Abstract
The use of reduced isomerised hop extracts to achieve both bitterness and light stability became very popular during the last decade. Changes during storage in four hop extracts and seven commercial lager beers are studied using HPLC and spectrophotometric techniques. The degradation of the iso-α-acids and tetrahydro iso-α-acids as a function of time is represented by the ratio, in percentage, of the sum of trans-isomer concentration to the sum of the cis-isomer concentration (T/C). The results provided conclusive evidence that the gradual decreased of bitterness intensity in beer was due to the degradation of iso-α-acids, notably to the instability of the trans-iso-α-acids. When the beers were stored for 14 days at 45°C the decrease of the T/C average varied from 8.6 to 14.0%, except in lemon beer, in which case, T/C decreased 36.9%. The results allowed us to infer that in open storage and/or warm conditions the deterioration of beer was critical above 35°C. On the other hand, the results showed that tetrahydro iso-α-acids remained unaltered.
INTRODUCTION
The bitter taste of beer is derived from hops (Humulus lupulus L.) or hop extracts added to the wort during brewing.[Citation1] In the boiling process, the hop α-acids, which are mildly bitter, are isomerised into the bitter-tasting iso-α-acids. These products are not only responsible for the bitter taste of beer, but they also exhibit bacteriostatic properties and, furthermore, play an essential role in enhancing the foam stability of beer.[Citation2–5 Citation Citation Citation5
Activity-guided separation of a hop extract, before and after wort-boiling, revealed that, besides the α-acids, the β-acids are also potential bitter taste precursors in hop.[Citation6] Flavour changes in packaged beer constitute one of the most serious challenges in brewing. The deterioration of beer flavour, occurring during storage, was believed to be caused by the formation of long-chain unsaturated carbonyls with low flavour thresholds.[Citation7] Among the carbonyls responsible for these changes, trans-2-nonenal was very important, giving the product a cardboard off-flavor at a concentration as low as 0.035 ppb.[Citation8] An array of enzymatic and chemical reactions are supposed to be involved in the formation of aging carbonyls, including melanoidin-catalysed oxidation of higher alcohols, oxidative reactions of iso-α-acids, Strecker-type degradation of amino acids, autooxidation, photo-oxidation and enzymatic degradation of unsaturated fatty acids, and aldol-type condensations of short-chain aldehydes.[Citation8–11 Citation Citation Citation11 It is widely known that a decrease in bitter substances, as well as in the sensory impression of bitterness, occurs during the aging of beer. Storage of beer at elevated temperatures simultaneously resulted in a decrease in bitterness-producing substances and a pronounced deterioration of the quality of bitterness,[Citation12] even in beer previously prepared at the pilot brewery.[Citation13]
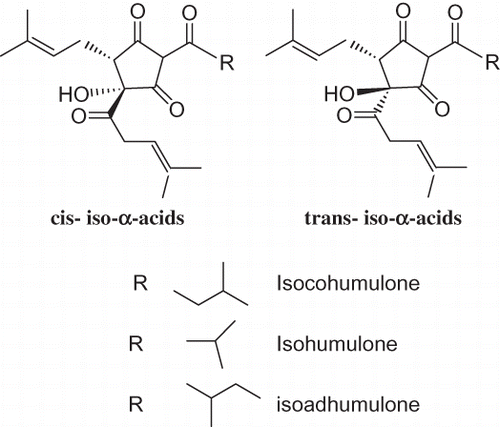
Six major iso-α-acids (cis- and trans-pairs of isocohumulones, isohumulones, and isoadhumulones) impart most of the bitter flavor to beer brewed with fresh hops (). The concentration of each hop-derived hop bitter acid varies from beer to beer. The choice of hop variety, the amount of hops and hop products used, and the conditions employed both in hop processing and beer production determine their concentration in beer.[Citation14] The bitterness of a beer can be profiled by chromatography, particularly by HPLC of the iso-α-acids and UV detection of individual constituents.[Citation15] These iso-α-acids were studied in great detail and their properties, as well as their chemical features, are quite well understood.[Citation16,Citation17] Some aspects of beer aging have recently been studied by the authors' group.[Citation18,Citation19] Partition coefficients of iso-α-acids and derivates, including hydrogenated molecules, such as dihydro-, tetrahydro-, or hexahydro, as well as their molecular sizes, have been used by us in order to establish the bacteriostatic effects of referred species.[Citation20]
Currently, much attention is being paid to reduced iso-α-acids, amongst others in the making of light-proof beers and beers with improved foam characteristics.[Citation21,Citation22] The use of reduced, isomerised hops extracts became very popular during the last decade in terms of achieving both better foam and light stability. Nowadays, one of the most common products are tetrahydro iso-α-acids.[Citation16] For their routine quality control, most brewers use a simple and rather empirical method to determine beer bitterness based on a simple spectrophotometric measurement of the light absorption by an iso-octane extract.[Citation23]
Hop products used in brewing are commercially present in various forms, such as hop cones, powdered hops, hop pellets, hop extracts, and isomerised extracts.[Citation24,Citation25] In Europe, hop extracts are the most often used products because they are the cheapest form of bittering. In this work, a comparative study of different samples of iso-α-acids and tetrahydro iso-α-acids was carried out using HPLC, a very popular technique in food analysis.[Citation26,Citation27] Effects of storage temperature on isomerization and degradation are studied both in commercial beers and in extracts.
