Abstract
This study aimed at investigating the effects of saccharose and its substitutes (sorbitol, acesulphame, aspartame) added to potato starch oxidized with sodium chlorate (I) on the physicochemical properties of starch and generation of free radicals at both 150 and 230°C. Oxidized starch with saccharose and its substitutes were analyzed by thermodynamic characterization of pasting, rheological properties, and susceptibility to degradation of 2% pastes. Moreover, a number of generated thermally free radicals in investigated samples was determined by quantitative electron paramagnetic resonance spectroscopy measurements. Saccharose and sorbitol increased considerably the onset temperature of transition (T0) and the peak temperature of transistion (Tp) as determined by differential scanning calorimetry. Among the sweeteners, only aspartame influenced the characteristic viscosities of the starch-sweetener-water systems, reducing the thermal stability of the obtained pastes. Acesulphame K and aspartame caused the largest increase in the susceptibility to retrogradation of 2% pastes of oxidized potato starch. In the presence of sorbitol, the number of radicals generated thermally in starch decreased. This effect, found by quantitative electron paramagnetic resonance spectroscopy measurements, indicates effective interaction of hydroxyl groups of the sorbitol molecule with starch matrix, leading to the stabilization of the starch structure.
INTRODUCTION
Using saccharose substitutes in the production of foods aims at lowering their energy value, which is important for people on diets. Another objective is to limit the use of saccharose or completely eliminate it from the products intended for diabetics. Therefore, the range of saccharose-free products offered by the food industry (labeled as “light”) is still expanding. Nevertheless, the presence of artificial sweeteners in foods influences their physicochemical, and especially rheological, properties.[Citation1,Citation2]
Many food products thermally treated during manufacturing or just before consumption contain starch. Native and modified starches, among them oxidized starches, have a variety of applications in the food industry as components of cake fillings and icings or as gelling agents for sauces, jellies, blancmanges, and creams.[Citation3] There is a question whether semi-artificial or artificial sweeteners increasingly used to replace saccharose would affect the physicochemical properties of a product during its thermal processing (e.g., by changing the number of thermally generated free radicals and/or their reactivity), which would have an impact on the consumer's health. The present study was aimed at investigating the effects of saccharose and its substitutes added to potato starch oxidized with sodium chlorate (I) on the physicochemical properties of the starch and the generation of free radicals during thermal treatment.
MATERIALS AND METHODS
The material used in the research was commercial potato starch produced by WPPZ S.A. Luboń (Luboń, Poland) and modified by oxidation with sodium chlorate (I). The sweeteners were saccharose (Chempur, Piekary Śląskie, Poland), sorbitol (Dakart, Kielce, Poland), aspartame (Nutra Sweet, Zug, Switzerland) and acesulphame K (Nutrinova, Sulzbach, Frankfurt, Germany). The modification of starch involved its oxidation in 40% water solution using NaClO in the amount equivalent to 40 g Cl per kg of starch. The solution of pH = 10 was stirred for 50 min at room temperature, neutralized to pH = 7, stirred, and washed. Then, the starch samples were dried and sieved.[Citation4] The efficiency of the oxidation process was estimated based on the increase in the contents of carboxyl groups[Citation5] and carbonyl groups[Citation6] determined in the starch. The amylose content of starch was established colorimetrically.[Citation7]
Saccharose was added to starch in a weight ratio of 1:1, while the amounts of saccharose substitutes were such that their addition ensured the same sweetness (1.8182 g sorbitol, 0.0050 g acesulfame K, 0.0055 g aspartame per 1 g of starch). To study the interactions between starch and saccharose or its substitutes, the following determinations were performed.
