Abstract
Flaxseed oil is rich in linolenic acid, and being accepted in diet by more and more people. The characteristics of flaxseed oil from two different varieties, namely fibre-flax seed and oil-flax seed, were evaluated. It was shown that fatty acids of flaxseed oil were mainly constituted by linolenic, oleic, linoleic, stearic, and palmitic acids, and the oil-flax seed oil contained more linolenic acid (58.03%) compared with the fibre-flax seed oil (47.37%). The fibre-flax seed oil showed a higher absorbance at 290–800 nm. Thermogravimetric curves showed that the oil-flax seed oil was more stable than the fibre-flax seed oil. Thermal profiles indicated that the fibre-flax seed oil had higher melting peak temperature and larger enthalpy. Rheological studies indicated that the apparent viscosity of the fibre-flax seed oil was higher than that of the oil-flax seed oil. It can be concluded that the oil-flax seed oil is better than the fibre-flax seed oil in terms of edibility.
INTRODUCTION
Flax (Linum usitatissimum L.) is one of the most important oil plants in the world, especially in Canada and China. In China, flax can be mainly divided into two types, namely oil-flax and fibre-flax, according to their use. Oil-flax is planted for oil from the seeds and mainly distributed in northwest of China. Fibre-flax is grown for fibre from its stem and mainly distributed in northeast of China. The seeds of both the oil-flax and fibre-flax can be used to obtain oils. Flax seed, also known as flaxseed or linseed, contains 20–40% oil.[Citation1–3 Citation Citation3 Flaxseed oil is a typical drying oil and mainly used for industrial purpose, such as the production of paints, linoleum, varnishes, inks, and cosmetics, in the past. However, flaxseed oil is becoming popular for its nutritional and pharmaceutical values. Flaxseed oil is known as the richest source of the n-3 fatty acid, alpha linolenic acid (ALA), which is one of the essential fatty acids.[Citation4] Studies have proven that flaxseed oil has positive effect on many diseases, such as hyperlipidemia,[Citation5] colon tumor,[Citation6] mammary cancer,[Citation7,Citation8] and atherosclerosis.[Citation9,Citation10]
Previous studies on flaxseed oil were mainly based on the industrial purpose, such as oxypolymerization[Citation11,Citation12] and oxidative degradation.[Citation13] Before flaxseed oil is introduced in the food field, its characteristics must be realized. Wiesenborn et al.[Citation14] have studied the sensory and oxidative quality of screw-pressed flaxseed oil. Choo et al.[Citation15] have reported the physicochemical and quality characteristics of seven cold-pressed flaxseed oils sold in New Zealand. The characteristics of flaxseed hull oil were reported by Oomah and Sitter.[Citation16] However, all of these studies did not report whether the oil came from oil-flax seeds (OFS) or fibre-flax seeds (FFS). And some important characteristics of flaxseed oil were not reported, for example, rheological properties and thermal stability. The aims of this study were to compare physico-chemical characteristics, fatty acid composition, spectroscopic properties, thermal properties, and rheological properties of the FFS oil with those of the OFS oil, and show the differences between these oils obtained from different sources.
MATERIALS AND METHODS
Material
Two varieties of flaxseed were purchased from the market. FFS was obtained from fibre-flax grown in the Heilongjiang province of China and OFS was obtained from oil-flax grown in the Hebei province of China. The samples were cleaned by hand carefully to remove the foreign materials, such as other seeds, stones, and small stalks. Then, they were ground into powder in a coffee grinder to pass a 1 mm screen. The samples of the ground flaxseed were preserved in hermetic bags at −20°C until use.
Oil Preparation
The ground flaxseeds (100 g) were mixed with 600 ml hexane in a flask. The mixture was agitated by a mechanical stirrer for 3 h at room temperature. The supernatent was then filtered through the filter paper under a vacuum. The extraction procedure was repeated three times. The solutions were then collected and concentrated with a rotary evaporator to acquire the flaxseed oil. After centrifugation, the flaxseed oil obtained was further dried in a vacuum drying oven to remove the residual hexane. Finally, the flaxseed oils were stored in a freezer (−20°C) for subsequent physico-chemical analyses.