MATERIALS AND METHODS
Four samples of different iso-α-acids and tetrahydro iso-α-acids extract provided from Germany were analysed using HPLC: two iso-α-acids (30%) and two tetrahydro iso-α-acids (10%), all of them pH = 9.0. They were stored in darkness at different temperatures (5, 15, and 35°C) for one month.
-
Extract A: An aqueous solution of the potassium salts of iso-α-acids.
-
Extract B: Potassium salt of iso-α-acids in aqueous alkaline solution.
-
Extract C: Aqueous solution of the potassium salts of tetrahydro iso-α-acids.
-
Extract D: Tetrahydro iso-α-acids in aqueous alkaline solution.
The following seven commercial beers were used: L1 NAC (Lager Non-Alcoholic Can) (0% vol), L1 NAB (Lager Non-Alcoholic Bottle) (0% vol), L2 B (Lager Pilsen Bottle) (5.0% vol), L2 C (Lager Pilsen Can) (5.0% vol), L3 CL (Lager Colourless glass) (4.6% vol), L4 WL (Lager with lemon) (0.9%vol), and L5 P (Lager Premium) (5.4% vol).
HPLC Conditions
The HPLC system consisted of a HP 1090 with a UV detector (Hewlett Packard, USA), which was set to measure absorbance at 270 nm. The injection volume was 10 μl. A Nucleosil column C 18 (250 × 4.6 mm, 5 μm) was used. The method accepted by the European Brewery Convention (EBC) was used (Sigma-Aldrich, Germany). This method has moments in gradient regime and moments in isocratic regime. For the complete separation, A was methanol pure and mixture B was acetonitrile-1% citric acid buffer (3:7) (v/v). The citric acid buffer was adjusted to pH 7.0 with 20% KOH solution and filtered separately before combination with the organic solvents. The percentages of cis- and trans-isomers were analysed. Data of trans- and cis-isoadhumulone were deliberately omitted because errors in the quantification of these minor constituents could be considerable. The reported HPLC results are the average of at least three determinations.
Beer Samples
Beers were bought at local stores, kept at different temperatures (35, 40, and 45°C), and freshly opened prior to analysis. They were stored in darkness for 14 days and degasification was done by ultrasonication. A portion sample of degasified beer was filtered through a syringe filter (0.2 μm) prior to injection.
Analysis of Bitterness
The bitterness of beer was analysed using the EBC method. The Institute of Brewing, Mitteleuropäischen Brautechnischen Analysenkommision, and American Society of Brewing Chemist organisations have accepted a standard method based on a simple spectrophotometric measure of the light absorption at 275 nm by an iso-octane extract of an acidified sample with HCl (22). The result of the analysis expresses bitterness in International Bitterness Units (IBU): Absorbance at 275 nm × 50. The factor of 50 is determined empirically and based on the assumption that about 70% of the absorbance reading of an iso-octane extract is caused by iso-α-acids. The spectrophotometric results were the average of at least three determinations.