| 1. | Thermodynamic characterization of pasting was done by using a Pelkin-Elmer DSC 7 scanning calorimeter (Perkin-Elmer Co., Branford, CT, USA). Starch samples of 11 to 12 mg in weight were placed in 60-mL steel calorimeter cells. An appropriate weight of water or saccharose solution (or solution of saccharose substitute) was added, the whole was air-tight sealed and left wet for 1 h. Then, the samples were heated in the calorimeter by raising the temperature from 20 to 120°C at a rate of 10°C/min. An identical empty calorimetric cell served as a reference. The onset temperature (T 0), peak temperature (Tp ), and enthalpy (ΔH) of transition were determined from thermograms. Temperature was measured with an accuracy of ±0.5 K, and enthalpy with an accuracy of ±0.05 J/g. | ||||
| 2. | Pasting characteristics of the water dispersions of starch with saccharose or its substitutes were obtained in a rotational viscometer Rheotest 2 (VEB Profgerate Werk Medingen/Dresden, Germany) at a concentration of 3.2 g/100 g with saccharose or its substitutes. Each sample was heated with continuous stirring at a rate of 27 rpm from 50 to 96°C, then held at this temperature for 20 min, cooled back to 50°C, and held at this temperature for 10 min. The rate of temperature increase and decrease was set at 1.5°C/min. The following characteristic values of viscosity and temperature were recorded: pasting temperature (Tp ) [°C], maximum viscosity (ηmax) [mPa s], temperature at maximum viscosity (T max) [°C], viscosity at 96°C (η96) [mPa s], viscosity after 20 min of heating at 96°C (η96/20) [mPa s], viscosity at 50°C (η50) [mPa s], and viscosity after 10 min of cooling at 50°C (η50/10) [mPa s].[Citation8] | ||||
| 3. | Susceptibility to retrogradation of 2% pastes was determined by turbidimetric analysis. The pastes of starch with saccharose or its substitutes were stored at a temperature of 8°C for 14 days. The measurements of turbidance were made on the 1st, 3rd, 5th, 7th, 10th, and 14th day of storage.[Citation9] | ||||
Quantitative Electron Paramagnetic Resonance Spectroscopy Measurements
Electron paramagnetic resonance spectroscopy (EPR) measurements were performed using an X-band (9.2 GHz) Bruker ELEXSYS 500 spectrometer (Bruker, Karlsruhe, Germany) with 100 kHz field modulation. The spectra were registered at room temperature with 0.3 mT modulation amplitude and 3 mW power level before and after heating the samples at temperatures commonly used for roasting, baking, and frying of food: 150°C (30 min) and 230°C (30 min). EPR parameters were determined using the SIM 14 program.[Citation10] The accuracy of determination was 0.0005 for g factor and 0.1 mT for hyperfine splitting constant A. The number of spins was calculated by a comparison method using the VOSO4 · 5 H2O/K2SO4 standard containing 5 × 1019 spins/gram. All necessary precautions, discussed in publication[Citation11] were followed, in order to assure good precision of the quantitative EPR measurements. For turbidance, the means and standard deviations were computed. The values of pasting parameters were subjected to a one-way analysis of variance and Tukey's test at significance level α = 0.05.
RESULTS AND DISCUSSION
The oxidized potato starch had 0.76 ± 0.01 g carboxyl groups and 0.22 ± 0.01 g carbonyl groups per 100 g d.m. and contained 27.96 ± 0.15% amylose. summarizes the results of calorimetric analysis (differential scanning calorimetry, DSC) of the preparations of oxidized potato starch without additives and with an addition of saccharose or its substitutes. The addition of saccharose increased the onset temperature (T 0) and the peak temperature (Tp ) of transition by about 10°C, compared to the values observed for the starch-water system. Sorbitol produced an even stronger effect with the increase approximating 20°C. The explanation for the rise in the temperature of the phase transitions of oxidized starch in the presence of both sweeteners may be sought in theories about substitution of water in the starch granule by molecules of the dissolved saccharide.[Citation12] Saccharide molecules penetrating into the starch granules may decrease their moisture content and consequently increase the pasting temperature of starch. The nearly two times bigger increase of T 0 and Tp for sorbitol than saccharose is probably due to the higher hygroscopicity of the former and its larger amount added to starch to reach a similar sweetness of the two pastes.