Fatty Acid Composition
Fatty acid composition was determined by gas chromatography after derivatization to fatty acid methyl esters (FAME). The preparation of FAME was performed according to the standard method.[Citation17] FAME separation and identification was carried out on the gas chromatograph (Model CP-3800, Varian Inc, Walnut Creek, CA, USA) equipped with a flame ionization detector and capillary column HP-Innowax (30 m × 0.32 mm × 0.25 μm). The amount of each sample injected was 1.0 μL. Nitrogen, at a constant flow 1.0 mL/min, was used as the carrier gas and a spilt/spiltless injector was used with a split ratio of 20:1. The injector temperature was 250°C and the detector temperature was 270°C. The column temperature was programmed from 100 to 180oC at 20°C/min, and then to 230°C at 10°C/min and held for 5 min at 230°C. Fatty acid methyl esters were identified by comparison with the standard fatty acid methyl esters (Sigma, St. Louis, MO, USA). Fatty acid methyl esters were quantified as percentages of the total methyl ester peak areas.
UV-Vis Spectrophotometric Analysis
Absorbance of oil solutions in hexane were measured with a UV-Vis spectrophotometer (Model TU-1810, Beijing Purkinje General Instrument Co., Ltd., Beijing, China) at wave lengths range from 200 to 800 nm.
Thermal Stability Analysis
Thermal stability of the flaxseed oils was analyzed using a thermogravimetric analyzer (Model Q600, TA Instruments, New Castle, DE, USA). The flaxseed oils (8 ± 0.5 mg) were added to an alumina pan. For thermogravimetric measurements, the samples were heated to 100°C at a heating rate of 20°C/min and equilibrated for 1 min at 100°C, and then heated to 600°C at a rate of 10°C/min in purge air flow of 100 ml/min (the air came from a compressor). The experiments were carried out three times and the representative thermogravimetric curves were reported.
Differential Scanning Calorimetry (DSC) Analysis
Thermal characteristics of the flaxseed oil were determined using a differential scanning calorimeter (DSC) (Model Q10, TA Instruments, New Castle, DE, USA). The oil samples (5 ± 0.5 mg) were weighed in a DSC-pan (aluminum hermetic pan, 900793.901; TA Instruments) and DSC runs were performed within the temperature range of −75 to 100°C at a heating rate of 5°C/min. An empty DSC-pan was used as an inert reference to balance the heat capacity of the sample pan. The instrument was calibrated for temperature and heat flow with indium (Melting temperature (Tm ) = 156.6°C, Enthalpy (ΔH) = 28.45 J/g).
Rheological Analysis
Rheological properties of the flaxseed oil were measured using a rheometer (Model AR2000ex; TA Instruments, New Castle, DE, USA) according to the method reported by Sathivel et al.[Citation18] with some modifications. All the rheological studies were conducted using cone and plate geometry (40 mm diameter and 1° cone, the gap was 27 μm between the two plates). The apparent viscosity of the flaxseed oil was measured at 5, 15, 25, and 35°C at shear rates ranging from about 15 to 800 s−1.
Physico-Chemical Characterization
The peroxide, iodine, and acidity values of the flaxseed oils were determined according to the AOCS official methods (Cd 8-53, Cd 1-25, and Cd 3d-63, respectively).[Citation19] The refractive index of flaxseed oil was determined using an Abbe refractometer. The density of the oils was measured using a densimeter. Chlorophyll content of flaxseed oil was quantified by spectrophotometry according to the AOCS method Cd 13d-55.[Citation19] Polyphenol content was determined according to the method described by Gutfinger.[Citation20]
RESULTS AND DISCUSSIONS
Fatty Acid Composition
Fatty acid composition of the FFS and OFS oils are given in . It is shown that the fatty acids of flaxseed oil were mainly constituted by linolenic (47–58%), oleic (16–21%), linoleic (15–16%), stearic (3–7%), and palmitic (5–9%) acids, which together comprised about 99% of the total fatty acids. This result was similar to that reported by others.[Citation15,Citation21] Fatty acid composition of the OFS oil was slightly different from our previous report,[Citation22] which may be due to the difference of detection conditions. Compared with the FFS oil, the OFS oil was higher in unsaturated fatty acids content (90.70% vs. 83.53%) and ratio of n-3/n-6 (3.61 vs. 3.08). Higher ratio of n-3/n-6 indicated that the OFS oil had higher nutritional value than the FFS oil.
