 – 244 .
- Soong , Y.-Y. and Barlow , P.J. 2004 . Antioxidant activity and phenolic content of selected fruit seeds . Food Chemistry , 88 : 411 – 417 .
- Le Bellec , F. , Vaillant , F. and Imbert , E. 2006 . Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future . Fruits , 61 : 237 – 250 .