Table 1 Thermodynamic characteristics of pasting of oxidized potato starch with saccharose and its substitutes
Based on the literature, it could be expected that both values of T 0 and Tp were increased and also ΔH value for the investigated starch-sugar-water systems. Higher values of T 0 and ΔH for starch-sugar-water systems in comparision to systems without sugar were observed by Kohyama et al.[Citation13] and Baek et al.[Citation14] The increase of T 0 and ΔH values was explained in Kohyama et al.[Citation13] as due to the strengthening of the crystalline region of starch by sugar molecules. In this work, an increase of ΔH values was observed only in the case of starch-sorbitol-water system. The transition enthalpy of the saccharose-containing system dropped (by 2.22 J/g), which agrees with the findings of Buck and Walker[Citation15] and Kim and Walker.[Citation16] The other saccharose substitutes, i.e., acesulphame K and aspartame, had practically no impact on T 0, Tp , or ΔH. This might be attributed to the fact that they were present in the systems in small amounts because of their very high sweetness as compared to saccharose.
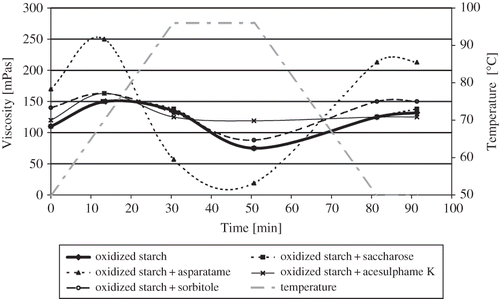
shows the main parameters of pasting curves, and displays the course of the curves for oxidized potato starch without and with sweetener additives. An analysis of the results revealed that the pastes differed in rheological properties. The addition of aspartame decreased the pasting temperature of oxidized starch, while saccharose and sorbitol increased this temperature, and acesulphame K had no effect. Only aspartame produced a significant effect on both maximum viscosity (increased ηmax compared to that of the paste of oxidized starch alone), viscosity at 96°C and viscosity after 20 min at this temperature (decreased η96°C and η96/20). The viscosities of the pastes of oxidized starch and all sweetener-containing systems decreased when they were held at 96°C for 20 min. This suggests that the pastes had a low stability and resistance to high temperatures and to mechanical stresses.[Citation2] Cooling the pastes down to 50°C resulted in the increased viscosity of all of them, with the increase being the largest (from 19 to 213 mPas) for the starch-aspartame-water system. Amino acids produced from aspartame decomposition would interact with starch, making starch gelation easier. As a result of this phenomenon the increase of viscosity during cooling was observed. After the 10-min holding at a temperature of 50°C, the paste viscosity of potato starch with an addition of saccharose or its substitutes did not differ considerably from η96°C, which shows that the sweeteners had no impact on the rate of starch retrogradation at this temperature.