Table 1 Fatty acid composition of FFS and OFS oils.Footnote*
UV–Vis Spectrophotometric Profile
The FFS and OFS oils showed some absorption in the UV-C (100–290 nm), UV-B (290–320 nm), UV-A (320–400 nm), and visible (400–800 nm) range (). The spectra profiles of the FFS and OFS oils were similar, but the FFS oil had stronger absorptivity. The absorptivity of both oils was nearly same at the range of 200–290 nm. In the UV-B and UV-A ranges, the wavelengths of the ultraviolet light are responsible for most of the cellular damage.[Citation23] The FFS and OFS oils can shield against UV-B and UV-A radiation induced damage by scattering, as well as by absorption. Thus, the FFS and OFS oils may be used in the formulation of UV protectors to provide the protection against both the UV-B and UV-A.
Figure 1 Ultraviolet/visible spectra of the FFS and OFS oils. Figure derived from scans (λ = 200–290) of oil diluted 1:800; from scans (λ = 290–400) of oil diluted 1:100; and from scans (λ = 400–800) of oil diluted 1:10, all in hexane.
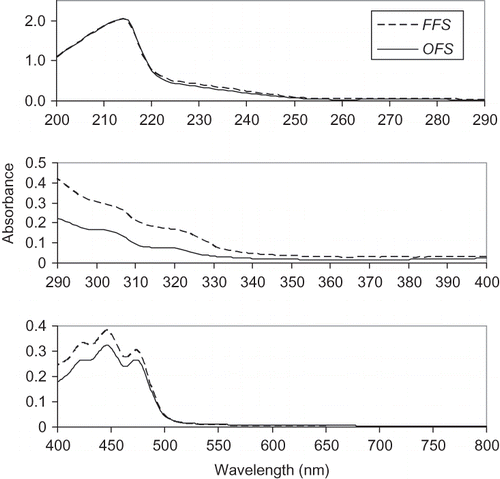
The FFS oil contained more yellow colouring than the OFS oil, as indicated by the absorbance at 440–460 nm for 10% oil in hexane (). This yellow colour, which includes carotenoids, is beneficial, since it stimulates the appearance of butter without the use of primary colourants, such as carotenes and annattos, and apocarotenals commonly used in the oil and fat industry.[Citation24]
The content of green pigments, particularly chlorophyll, was low as indicated by very low absorbance (0.005–0.009) in the 600–750 nm range for the FFS and OFS oils (10% oil in hexane). The values of absorbance were similar with that (0.003–0.007) of raspberry seed oil,[Citation24] but lower than that (0.014–0.095) of Echinacea seed oil.[Citation25] The actual chlorophyll content of the FFS and OFS oils were 0.59 and 0.39 mg/kg of oil (), respectively. The spectra profiles of flaxseed oil were similar with that of raspberry seed oil reported by Oomah et al.,[Citation24] particularly at UV-B, UV-A, and visible range. Moreover, flaxseed oil showed higher absorbance than raspberry seed oil.
Table 2 Physicochemical characteristics of FFS and OFS oils.Footnote*
Thermal Stability
The thermogravimetric curves of the FFS and OFS oils are shown in . When the thermogravimetric curves of the FFS and OFS oils were compared, some differences were noted. A mass increase in the temperature range 120–220°C followed by a rapid mass loss was observed for the OFS oil. The mass increase may be attributed to lipid oxidation process, which absorbed oxygen to form peroxides.[Citation26] The following mass loss may be due to the thermal-oxidative decomposition. The similar results were obtained for flaxseed oil at oxygen atmosphere.[Citation27] The mass increase was not found for the FFS oil, which may be attributed to the thermal-oxidative decomposition process and oxidation process proceeded at the same time. The similar result was observed for salmon oil and pollock oil under air atmosphere,[Citation18,Citation28] and they attributed this phenomenon to that the thermal decomposition of the oils was not related to oxygen absorption. The TGA result suggests that the OFS oil had higher thermal stability compared with the FFS oil.