RESULTS AND DISCUSSION
Firstly, all isomers in each sample were separated by HPLC. Four isomers were identified at different retention times: cis-isocohumulone 15.88 min, trans-isocohumulone 16.67 min, cis-isohumulone 20.75 min, and trans-isohumulone 21.52 min. Data of trans- and cis-isoadhumulone was deliberately omitted because errors in the quantification of these minor constituents could be considerable.[Citation21]
Hop Extracts
A study of temperature effects (5, 15, and 35°C) in isomer variation on hop extracts was carried out. shows the variation of each isomer with temperature. shows that cis-isohumulone remained largely unaltered, whereas the trans-iso-α-acids deteriorated at a much faster rate than the cis-iso-α-acids. These results might be justified since the propensity of trans-iso-α-acids to auto-oxidation was associated to the cis-configuration of the side chains at C(4) and C(5), therefore creating a region of high electron density, which allows ready initiation of radical-type oxidation reaction.
Figure 2 Temperature effects on iso extract isomer areas: (A): Extract A; (B): Extract B, (C): Extract C, (D): Extract D. CIC: Cis-isocohumulone; TIC: Trans-isocohumulone; CIH: Cis-isohumulone; TIH: Trans-isohumulone; CTC: Cis-tetrahydroisocohumulone; TTC: Trans-tetrahydroisocohumulone; CTH: Cis-tetrahydroisohumulone; TTH: Trans-tetrahydroisohumulone. (a) iso-α-acids; (b) tetrahydro iso-α-acids.
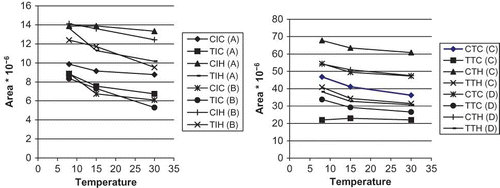
Trans-iso-α-acids are lost preferentially during primary fermentation and are enriched in the beer foam with respect to the cis-iso-α-acids. Differences in the properties of the trans-and cis-iso-α-acids were previously observed.[Citation28,Citation29] The differential behaviour may be explained on the basis of small, but significant, entropy differences between trans- and cis-iso-α-acids related to the respective geometrical configuration. These entropy differences were caused by the position of the prenyl and isohexenoyl side chains. These side chains are on the same side of the five-membered hop acid ring in trans-iso-α-acids but they are on the different side in cis-isomers. Parallel studies were made with tetrahydro iso-α-acids samples. shows the variation of different isomers with varying temperatures. In this case, we can observe that the cis- and trans-tetrahydro iso-α-acids remained unaltered.
Commercial Beers
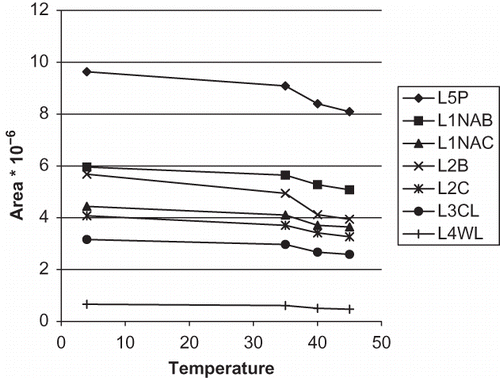
Several studies demonstrated that auto-oxidation, including decomposition of iso-α-acids, played a pivotal role in the deterioration of taste and flavour qualities of beer during aging.[Citation21] In order to continue with our previous research, the effect of temperature of storage on isomers variation in seven commercial beers was studied (). Full results obtained are shown in . Percentage variation of each iso-α-acid with the temperature in beers is shown in .
Figure 4 Total area variation of iso-α-acids for any beer at different temperature ranges (color figure available online).
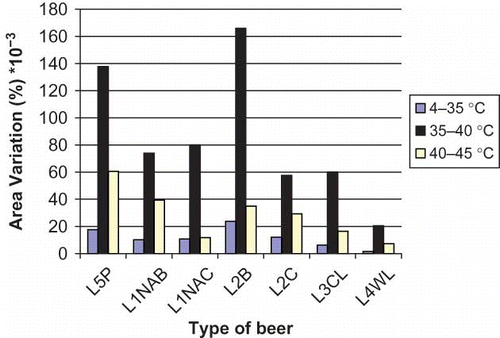
Table 1 Areas of different isomers of iso-α-acids and tetrahydro iso-α-acids found in commercial packaged beers stored for 14 days at 4, 35, 40, and 45°C
The storage properties of trans- and cis-iso-α-acids may be described as the ratio of the sum of the concentrations of trans-isohumulone and trans-isocohumulone to the sum of the concentrations of cis-isohumulone and cis-isocohumulone, expressed as a percentage (T/C ratio %). shows the variation of this ratio for analysed beers. It was observed that in analysed beers the cis-isomer predominates, however, in non-alcoholic beer, the concentration of cis- and trans-forms is similar. In fact, the ratio trans-/cis- decreased with aging, indicating a pronounced degradation of the trans-iso-α-acids.