Table 2 Basic parameters of pasting curves of oxidized potato starch with saccharose and its substitutes
The addition of saccharose, sorbitol, and acesulphame K did not affect (or affected only slightly) the course of pasting of oxidized starch at the temperatures applied (). By contrast, aspartame produced a marked effect: the pasting curve of oxidized starch with this sweetener had a similar course to those of native potato starches or potato starches with a small degree of oxidation, i.e., a large increase of viscosity during the heating to 70°C, then a decrease of viscosity during the heating to 96°C and keeping at this temperature for about 14 min. Then a slight increase of viscosity was observed during the last 6 min of the hold period, followed by an intensive increase during the cooling to 50°C and holding at this temperature.[Citation17,Citation18] Such a pattern indicates that aspartame greatly influences the stability of the starch-water system, decreasing its resistance to temperatures and mechanical shear stresses. The low stability of the paste of starch with this sweetener may be due to the cleavage of the peptide bond of aspartame caused by high temperature (96°C).[Citation19]
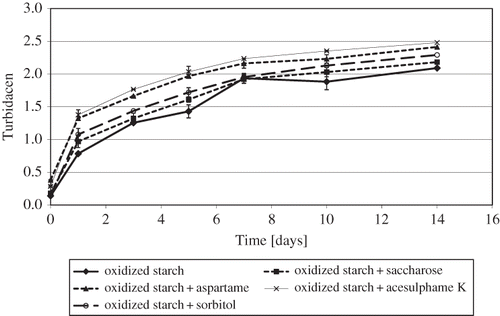
The susceptibility to retrogradation of the starch pastes stored at a temperature of 8°C for 14 days changed with time (). During the whole storage period (i.e., from the cooling of samples after pasting to the 14th day of storage), the pastes of oxidized potato starch with an addition of saccharose or its substitutes were more susceptible to retrogradation than the paste of starch alone. The increase in retrogradation was biggest for aspartame and acesulphame K, and smallest for saccharose. It is likely that already in the course of cooling some amount of saccharose (or its substitutes) became linked (e.g., by hydrogen bonds) to amylose chains present in the solution or got adsorbed on the remaining starch granules, which accelerated the retrogradation process. As time went by, the other part of the sweetener present in the solution gradually got involved in this process, thus contributing to a faster linkage of amylose chains and their removal from the solution.[Citation20,Citation21] Our findings about the effect of monosaccharides on the retrogradation of starch disagree with those of Prokopowich and Biliaderis.[Citation22] The latter authors claim that monosaccharides dissolved in water stabilize starch structure, thus reducing the rate of starch retrogradation processes. It should be mentioned, however, that they investigated a starch-sugar-water system with much larger starch and sugar contents than those used in our studies.
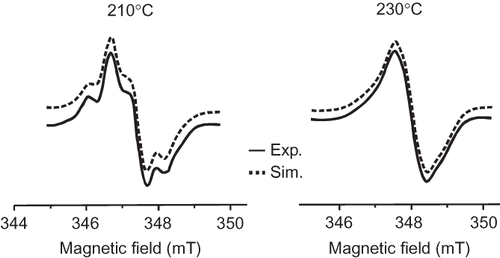
No EPR signals were observed in the native and oxidized starch before the thermal treatment. The heating of the starch at temperature 150°C led to signals with very small intensity which increases with increasing temperature. shows the signals generated at higher temperatures of 210 and 230°C. The EPR spectrum consisted of two anisotropic signals, with similar g factor values, both attributed to an unpaired electron localized at C(1) atom of the glucose unit.[Citation23] The signals appearing after thermal treatment of the oxidized starch at 210°C exhibited the following g factor values: g 1 = 2.0030 − 2.0036, g 2 = 2.0060 − 2.0064, g 3 = 2.0098 − 2.0099. One of the signals revealed additionally the hyperfine structure, with A 1 = 1.0 mT, A 2 = 1.4 mT, A 3 = 1.5 mT, resulting from interaction between magnetic moments of the electron and nucleus of the β hydrogen (localized at C(2)). With increasing temperature of the thermal treatment (up to 230°C), the spectrum became less anisotropic () and the contribution of the signal with hyperfine structure decreased. This effect is connected with the abstraction of β hydrogen during dehydration of the starch occurring upon thermal treatment.[Citation23] Simultaneously, the EPR parameters slightly changed and became closer themselves (g 1 = 2.0037, g 2 = 2.0060 − 2.0062, g 3 = 2.0083 − 2.0084).
Figure 3 Experimental and simulated EPR spectra of radicals generated during thermal treatment at 210 and 230°C (registration at room temperature).