Figure 2 Thermogravimetric curves and corresponding derivative curves of the FFS and OFS oils. The inset shows the detailed section of the thermogravimetric curves at temperatures between 120 and 240°C (color figure available online).
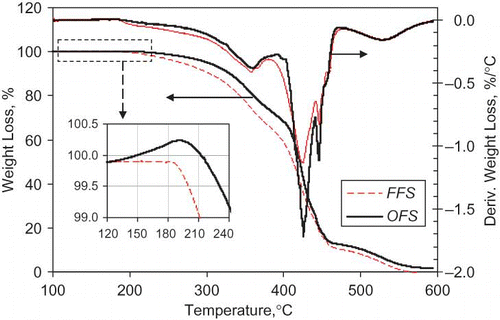
According to the derivative of thermogravimetric curves between 100 and 600°C (), the weight losses could be divided into three periods. For the FFS oil, the weight losses were 32.73% from 100 to 378°C, 56.25% from 378 to 474°C, and 11.02% from 474 to 600°C. For the OFS oil, the weight losses were 29.14% from 100 to 389°C, 59.78% from 389 to 470°C, and 11.08% from 470 to 600°C. At first period, the FFS oil had a lower terminate temperature (378°C vs. 389°C), but had a larger weight loss (32.73% vs. 29.14%). This indicated that the FFS oil had less stability and was easier to decompose compared to the OFS oil. During the second period, the weight of both flaxseed oils lost very rapidly. Simultaneously, a thick smoke and a pungent odor were found. It's shown that flaxseed oil decomposed into volatile compounds and others at this stage. The third period, FFS and OFS oils had similar weight loss and initial temperature, which showed that both oils might have similar composition at this time.
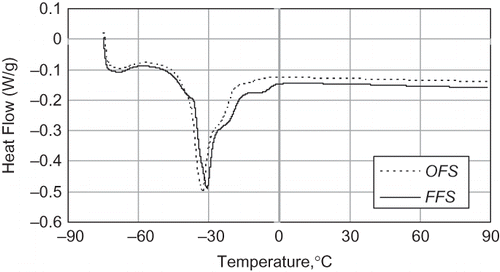
Thermal Profile
Melting thermograms for the FFS and OFS oils are shown in . The thermograms of both varieties show some small differences. The characteristics of the thermogram of the OFS oil were as follows: the onset temperature of −38.76°C and the melting peak temperature of −32.53°C. The characteristics of thermogram of the FFS oil were as follows: the onset temperature of −37.30°C and the melting peak temperature of −30.65°C. The lower melting peak temperature of the OFS oil may be due to unsaturated fatty acids, which was more for the OFS oil. Compared with the OFS oil, the thermogram of the FFS oil showed a more pronounced shoulder peak around the temperature of −5.2°C. These shoulder events may be attributed to the presence of higher-melting triacylglycerols or the transformation of the metastable phase to the more stable polymorphic forms.[Citation29] The enthalpies of the FFS and OFS oils were 62.15 J/g and 57.85 J/g, respectively. These values were similar with those (59.52 J/g and 60.68 J/g) reported for Nigella seed oils,[Citation29] but was lower than that (75 J/g) reported for raspberry seed oil.[Citation24]
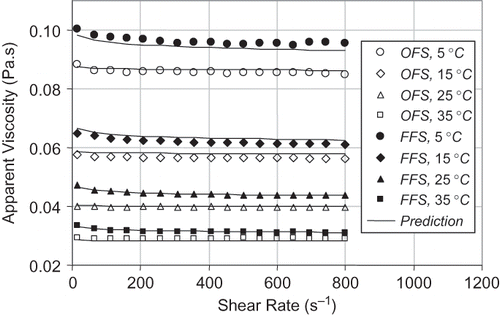
Rheological Properties
shows the apparent viscosities of flaxseed (OFS and FFS) oils as a function of shear rate ranging from about 15 to 800 s−1. As can be seen from , the apparent viscosity of the FFS oil was larger than that of the OFS oil at the same temperature. The influences of temperature and shear rate on the apparent viscosity can be combined into the following single expression, which is known as the power law model.