Table 2 Level of cis- and trans-isomers in beers after storage under specified conditions
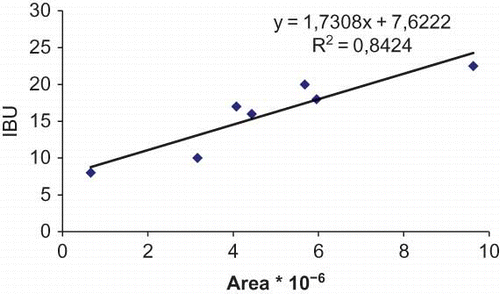
Studies about bitterness of the iso-α-acids[Citation14,Citation17] demonstrated that there were large differences in the bitterness of the individual compounds: cis-isohumulone > trans-isohumulone = cis-isocohumulone > trans-isocohumulone. There are two trials that are worthy of noting. Firstly, for a given pair of cis- and trans-isomers, the cis- component was significantly more bitter than its trans- counterpart. Secondly, the isohumulones were more bitter than the isocohumulone. This latter was expected on the basis of polarity, but the differences in the behaviour of the cis- and trans-isomers cannot be explained in terms of polarity as there is little, if any, difference between them. Thus, the bitterness of beers was analysed using a spectrophotometric method. The result of this analysis expressed the bitterness in IBU. In fact, spectrophotometric measurements have corroborated the results obtained using HPLC. It was found that the beers analysed had bitterness in the range of 8–22 IBU and an approximate equivalence between IBU and percentage of iso-α-acids was achieved ( ).
CONCLUSION
Decomposition of iso-α-acids, the main bittering agents of beer during the beer aging, determines the consistence of the bitter taste in beer. This study demonstrated that the decrease of trans-isohumulone concentration by temperature effect was rather more important than in other isomers. Being the second most important compound in bitterness intensity, it is expected that the decrease of bitterness will be notable.
ACKNOWLEDGMENT
The authors would like to acknowledge the financial support from the Consejería de Educación de la Junta de Castilla y Leon.
REFERENCES
- Hough , J.S. , Briggs , D.E. , Stevens , R. and Young , T.W. 1992 . Malting and Brewing Science , 2nd , London : Chapman and Hall .
- Simpson , W.J. and Fernandez , J.L. 1994 . Mechanism of resistance of lactic bacteria to trans-isohumulone . Journal of the American Society of Brewing Chemists , 52 ( 1 ) : 9 – 11 .
- Haikara , A. 1995 . Detection of pectinatus contaminants in beer . Monatsschr. Brauwiss , 38 : 239 – 243 .
- De Keukeleire , D. , Vindevogel , J. , Szucs , R. and Sandra , P. 1992 . The history and analytical chemistry of beer bitter acids . Trends in Analytical Chemistry , 11 : 275 – 280 .
- Schönberger , C. 2006 . Bitter is better: A review on the knowledge about bitterness in beer . Monatsschr. Brauwissensch , 59 : 56 – 66 .
- Haseleua , G. , Intelmanna , D. and Hofmann , T. 2009 . Structure determination and sensory evaluation of novel bitter compounds formed from β-acids of hop (Humulus lupulus L.) upon wort boiling . Food Chemistry , 116 ( 1 ) : 71 – 81 .
- Wheeler , R.E. , Pragnell , M.J. and Pierce , J.S. 1971 . The identification of factors affecting flavour stability in beer . Proceedings of the European Brewery Convention Congress , : 423 – 436 .
- Meilgaard , M.C. 1993 . Individual differences in sensory threshold for aroma chemicals added to beer . Food Quality and Preference , 4 : 153 – 167 .
- Hambraeus , G and Nyberg , N. 2005 . Enzymatic hydrogenation, trans-2-nonenal in barley . Journal of Agricultural and Food Chemistry , 53 : 8714 – 8721 .
- Devreux , A. , Blockmans , C. and van de Meerssche , J. 1981 . Monograph VII . EBC Flavor Symposium, EBC, Copenhagen , : 191 – 201 .