The introduction of various sweeteners into the oxidized starch caused similar changes in EPR parameters as observed after increasing temperature of the thermal treatment. The signals became more isotropic, and in the case of addition of saccharose the only one signal without hyperfine structure was observed. The character of the signals in oxidized starch containing sweeteners was similar to that observed in the native one with the same additives.[Citation24]
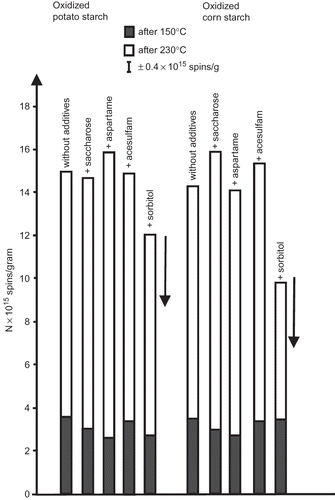
The results of quantitative EPR measurements of radicals generated thermally in oxidized potato starch with additives of saccharose and its substitutes are shown in The samples heated at 230°C exhibited EPR signals of the total intensity about 15 × 1015 spins/gram, whereas after heating at 150°C the intensity of the signals was much lower. The error of the measurements was ±0.5 × 1015 spins/gram. The number of spins in the starch samples without additives and those containing saccharose, acesulfam or aspartame did not differ significantly, compared to the experimental error. The only samples, in which the amount of generated radicals was considerably smaller than in the absence of any additive, were those containing sorbitol. A similar effect of sorbitol was observed by us[Citation24] for native, non-oxidized, potato and corn starch (Sigma, Steinheim, Germany). We ascribed the decrease in the number of thermally generated radicals in starch samples containing sorbitol to the particular features of this sweetener: small size and six OH groups (C6H14O6, 182.2g/mol). Small dimensions of sorbitol molecule facilitate its penetration into the starch matrix whereas numerous hydroxyl groups of this additive can effectively interact with hydroxyl groups of the starch leading to formation of hydrogen bridges stabilizing the starch structure and hindering radical formation.
CONCLUSION
The sweeteners added to oxidize potato starch differed in the effects on its physicochemical parameters investigated in this study. Saccharose and sorbitol increased considerably the onset temperature of transition (T 0) and the peak temperature of transition (Tp ) as determined by differential scanning calorimetry. Among the sweeteners, only aspartame influenced the characteristic viscosities (ηmax, η96°C, and η96/20) of the starch-sweetener-water systems, reducing the thermal stability of the pastes obtained. Acesulphame K and aspartame caused the largest increase in the susceptibility to retrogradation of 2% pastes of oxidized potato starch. In the presence of sorbitol the number of radicals generated thermally in starch decreased. This effect, found by quantitative EPR measurements, indicates effective interaction of hydroxyl groups of the sorbitol molecule with the starch matrix, leading to the stabilization of the starch structure.
ACKNOWLEDGMENT
The work was financed by Grant No. 2 P06T 087 27 awarded by the Polish Ministry of Science and Higher Education
REFERENCES
- Gaudin , S. , Lourdin , D. , Le Botlan , D. , Ilari , J.L. and Colonna , P. 1999 . Plasticisation and mobility in starch-sorbitol films . Journal of Cereal Science , 29 : 273 – 284 .
- Fortuna , T. and Gałkowska , G. 2006 . Effect of saccharides on rheological properties of modified starches . Żywność, Nauka, Technologia, Jakość , 4 ( 49 ) : 5 – 17 . in Polish
- Nadison , J. 1995 . Modified starch kind, properties, application of product . Przemysł. Spożywczy , 6 : 209 – 212 . in Polish
- Forssell , P. , Hamunen , A. , Autio , K. , Suorti , T. and Poutanen , K. 1995 . Hypochlorite oxidation of barley and potato starch . Starch/Stärke , 47 : 371 – 377 .
- 5. ISO standard 11214. Modified starch—Determination of carboxyl group content of oxidized starch, 1996.
- Whistler , R.L. 1967 . Starch Chemistry and Technology , New York and London : Academic Press .
- Morrison , W.R. and Laignelet , B. 1983 . An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches . Journal of Cereal Science , 1 : 9 – 20 .