Figure 4 The apparent viscosities of the FFS and OFS oils at various temperatures. The symbols represent the measured values and the solid lines represent the predicted values using EquationEq. (3).
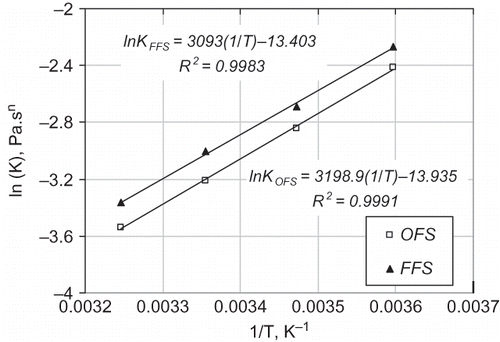
The natural logarithm of K against the reciprocal of temperature plots for the flaxseed oils, which are given in , show a linear relationship (R 2 > 0.998), suggesting that the temperature dependence of K can be conveniently described by the Arrhenius equation (EquationEq. 2). The magnitudes of Ea were determined as 25.72 and 26.60 kJ/mol for the FFS and OFS oils, respectively. And the values of A were determined as 1.5 × 10−6 and 8.9 × 10−7 for the FFS and OFS oils, respectively. The Ea values of the FFS and OFS oils were similar with that (Ea = 27.65 kJ/mol) of pollock oil reported by Sathivel et al.,[Citation18] and higher than that (Ea = 21.80 kJ/mol) of salmon oil reported by Huang and Sathivel.[Citation30]
After inserting the EquationEq. (2) into EquationEq. (1), the apparent viscosity of the FFS and OFS oils can be predicted as a function of temperature and shear rate using the following equation with the calculated parameters (A, Ea
, and ).
The solids lines in represent the predicted apparent viscosities for the FFS and OFS oils. As can be seen from , EquationEq. (3) is suitable for describing the apparent viscosity of flaxseed oils as a function of temperature and shear rate. This study indicated that magnitude of the apparent viscosity of flaxseed oils was greatly influenced by temperature. However, the influence of shear rate on the apparent viscosity was not significant, suggesting that these flaxseed oils showed almost a Newtonian behavior at the shear rates between 15 and 800 s−1.
Physicochemical Characteristics
The physicochemical characteristics of flaxseed oils are shown in . The lower acidity value and peroxide value of the OFS oil indicate that the OFS oil have a better quality and longer shelf life than the FFS oil. This result was conflicted with higher saturated fatty acids content of the FFS oil. The conflict may be due to longer storage period of the FFS and more total polyphenol in the OFS oil (114.67 mg/kg against 76.33 mg/kg). Both total polyphenol content of the OFS and FFS oils were in the range of 76–307 mg/kg reported by Choo et al.[Citation15] The iodine value of the OFS oil was higher than that of the FFS oil, which indicated that the OFS oil contained more linolenic acid, as previously shown in . The relationship between iodine value and refractive index was similar with that reported by Hopper and Nesbitt.[Citation31] The chlorophyll (0.39–0.59 mg/kg) was lower than that (0.80–5.76 mg/kg) of cold-pressed flaxseed oil reported by Choo et al.[Citation15] The density of the OFS oil was larger than that of the FFS oil.
CONCLUSION
In this study, the characteristics of the FFS and OFS oils were evaluated. It was found that the OFS oil contained more unsaturated fatty acid content (USFA), especially the linolenic acid content of the OFS oil was higher than that of the FFS oil. Thermogravimetric curves showed that the higher USFA did not affect the stability of the OFS oil relative to the FFS oil. This means that the OFS oil has higher quality and longer shelf life. Higher absorbance of the FFS oil indicated that the FFS oil fit to be used in cosmetics as a formulation of UV protector. The apparent viscosity of the FFS oil was larger than that of the OFS oil. The FFS and OFS oils showed almost a Newtonian behavior at the shear rates between 15 and 800 s−1. Lower acid and peroxide values also indicated that the OFS oil was more suitable as edible oil than the FFS oil.