- Miedaneer , H. , Narziss , L. and Eichhorn , P. 1991 . Proceedings EBC Congress, Oxford, Oxford University Press , : 401 – 408 .
- Vanderhaegen , B. , Neven , H. , Coghe , S. , Verstrepen , K.J. , Verachtert , H. and Derdelinckx , G. 2003 . Evolution of chemical and sensory properties during aging of top-fermented beer . Journal of Agricultural and Food Chemistry , 51 : 6782 – 6790 .
- Jaskula , B. , Syryn , E. , Goiris , K. , De Rouck , G. , Van Opstaele , F. , De Clippeleer , J. , Aerts , G. and De Cooman , L. 2007 . Hopping technology in relation to beer bitterness consistency and flavor stability . Journal of the American Society of Brewing Chemists , 65 ( 1 ) : 38 – 46 .
- Hughes , P.S. and Simpson , W.J. 1996 . Bitterness of congeners and stereoisomers of hop-derived bitter acids found in beer . Journal of the American Society of Brewing Chemists , 54 ( 4 ) : 234 – 237 .
- Vanhoenacker , G. , De Keukeleire , D and Sandra , P. 2004 . Analysis of iso-α-acids in beer by direct injection and liquid chromatography with ultraviolet absorbance detection or with mass spectrometry . Journal of Chromatography A , 1035 : 53 – 61 .
- Smith , R. and Davidson , D. 1998 . Natural foam stabilizing and bittering compounds derived from hops . Journal of the American Society of Brewing Chemists , 56 ( 2 ) : 52 – 57 .
- Hughes , P. 2000 . The significance of iso-α-acids for beer quality cambridge prize paper . Journal of Institute of Brewing , 106 : 271 – 276 .
- Blanco , C.A. , Caballero , I. , Rojas , A. , Gómez , M. and Álvarez , J. 2003 . Chelation of aqueous iron (III) by 2-acetyl-1,3-cyclohexanedione and beer ageing . Food Chemistry , 81 : 561 – 568 .
- Blanco , C.A. , Rojas , A. , Caballero , P. , Ronda , F. , Gomez , M. and Caballero , I. 2006 . A better control of beer properties by predicting acidity of hop iso-α-acids . Trends in Food Science and Technology , 17 : 373 – 377 .
- Blanco , C.A. , Rojas , A and Nimubona , D. 2007 . Effects of acidity and molecular size on bacteriostatic properties of beer hop derivates . Trends in Food Science and Technology , 18 : 144 – 149 .
- De Cooman , L. , Aerts , G. , Overmeire , H. and De Keukeleire , D. 2000 . Alterations of profiles of iso-α-acids during beer ageing, marked instability of trans-α-acids, and implications for beer bitterness consistency in relation to tetrahydro iso-α-acids . Journal of Institute of Brewing , 106 : 169 – 178 .
- Verzele , M. and de Keukeleire , D. 1999 . Chemistry and analysis of hop and beer bitter acids , Amsterdam, , the Netherlands : Elsevier .
- American Society of Brewing Chemist . 1994 . Métodos Oficiales de Análisis de la Cerveza 9.6 ,
- Lewis , M.J. and Young , T.W. 1995 . Brewing , London : Chapman and Hall .
- Moir , M. 2000 . Hop: A millennium review . Journal of the American Society of Brewing Chemists , 58 ( 4 ) : 131 – 146 .
- Liang , Y.R. , Ye , Q. , Jin , J. , Liang , H. , Lu , J.L. , Du , Y.Y. and Dong , J.J. 2008 . Chemical and instrumental assessment of green tea sensory preference . International Journal of Food Properties , 11 ( 2 ) : 258 – 272 .
- Mandic , A.I. , Dilas , S.M. , Cetkovic , G.S. , Canadanovic-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 ( 4 ) : 713 – 726 .
- Forster , A. , Beck , B. and Schmidt , R. 1995 . “ Untersuchungen zu Hopfenpolyphenolen ” . In Proceedings of the 25th European Brewery Convention, Brussels , 143 – 150 . Oxford, , England : Oxford University Press .
- Hashimoto , N. 1976 . Contribution of hop bitter substances to head formulation of beer. Report of the Research Laboratory of the Kirin Brewing Company , 19 : 1