- Winkler , S. , Luckowi , J. and Donic , H. 1971 . Absolute und Relative Verkleisterungscharakterik von Stärke, Teil 1, Absolute Viskositätsmessung nach dem Cearte-Princip . [The Absolute and the Relative Gelatinization Characteristics of starches. Part 1. Absolute Viscosity Measurings (Searle Principle).] Starch/Stärke , 23 : 235
- Jacobson , M.R. , Obanui , M. and Becuiler , J.M. 1997 . Retrogradation of starch from different botanic sources , 74 ( 5 ) : 511 – 518 .
- Lozos , G.P. , Hoffman , B.M. and Franz , C.G. SIM 14 Program. Chemistry Department, Northwestern University , Evanston, IL : QCPE, 1974, No 265 .
- Dyrek , K. , Rokosz , A. and Madej , A. 1994 . Spin dosimetry in catalysis research . Applied Magnetic Resonance , 6 : 309 – 332 .
- Beleia , A. , Miller , R.A. and Hoseney , R.C. 1996 . Starch gelatinization in sugar solution . Starch/Starke , 48 ( 7/8 ) : 259 – 262 .
- Kohyama , K. and Nishinari , K. 1991 . Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch . Journal of Agriculture and Food Chemistry , 39 ( 8 ) : 1406 – 1410 .
- Baek , M.H. , Yoo , B. and Lim , S.T. 2004 . Effects of sugars and sugar alcohols on thermal transition and cold stability of corn starch gel . Food Hydrocolloids , 18 ( 1 ) : 133 – 142 .
- Buck , J.S. and Walker , C.E. 1988 . Sugar and sucrose ester effects on maize and wheat starch gelatinization patterns by differential scanning calorimeter . Starch/Stärke , 40 ( 9 ) : 353 – 356 .
- Kim , C.S. and Walker , C.E. 1992 . Effects of sugars and emulsifiers on starch gelatinization evaluated by differential scanning calorimetry . Cereal Chemistry , 69 ( 2 ) : 212 – 217 .
- Swinkels , J.J.M. 1985 . Composition and properties of commercial native starch . Starch/Stärke , 37 : 1 – 5 .
- Pietrzyk , S. , Fortuna , T. and Ras , Ł. 2007 . The influence of pH and Fe(II) ions on physicochemical properties of oxidised potato starch . Electronic Journal of Polish Agricultural Universities (Food Science and Technology) , 10 ( 4 ) : 16
- Leliwa , S. 1995 . Sweeteners and Solvents , Gdańsk, , Poland : EKOS .
- Wang , Y.J. and Jane , J. 1994 . Correlation between glass transition temperature and starch retrogradation in the presence of sugar and maltodextrins . Cereal Chemistry , 71 : 527 – 531 .
- Sandhu , K.S. and Singh , N. 2007 . Some properties of corn starches II: Physicochemical gelatinization, retrogradation, pasting and gel texture properties . Food Chemistry , 101 : 1499 – 1507 .
- Prokopowich , D.J. and Biliaderis , C.G.A. 1995 . Comparative study of the effect of sugars on the thermal and mechanical properties of concentrated waxy maize, wheat, potato and pea starch gels . Food Chemistry , 52 : 255 – 262 .
- Dyrek , K. , Bidzińska , E. , Łabanowska , M. , Fortuna , T. , Przetaczek , I. and Pietrzyk , S. 2007 . EPR study of radicals generated in starch by microwaves and conventional heating . Starch/Stärke , 59 : 318 – 325 .
- Łabanowska , M. , Dyrek , K. , Bidzińska , E. , Fortuna , T. , Pietrzyk , S. , Przetaczek , I. and Rożnowski , J. 2009 . Effect of sweeteners on radical formation in the starch studied by electron paramagnetic resonance spectroscopy . Food Sciences and Technology International , 15 ( 4 ) : 357 – 365 .