ACKNOWLEDGMENTS
This work was supported by the Program for New Century Excellent Talents in University of China (NCET-08-0537), National Natural Science Foundation of China (30800662), Research and Development Fund for University's Doctoral Discipline of China (20050019029), and Science and Technology Research Key Program of China (105014).
Notes
Li-Jun Wang and Zhen-Shan Zhang contributed equally to this work.
REFERENCES
- Jiao , S.-S. , Li , D. , Huang , Z.-G. , Zhang , Z.-S. , Bhandari , B. , Chen , X.D. and Mao , Z.-H. 2008 . Optimization of supercritical carbon dioxide extraction of flaxseed oil using response surface methodology . International Journal of Food Engineering , 4 ( 4 ) : 1 – 17 .
- Wu , M. , Li , D. , Wang , L.-J. , Zhou , Y.-G. , Brooks , M.S.-L. , Chen , X.D. and Mao , Z.-H. 2008 . Extrusion detoxification technique on flaxseed by uniform design optimization . Separation and Purification Technology , 61 ( 1 ) : 51 – 59 .
- Wang , Y. , Wang , L.-J. , Li , D. , Özkan , N. , Chen , X.D. and Mao , Z.-H. 2008 . Effect of flaxseed gum addition on rheological properties of native maize starch . Journal of Food Engineering , 89 ( 1 ) : 87 – 92 .
- Carter , J.F. 1993 . Potential of flaxseed and flaxseed oil in baked goods and other products in human nutrition . Cereal Foods World , 38 ( 10 ) : 753 – 759 .
- Vijaimohan , K. , Jainu , M. , Sabitha , K.E. , Subramaniyam , S. , Anandhan , C. and Shyamala Devi , C.S. 2006 . Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats . Life Sciences , 79 ( 5 ) : 448 – 454 .
- Dwivedi , C. , Natarajan , K. and Matthees , D.P. 2005 . Chemopreventive effects of dietary flaxseed oil on colon tumor development . Nutrition and Cancer , 51 ( 1 ) : 52 – 58 .
- Thompson , L.U. , Rickard , S.E. , Orcheson , L.J. and Seidl , M.M. 1996 . Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis . Carcinogenesis , 17 ( 6 ) : 1373 – 1376 .
- Wang , L. , Chen , J. and Thompson , L.U. 2005 . The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenografts is attributed to both its lignan and oil components . International Journal of Cancer , 116 ( 5 ) : 793 – 798 .
- Prasad , K. 1997 . Dietary flax seed in prevention of hypercholesterolemic atherosclerosis . Atherosclerosis , 132 ( 1 ) : 69 – 76 .
- Yamashita , T. , Oda , E. , Sano , T. , Yamashita , T. , Ijiru , Y. , Giddings , J.C. and Yamamoto , J. 2005 . Varying the ratio of dietary n-6/n-3 polyunsaturated fatty acid alters the tendency to thrombosis and progress of atherosclerosis in apoe−/− LDLR−/− double knockout mouse . Thrombosis Research , 116 ( 5 ) : 393 – 401 .
- Güler , Ö.K. , Güner , F.S. and Erciyes , A.T. 2004 . Some empirical equations for oxypolymerization of linseed oil . Progress in Organic Coatings , 51 ( 4 ) : 365 – 371 .
- Mallégol , J. , Lemaire , J. and Gardette , J.-L. 2000 . Drier influence on the curing of linseed oil . Progress in Organic Coatings , 39 ( 2–4 ) : 107 – 113 .
- Lazzari , M. and Chiantore , O. 1999 . Drying and oxidative degradation of linseed oil . Polymer Degradation and Stability , 65 ( 2 ) : 303 – 313 .
- Wiesenborn , D. , Kangas , N. , Tostenson , K. , Hall , C. III and Chang , K. 2005 . Sensory and oxidative quality of screw-pressed flaxseed oil . Journal of the American Oil Chemists’ Society , 82 ( 12 ) : 887 – 892 .
- Choo , W.-S. , Birch , J. and Dufour , J.-P. 2007 . Physicochemical and quality characteristics of cold-pressed flaxseed oils . Journal of Food Composition and Analysis , 20 ( 3–4 ) : 202 – 211 .
- Oomah , B.D. and Sitter , L. 2009 . Characteristics of flaxseed hull oil . Food Chemistry , 114 ( 2 ) : 623 – 628 .
- ISO . 2000 . “ ISO Paper 5509 ” . In Animal and Vegetable Fats and Oils-Preparation of Methyl Esters of Fatty Acids , 2nd , ISO : Geneva .
- Sathivel , S. , Huang , J. and Prinyawiwatkul , W. 2008 . Thermal properties and applications of the arrhenius equation for evaluating viscosity and oxidation rates of unrefined pollock oil . Journal of Food Engineering , 84 ( 2 ) : 187 – 193 .
- Association of Official Analytical Chemists . 1997 . Official Methods and Recommended Practices of the American Oil Chemists’ Society , 5th , Champaign, Illinois : AOCS .
- Gutfinger , T. 1981 . Polyphenols in olive oils . Journal of the American Oil Chemists Society , 58 ( 11 ) : 966 – 968 .
- Vereshchagin , A.G. and Novitskaya , G.V. 1965 . The triglyceride composition of linseed oil . Journal of the American Oil Chemists’ Society , 42 ( 11 ) : 970 – 974 .
- Zhang , Z.-S. , Wang , L.-J. , Li , D. , Jiao , S.-S. , Chen , X.D. and Mao , Z.-H. 2008 . Ultrasound-assisted extraction of oil from flaxseed . Separation and Purification Technology , 62 ( 1 ) : 192 – 198 .
- Elleuch , M. , Besbes , S. , Roiseux , O. , Blecker , C. and Attia , H. 2007 . Quality characteristics of sesame seeds and by-products . Food Chemistry , 103 ( 2 ) : 641 – 650 .
- Oomah , B.D. , Ladet , S. , Godfrey , D.V. , Liang , J. and Girard , B. 2000 . Characteristics of raspberry (Rubus idaeus L.) seed oil . Food Chemistry , 69 ( 2 ) : 187 – 193 .
- Oomah , B.D. , Dumon , D. , Cardador-Martínez , A. and Godfrey , D.V. 2006 . Characteristics of echinacea seed oil . Food Chemistry , 96 ( 2 ) : 304 – 312 .
- Hassel , R. L. 1976 . Thermal analysis: An alternative method of measuring oil stability . Journal of the American Oil Chemists’ Society , 53 ( 5 ) : 179 – 181 .
- Rudnik , E. , Szczucinska , A. , Gwardiak , H. , Szulc , A. and Winiarska , A. 2001 . Comparative studies of oxidative stability of linseed oil . Thermochimica Acta , 370 ( 1–2 ) : 135 – 140 .
- Sathivel , S. 2005 . Thermal and flow properties of oils from salmon heads . Journal of the American Oil Chemists’ Society , 82 ( 2 ) : 147 – 152 .
- Cheikh-Rouhou , S. , Besbes , S. , Hentati , B. , Blecker , C. , Deroanne , C. and Attia , H. 2007 . Nigella Sativa L.: Chemical composition and physicochemical characteristics of lipid fraction . Food Chemistry , 101 ( 2 ) : 673 – 681 .
- Huang , J. and Sathivel , S. 2008 . Thermal and rheological properties and the effects of temperature on the viscosity and oxidation rate of unpurified salmon oil . Journal of Food Engineering , 89 ( 2 ) : 105 – 111 .
- Hopper , T.H. and Nesbitt , L.L. 1937 . Relation between the refractive index and iodine number of raw linseed oil . Oil and Soap , 14 ( 2 ) : 34 – 36 .